- 1School of Public Health, Jiamusi University, Jiamusi, China
- 2Department of Food Hygiene Monitoring, Jiamusi City Center for Disease Control and Prevention, Jiamusi, China
- 3Director, Jiamusi City Center for Disease Control and Prevention, Jiamusi, China
Background: Levetiracetam (LEV) and oxcarbazepine (OXC) are new antiseizure medications (ASMs). In recent years, OXC monotherapy is widely used in children with epilepsy; however, no consensus exists on applying LEV monotherapy among children with epilepsy.
Objective: The present work focused on comparing the efficacy and safety of LEV and OXC monotherapy in treating children with epilepsy.
Methods: We conducted a comprehensive search across multiple databases including PubMed, Cochrane Library, Embase, Web of Science, CNKI, Wanfang Database, VIP, and China Biology Medicine disc, covering studies from inception to August 26, 2023. We included randomized controlled trials (RCTs) and cohort studies evaluating the efficacy and safety of LEV and OXC monotherapy for treating epilepsy in children. We utilized Cochrane Risk of Bias Tool in RevMan 5.3 software for assessing included RCTs quality. In addition, included cohort studies quality was determined using Newcastle-Ottawa Scale (NOS). A random-effects model was utilized to summarize the results.
Results: This meta-analysis included altogether 14 studies, including 893 children with epilepsy. LEV and OXC monotherapy was not statistical different among children with epilepsy in seizure-free rate (relative risk [RR] = 1.010, 95% confidence interval [CI] [0.822, 1.242], P > 0.05) and seizure frequency decrease of ≥50% compared with baseline [RR = 0.938, 95% CI (0.676, 1.301), P > 0.05]. Differences in total adverse reaction rate [RR = 1.113, 95% CI (0.710, 1.744), P > 0.05] and failure rate because of serious adverse reaction [RR = 1.001, 95% CI (0.349, 2.871), P > 0.05] were not statistical different between LEV and OXC treatments among children with epilepsy. However, the effects of OXC monotherapy on thyroid among children with epilepsy was statistically correlated than that of LEV (thyroid stimulating hormone: standardized mean difference [SMD] = −0.144, 95% CI [−0.613, 0.325], P > 0.05; free thyroxine: SMD = 1.663, 95% CI [0.179, 3.147], P < 0.05).
Conclusion: The efficacy of LEV and OXC monotherapy in treating children with epilepsy is similar. However, OXC having a more significant effect on the thyroid than that of LEV. Therefore, LEV may be safer for children with epilepsy who are predisposed to thyroid disease than OXC.
Systematic Review Registration: https://www.crd.york.ac.uk/, PROSPERO (CRD42024514016)
1 Introduction
Epilepsy represents the commonly occurring chronic brain disease causing mortality in approximately 125,000 individuals annually (1). It is characterized by recurrent seizures, and children's brains are not completely developed; repeated seizures often cause critical neurological damage, and can lead to intellectual disability in severe cases (2). Based on the statistics, the global incidence of childhood epilepsy is 41–187/100,000; and its global prevalence is higher than its incidence. To be specific, the prevalence of childhood epilepsy in developed and less developed countries is 3.2–5.5/1,000 and 3.6–44/1,000, respectively (3). Epilepsy in children is primarily treated by antiseizure medications (ASMs). ASMs have exhibited good efficacy in approximately 70% of pediatric patients (4).
Since 1990, more than 20 novel ASMs have been introduced, with similar efficacy but better safety compared to traditional ASMs (5). A novel ASM, oxcarbazepine (OXC), is a derivative that improves the safety and pharmacokinetic characteristics of carbamazepine (CBZ) and reduces the interaction between CBZ and other ASMs (6). OXC's mechanism of action blocks sodium channels and controls the abnormal firing of neurons (7). Another novel ASM, levetiracetam (LEV), demonstrates the antiepileptic mechanism of action through combination with synaptic vesicle protein SV2A, interference with neurotransmitter release in vesicles, control of rapid firing of neurons, inhibition of Ca2+ release and blockage of Ca2+ channels, and control of the excessive synchronization between neurons (8). It is advantageous owing to its high bioavailability, linear pharmacokinetics, low plasma protein binding, no liver metabolism, renal excretion, and good tolerance (9).
In recent times, monotherapy with OXC has gained widespread use in treating childhood epilepsy. Nevertheless, there is no consensus regarding the utilization of LEV as a monotherapy in this same patient population. OXC has received global registration in over 50 countries. OXC monotherapy is extensively employed in numerous nations, such as the United States, China, and Europe, to manage epilepsy in children (10–12). While LEV has been granted approval for monotherapy use in children with epilepsy in China, it has only been sanctioned for monotherapy for this purpose in European children aged ≥16 years. Notably, it has not received approval for treating children with epilepsy as a monotherapy in the USA (13). The 2018 American Academy of Neurology Guidelines and recommendations from Belgian epilepsy experts in 2020 advise OXC being used alone or as adjuvant treatment for childhood epilepsy, whereas LEV is suggested for adjunctive treatment (14, 15).
Systematic reviews and meta-analyses of mixed ASMs types, which encompass not only LEV and OXC but also various other ASMs, has revealed that LEV and OXC are comparable in terms of the efficacy and safety on epilepsy in pediatric patients (16, 17). Nevertheless, recent studies published within the last five years have produced varying results concerning both the efficacy and safety of these medications. Zhao et al. (18) indicated that OXC monotherapy was more high than LEV on treating focal epileptic infants in seizure freedom and the 12-month retention rate. Shi et al. (19) demonstrated that, in the context of childhood epilepsy, OXC monotherapy was more associated with adverse effects on thyroid function and bone metabolic disturbances when compared to LEV monotherapy.
Additionally, when it comes to systematic reviews and meta-analyses of mixed ASMs types, there is a scarcity of literature comparing the efficacy and safety of LEV and OXC treatments alone in treating epilepsy in pediatric patients. Only a maximum of six articles available, and have not provided comparison between LEV and OXC treatments alone on managing childhood epilepsy, in terms of failure rate because of serious adverse reaction, and effects on the thyroid gland. Therefore, this study has encompassed data from 14 different studies involving 893 participants to provide a comprehensively compare the efficacy and safety of LEV and OXC monotherapy in the treatment of childhood epilepsy. This endeavor seeks to offer updated and more dependable evidence to inform the clinical application of LEV treatment alone in treating childhood epilepsy.
2 Methods
2.1 Study screening procedure
Our systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20). Our research protocol has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024514016). Four English databases (PubMed, Cochrane Library, Embase, Web of Science) together with four Chinese databases (China National Knowledge Infrastructure [CNKI], Wanfang Database, China Science and Technology Journal Database [VIP], China Biology Medicine disc), were comprehensively searched to identify published studies examining the efficacy and safety of LEV and OXC treatments alone in treating pediatric epilepsy from their inception to August 26, 2023. Search terms included “Levetiracetam”, “Keppra”, “Oxcarbazepine”, “Trileptal”, “Child”, “Children”, “Infant”, “Adolescent”, “Pediatrics”, “Epilepsy”, and “Seizure Disorder.” Supplementary Appendix S1 displays detailed results regarding PubMed search strategy. Additionally, relevant reviews, systematic reviews, meta-analyses from last three years, as well as references from the included studies, were manually scrutinized to avoid overlooking qualified studies.
2.2 Inclusion and exclusion criteria
Studies below were included: (1) Participants: Children with epilepsy under 18 years, irrespective of epilepsy type, gender, ethnicity, or geographic location. (2) Interventions: The LEV and OXC groups received LEV and OXC monotherapies, respectively, without restrictions on dosage form, dosage, route of administration, frequency, or treatment duration. (3) Outcomes: seizure-free rate (children with epilepsy treated with LEV and OXC monotherapy had no more seizures or breakthrough seizures only with missed doses of medication), seizure frequency decrease of ≥50% compared with baseline (seizure frequency decrease of ≥50% compared with baseline in children with epilepsy treated with LEV and OXC monotherapy), total adverse reaction rate (the total number of adverse effects observed in children with epilepsy treated with LEV and OXC monotherapy), failure rate because of serious adverse reaction (defined as adverse effects leading to the addition of a second ASM, change to another ASM, or discontinuation of ASM treatment), and effects on the thyroid (thyroid stimulating hormone[TSH] and free thyroxine [fT4] levels before and after LEV and OXC monotherapy in children with epilepsy). One or more primary and secondary outcome indicators must be reported. (4) Study Types: Randomized controlled trials (RCTs) and cohort studies conducted in both Chinese and English languages.
Studies below were excluded: (1) Duplicates. (2) Reviews, systematic reviews, meta-analyses, guidelines, commentaries, conference abstracts, case reports, letters, and animal studies. (3) Unavailability of full-text, unpublished literatures. (4) Incorrect or incomplete data, or inability to provide data that can be transformed into aggregated data. (5) Chinese literatures not published in Peking University's “A Guide to the Core Journal of China.”
2.3 Study selection and data collection
Those searched articles were imported in EndNote 20 software. Two researchers were responsible for study selection and data collection according to inclusion and exclusion criteria. A third researcher assisted in making the final decision for any disputed sections. Using Excel 2019, data below were collected, first author, publication year, country, study type, sample size per group, sex, age, therapeutic time, and outcome.
2.4 Study quality assessment
We utilized Cochrane Risk of Bias Tool (21) in RevMan 5.3 software for assessing included RCTs quality. In addition, included cohort studies quality was determined using Newcastle-Ottawa Scale (NOS) (22). The quality assessment process was performed independently by two researchers. A third researcher assisted in resolving any disputes. Seven items were included in Cochrane Risk of Bias Assessment Tool: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, together with other bias. Besides, every item was classified into unclear, low, or high bias risk. NOS consisted of three sections: selection, comparability, and outcome, its total score was 9 and ≥6 scores indicated high-quality studies.
2.5 Statistical analysis
Stata 15.1 software was employed for statistical analysis. Dichotomous variables were analyzed by relative risk (RR) together with 95% confidence intervals (95% CIs) for effect sizes, and P < 0.05 stood for statistical significance. Continuous variables were analyzed by standardized mean difference (SMD) and 95% CIs for effect sizes, and P < 0.05 stood for statistical significance. At least three original studies for each outcome indicator were combined. Continuous variables were combined directly using post-treatment in the absence of significant difference before treatment; otherwise, changes before and after treatment were combined. Effect sizes were combined with random-effects models.
3 Results
3.1 Study selection results and basic study characteristics
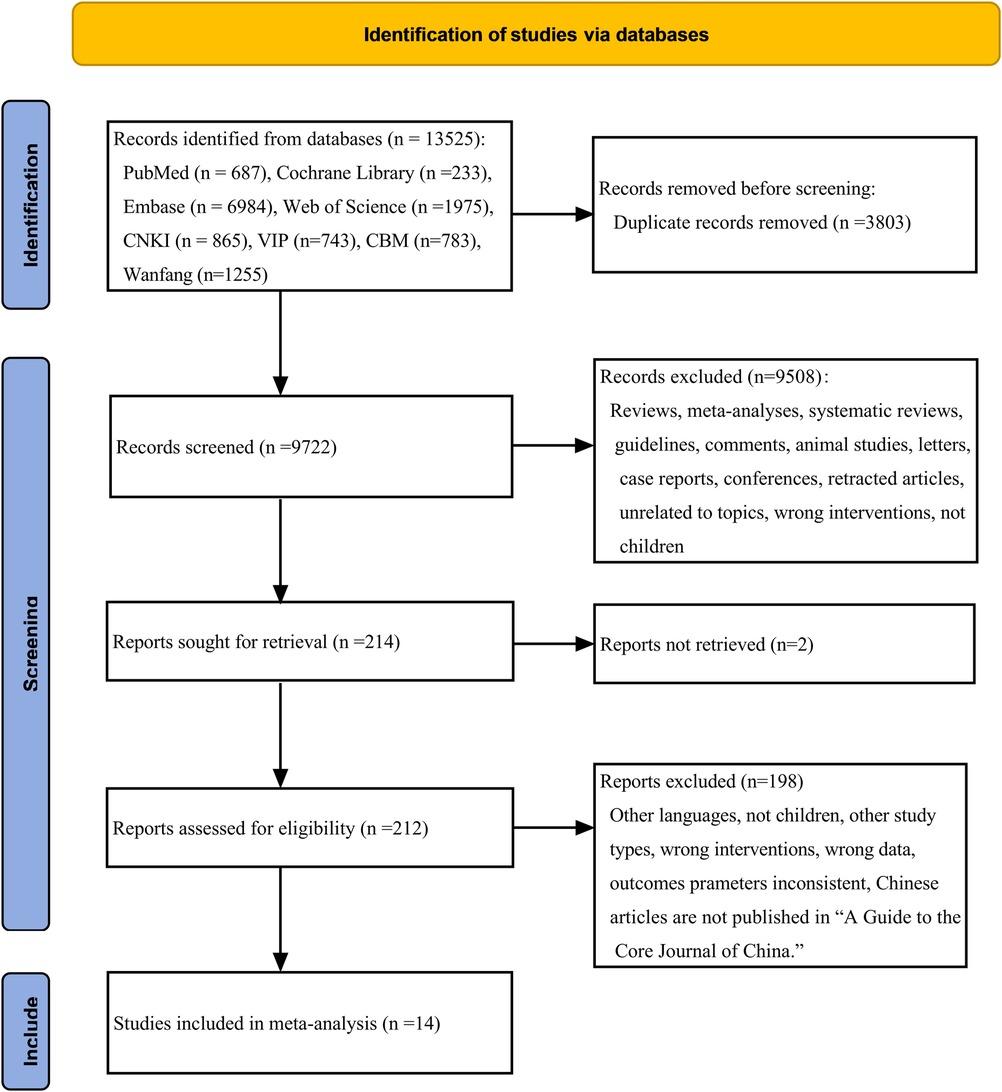
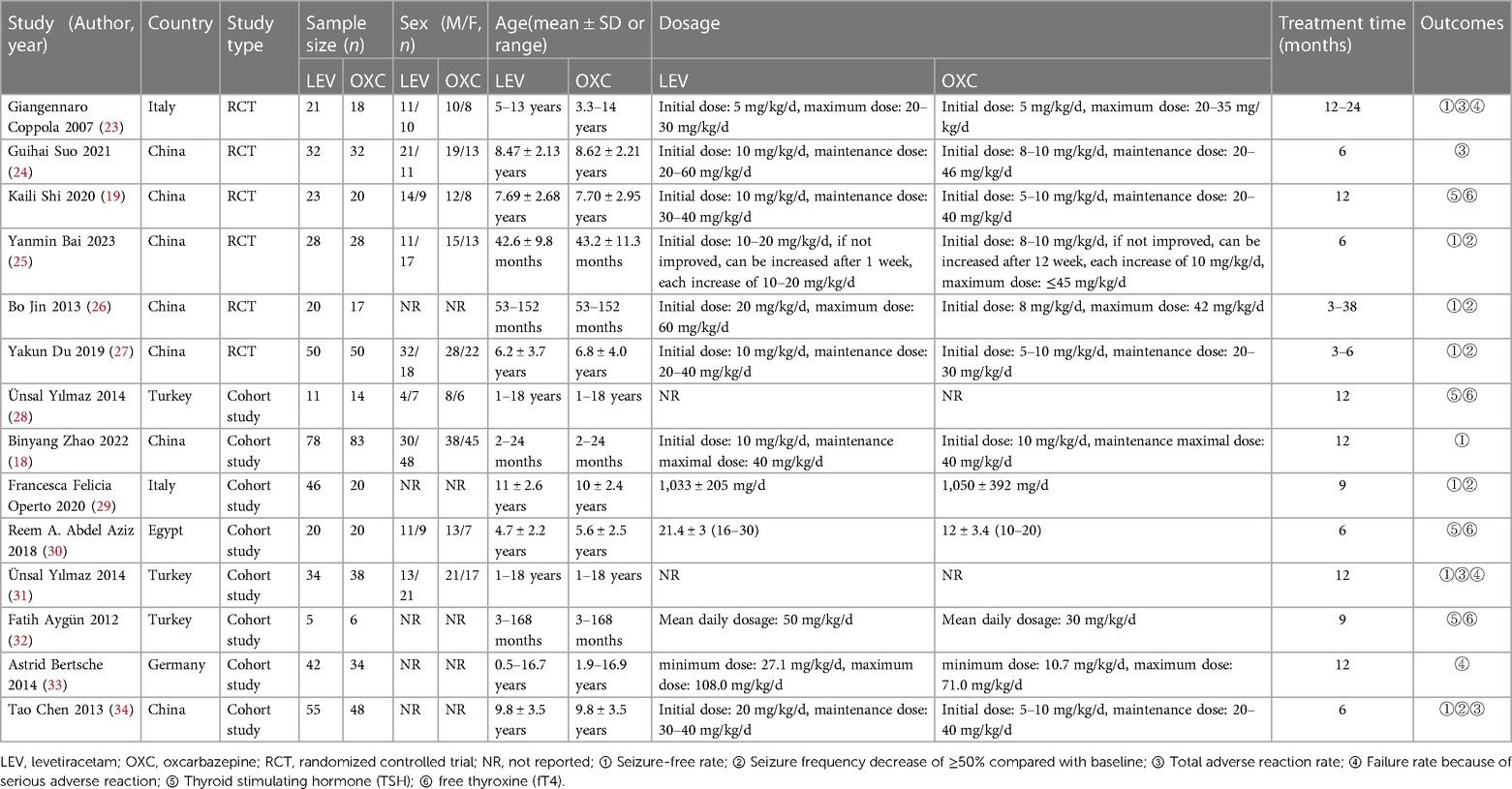
13,525 studies were initially identified. After duplicate removal and thorough title-, abstract- and full-text-reading, we ultimately chose 14 articles for this meta-analysis. This specific process is outlined in Figure 1. This comprised 893 children with epilepsy, with 465 of them undergoing LEV treatment and 428 receiving OXC. The selected literature ranged from 2007 to 2023 and encompassed six RCTs (19, 23–27) and eight cohort studies (18, 28–34). Among these, eight studies reported on the efficacy (18, 23, 25–27, 29, 31, 34), while nine studies presented data on safety (19, 23, 24, 28, 30–34). The required details about those enrolled articles are displayed in Table 1.
3.2 Quality evaluation of the literature
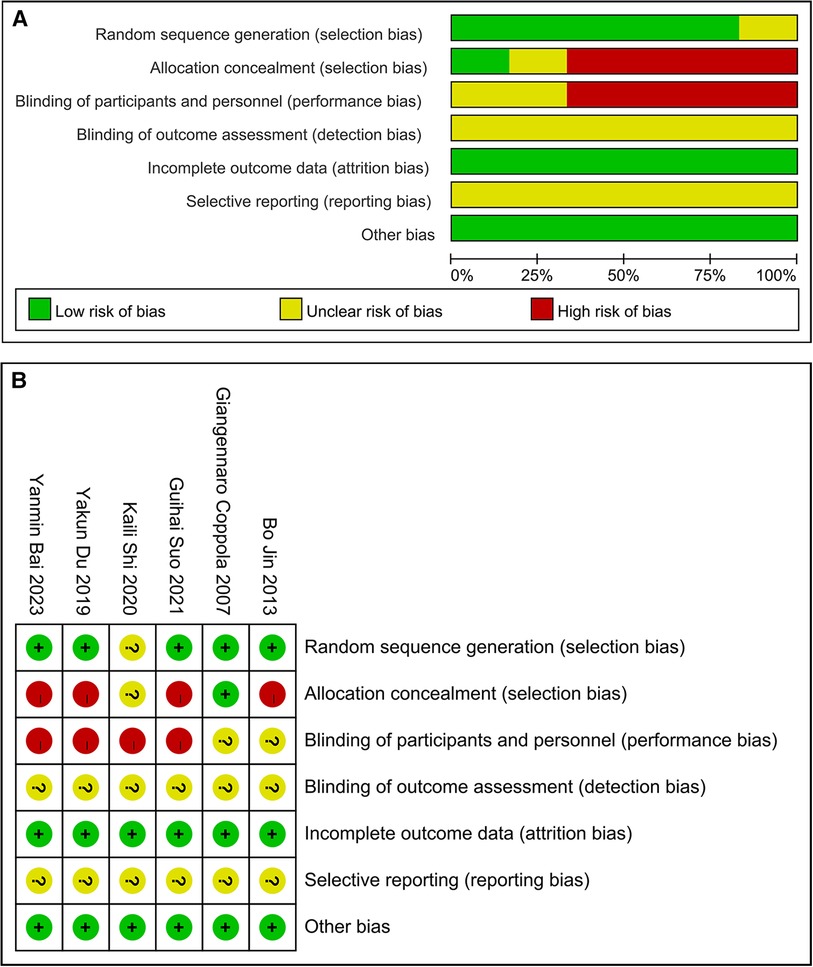
The assessment of the quality of six RCTs revealed that in five of them, generation of random sequence and in one, concealment of allocation showed a low bias risk. In four RCTs, a high bias risk was detected in blinding participants and personnel, while in two, there remained an unclear bias risk. All the articles had an unclear bias risk regarding blinding of outcome assessment. All the articles had a low bias risk regarding incomplete outcome data. All the articles had an unclear bias risk regarding selective reporting. Furthermore, in these articles, other bias risk showed a low bias risk. These findings can be observed from Figure 2.
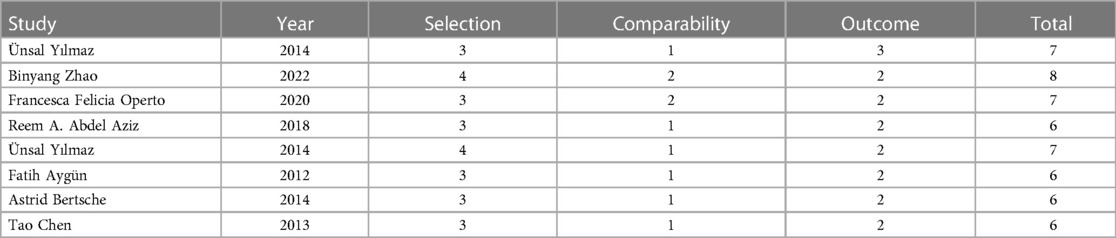
Quality evaluation for the eight cohort studies demonstrated one, three, and four studies with a total score of eight, seven, and six (Table 2).
3.3 Meta-analysis results
3.3.1 Efficacy outcomes
3.3.1.1 Seizure-free rate
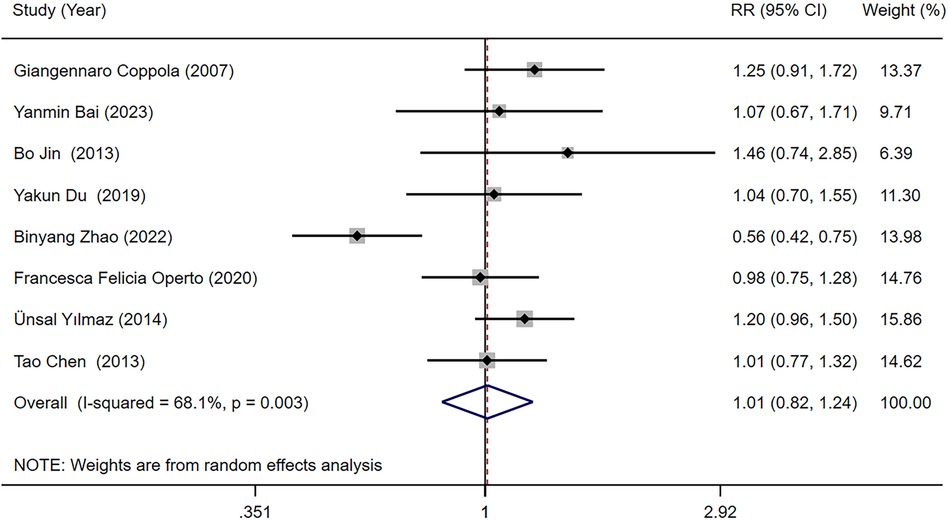
Eight studies (18, 23, 25–27, 29, 31, 34) reported the seizure-free rate in LEV and OXC treatments alone for children with epilepsy. Our meta-analysis results demonstrated that seizure-free rate [RR = 1.010, 95% CI (0.822, 1.242), P = 0.923 > 0.05] was not significantly different between two treatments (Figure 3). We conducted subgroup analysis based on study type, which indicated no statistically difference in seizure-free rate between LEV and OXC in the treatment of childhood epilepsy, either in RCTs or cohort studies (RCTs, RR = 1.171, 95% CI [0.950, 1.443], P = 0.139 > 0.05; cohort studies, RR = 0.908, 95% CI [0.651, 1.266], P = 0.569 > 0.05) (Figure 4).
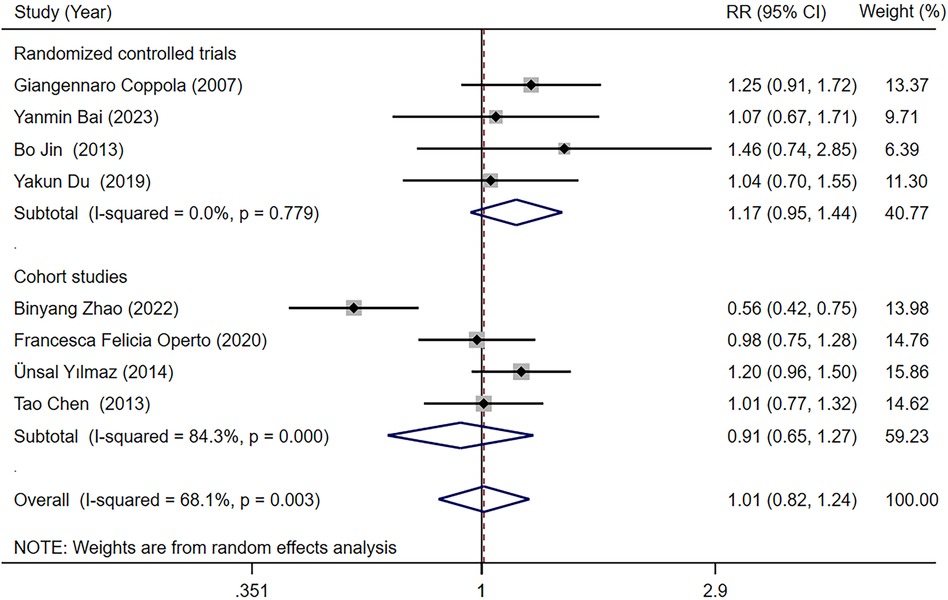
Figure 4. A forest plot of subgroup analysis for the seizure-free rate of levetiracetam (LEV) vs. oxcarbazepine (OXC).
3.3.1.2 Seizure frequency decrease ≥50% compared with baseline
Five studies (25–27, 29, 34) reported the seizure frequency decrease ≥50% compared with baseline in LEV and OXC treatments alone for children with epilepsy. Our meta-analysis results suggested that seizure frequency decrease ≥50% compared with baseline was not significantly different between two treatments [RR = 0.938, 95% CI (0.676, 1.301), P = 0.700 > 0.05] (Figure 5).
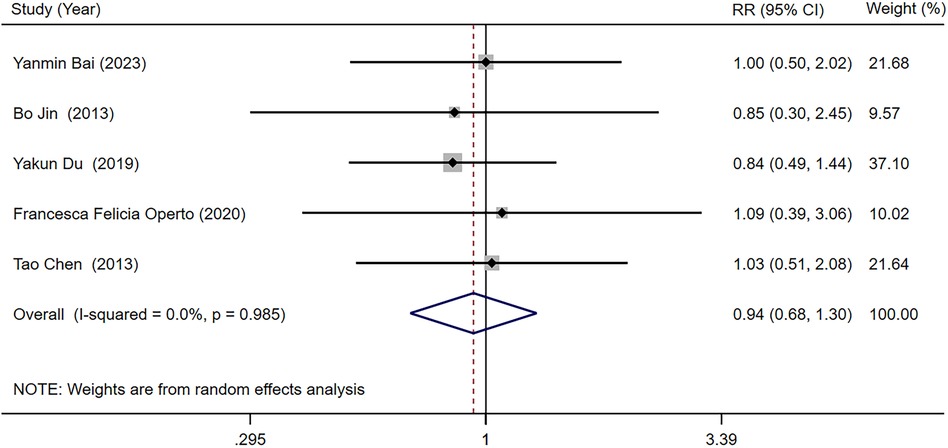
Figure 5. A forest plot of the seizure frequency decrease ≥50% compared with baseline of levetiracetam (LEV) vs. oxcarbazepine (OXC).
3.3.2 Safety outcomes
3.3.2.1 Total adverse reaction rate
Four studies (23, 24, 31, 34) provided information regarding total adverse reaction rate in children with epilepsy receiving LEV and OXC monotherapy. Our meta-analysis outcomes suggested that total adverse reaction rate was not significantly different between two treatments [RR = 1.113, 95% CI (0.710, 1.744), P = 0.640 > 0.05] (Figure 6).

Figure 6. A forest plot of the total adverse reaction rate of levetiracetam (LEV) vs. oxcarbazepine (OXC).
3.3.2.2 Failure rate because of serious adverse reaction
Three studies (23, 31, 33) provided data on the failure rate because of serious adverse reaction among children with epilepsy undergoing LEV and OXC monotherapy. Our meta-analysis results indicated that the failure rate because of serious adverse reaction was not significantly different between two groups [RR = 1.001, 95% CI (0.349, 2.871), P = 0.999 > 0.05] (Figure 7).
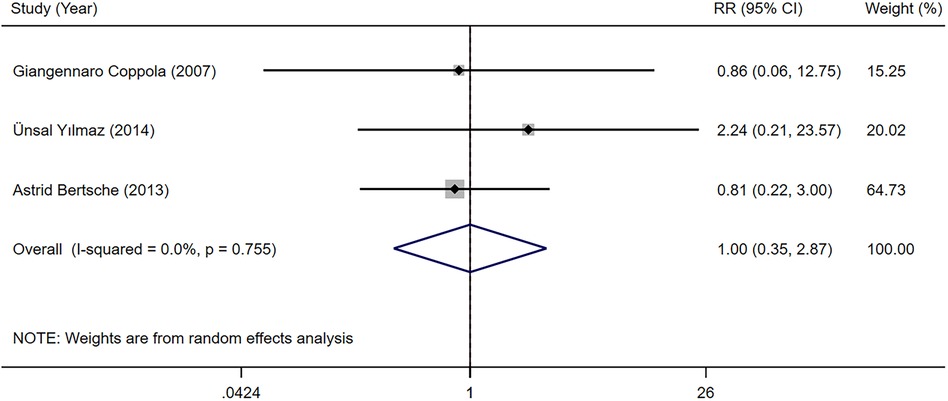
Figure 7. A forest plot of the failure rate because of serious adverse reaction of levetiracetam (LEV) vs. oxcarbazepine (OXC).
3.3.2.3 Effects on the thyroid gland
The TSH and fT4 levels in children with epilepsy before and after LEV and OXC monotherapy were reported in four studies (19, 28, 30, 32). According to our meta-analysis finding, TSH and fT4 levels were not significantly different between LEV and OXC before treatment (Supplementary Appendix S2, S3). Therefore, the levels of TSH and fT4 after treatment were directly combined. According to our meta-analysis finding, the effects of LEV and OXC on TSH was not significantly different [SMD = −0.144, 95% CI (−0.613, 0.325), P = 0.548 > 0.05] (Figure 8). However, OXC-reduced fT4 levels were statistically correlated than that of LEV [SMD = 1.663, 95% CI (0.179, 3.147), P = 0.028 < 0.05] (Figure 9).
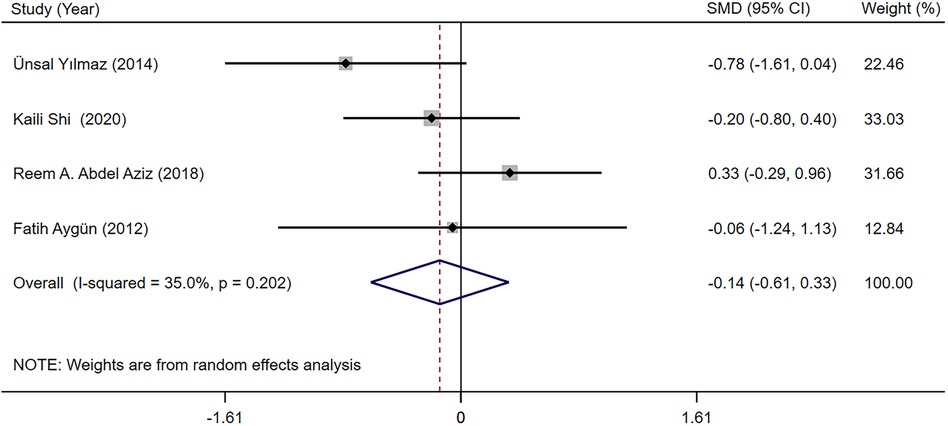
Figure 8. A forest plot of the effect on thyroid stimulating hormone (TSH) levels of levetiracetam (LEV) vs. oxcarbazepine (OXC).
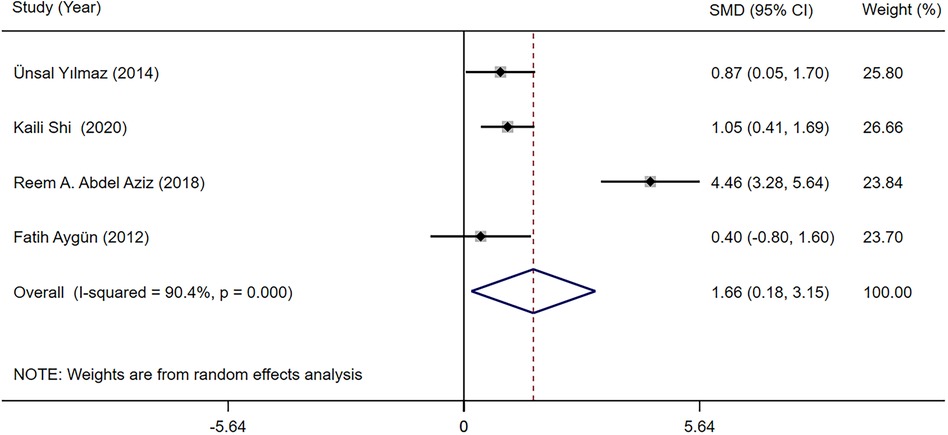
Figure 9. A forest plot of the effect on free thyroxine (fT4) levels of levetiracetam (LEV) vs. oxcarbazepine (OXC).
3.4 Sensitivity analysis and publication bias
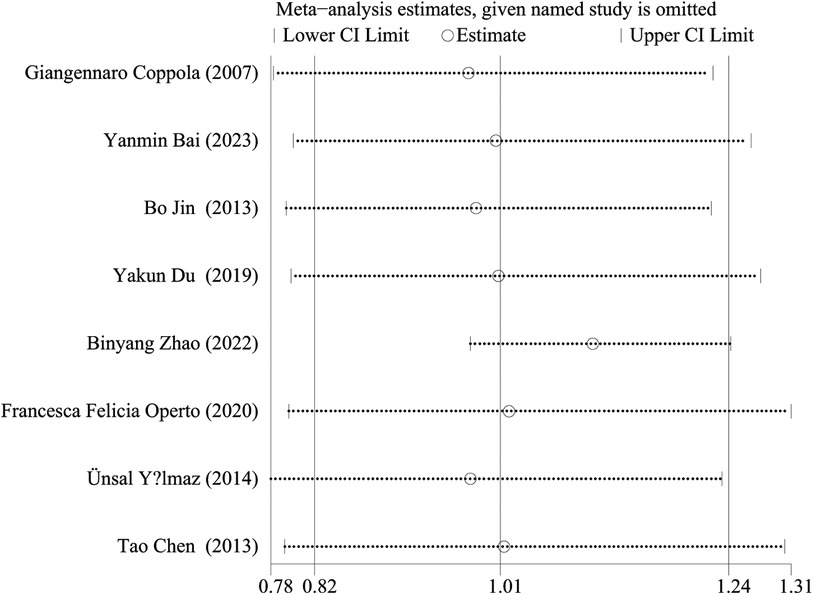
We performed a sensitivity analysis by comparing the results of the meta-analysis after the exclusion of each study with the results of the meta-analysis before the exclusion. The results showed that there was no statistical difference in seizure free rate between LEV and OXC monotherapy in children with epilepsy remained stable and reliable (Figure 10).
A minimum of 10 studies should be included to use the funnel plot asymmetry test, based on Cochrane Handbook for Systematic Reviews of Interventions (35), and if too few studies are included, the efficacy of the test will be too low, failing to truly differentiate between symmetry or not. Therefore, we did not assess publication bias in the present work.
4 Discussion
Our systematic review and meta-analysis yielded the following insights: (1) Seizure-free rate and seizure frequency decrease ≥50% compared with baseline were not significantly different in children with epilepsy when comparing LEV and OXC monotherapy. (2) Total adverse reaction rate, failure rate because of severe adverse reaction, between LEV and OXC treatments alone were not significantly different. However, the effects of OXC on the thyroid was greater than that of LEV.
LEV and OXC are considered as new ASMs. LEV is primarily utilized to be adjuvant treatment in children with epilepsy, whereas OXC is applied as monotherapy or adjuvant treatment in pediatric epilepsy. Numerous studies have indicated that OXC, when employed as a monotherapy or adjunctive treatment for childhood epilepsy, can yield favorable outcomes (36–38). We compared the efficacy of LEV and OXC monotherapy in children with epilepsy, and our results indicated that there were no statistical differences in seizure-free rate and seizure frequency decrease of ≥50% compared with baseline. Some prior studies have reported results consistent with our findings. Geng et al. (16) discovered that seizure-free rates and seizure frequency decrease of more than 75%, 50%–75%, or less than 50% compared with baseline were not significantly different between OXC and LEV monotherapies among children with epilepsy. Similarly, Zhang et al. (17) suggested that seizure-free rate and seizure frequency decrease ≥50% compared with baseline were not significantly different when comparing LEV and OXC monotherapy among children with epilepsy. Coppola et al. (23) also observed that seizure-free rate was not significantly different in LEV vs. OXC treatment alone for children with benign epilepsy with centrotemporal spikes.
ASMs-related adverse reactions notably affect patient life quality and treatment adherence among individuals with epilepsy, potentially leading to discontinuation of therapy (39). In this study, the incidence of total adverse reaction for LEV and OXC was 22.53% and 20.59%, respectively. Both LEV and OXC displayed a favorable safety profile, with the majority of adverse effects being mild. Nonetheless, some serious adverse reactions, including headache, behavioral and emotional disturbances, diplopia, and rashes, resulted in treatment failure. Our findings revealed no statistical difference in total adverse reaction rate, and failure rate because of serious adverse reaction, in LEV vs. OXC when treating children with epilepsy. These results align with some previous studies. For instance, Suo et al. (24) demonstrated that LEV and OXC monotherapies were not significantly different with regard to total adverse reaction rate, among children with benign epilepsy with centrotemporal spikes. Bertsche et al. (33) found that the treatment failure rate because of adverse drug reaction was not significantly different between LEV vs. OXC monotherapy in children with focal epilepsy. Therefore, it is essential for children with epilepsy receiving LEV or OXC for initiating treatment with a low dose, progressively increase the dosage, monitor blood drug concentrations as needed, and maintain close follow-up to promptly detect and address adverse effects as they arise.
Thyroid hormones have a crucial effect on central nervous system development, normal physiological functions of the brain, and repair mechanisms (40). Even minor alterations in thyroid hormones, including subclinical hypothyroidism, can hinder growth and development in children (41). ASMs can influence thyroid hormone biosynthesis, production, transportation, metabolism, and excretion, causing varying degrees of harm to in thyroid-hormone homeostasis (42). Thyroid irregularities have been reported in one-third of epilepsy patients taking ASMs (43). Our research found no statistical notable difference in TSH levels between LEV and OXC monotherapies among children with epilepsy. However, OXC is linked to more reduction in fT4 levels compared to LEV. Some prior studies have reported findings in line with our study. For instance, Yılmaz et al. (28) revealed that serum fT4 levels did not significantly change after 12 months of LEV monotherapy among children with epilepsy, and the incidence of subclinical hypothyroidism was 0%. In contrast, the level of fT4 decreased after 12 months of OXC monotherapy, with an incidence rate of 21.4% for subclinical hypothyroidism. Aziz et al. (30) suggested that TSH and fT4 levels were not significantly changed after LEV treatment for over 6 months in children with epilepsy. They noted that TSH level were not significantly changed when treating childhood epilepsy with OXC for over 6 months. However, fT4 levels decreased. Therefore, it is advisable to closely monitor thyroid function in children with epilepsy who are administered OXC. For children with epilepsy prone to thyroid issues, OXC treatment should be approached with caution.
This systematic review and meta-analysis have some limitations: (1) We included 14 studies, but some had relatively small sample sizes, potentially affecting result accuracy. (2) The limited number of included studies makes it challenging to compare the efficacy and safety of LEV and OXC monotherapies in children across different age groups. (3) There was heterogeneity due to differences in epilepsy diagnostic criteria, epilepsy type, dosage, and treatment duration, which may weaken the strength of the evidence. (4) The lack of uniform criteria and quantitative evaluation for adverse reactions of LEV and OXC, coupled with the limited number of studies included, pose challenges in gathering comprehensive data on adverse reactions of LEV and OXC monotherapy in the treatment of children with epilepsy. Hence, additional large-sample, high-quality RCTs should be conducted for confirming efficacy and safety of LEV compared to OXC in treating children with epilepsy.
5 Conclusion
To sum up, the present systematic review and meta-analysis indicate that LEV and OXC monotherapies achieve comparable efficacy in treating childhood epilepsy. However, the effects of OXC on the thyroid was greater than that of LEV. As a result, LEV may be a preferable choice for children with epilepsy who have a predisposition to thyroid issues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YL: Data curation, Formal Analysis, Software, Writing – original draft. YW: Data curation, Investigation, Software, Writing – review & editing. XL: Conceptualization, Supervision, Writing – review & editing. XW: Data curation, Funding acquisition, Investigation, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Health Commission of Heilongjiang Province (No. 20211212050384).
Acknowledgments
The authors thank the authors of the original studies in this meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1336744/full#supplementary-material
References
1. Singh G, Sander JW. The global burden of epilepsy report: implications for low- and middle-income countries. Epilepsy Behav. (2020) 105:106949. doi: 10.1016/j.yebeh.2020.106949
2. Zhu YF, Yang JL, Zhu XJ. Combined effects of levetiracetam and sodium valproate on paediatric patients with epilepsy: a systematic review and meta-analysis. Seizure. (2022) 95:17–25. doi: 10.1016/j.seizure.2021.12.003
3. Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. (2015) 17:117–23. doi: 10.1684/epd.2015.0736
4. Moosa ANV. Antiepileptic drug treatment of epilepsy in children. Continuum (Minneap Minn). (2019) 25:381–407. doi: 10.1212/CON.0000000000000712
5. Pong AW, Xu KJ, Klein P. Recent advances in pharmacotherapy for epilepsy. Curr Opin Neurol. (2023) 36:77–85. doi: 10.1097/WCO.0000000000001144
6. Beydoun A, DuPont S, Zhou D, Matta M, Nagire V. Current role of carbamazepine and oxcarbazepine in the management of epilepsy. Seizure. (2020) 83:251–63. doi: 10.1016/j.seizure.2020.10.018
7. Bresnahan R, Atim-Oluk M, Marson AG. Oxcarbazepine add-on for drug-resistant focal epilepsy. Cochrane Database Syst Rev. (2020) 3:CD012433. doi: 10.1002/14651858.CD012433.pub2
8. Sharma D, Hussain AM, Sharma SS. Efficacy of Levetiracetam in neonatal seizures: a systematic review. J Matern Fetal Neonatal Med. (2022) 35:3923–30. doi: 10.1080/14767058.2020.1844651
9. Yıldırım M, Bektaş Ö, Göktaş AÖ, Yüksel MF, Şahin S, Teber ST. Levetiracetam monotherapy in children with epilepsy: experience from a tertiary pediatric neurology center. Epilepsy Behav. (2021) 116:107745. doi: 10.1016/j.yebeh.2020.107745
10. Glauser TA, Nigro M, Sachdeo R, Pasteris LA, Weinstein S, Abou-Khalil B, et al. Adjunctive therapy with oxcarbazepine in children with partial seizures. The oxcarbazepine pediatric study group. Neurology. (2000) 54:2237–44. doi: 10.1212/wnl.54.12.2237
11. Bourgeois BFD, D’Souza J. Long-term safety and tolerability of oxcarbazepine in children: a review of clinical experience. Epilepsy Behav. (2005) 7:375–82. doi: 10.1016/j.yebeh.2005.07.017
12. Qin J, Wang Y, Huang XF, Zhang YQ, Fang F, Chen YB, et al. Oxcarbazepine oral suspension in young pediatric patients with partial seizures and/or generalized tonic-clonic seizures in routine clinical practice in China: a prospective observational study. World J Pediatr. (2018) 14:280–9. doi: 10.1007/s12519-017-0114-6
13. Ansari ZAH, Levetiracetam: pharmacological properties, safety and efficacy in the pediatric population with epilepsy. J Pediatr Neurol. (2014) 12:1–13. doi: 10.3233/JPN-140633
14. Kanner AM, Ashman E, Gloss D, Harden C, Bourgeois B, Bautista JF, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment-resistant epilepsy: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology and the American epilepsy society. Neurology. (2018) 91:82–90. doi: 10.1212/WNL.0000000000005756
15. Boon P, Santos SF, Jansen AC, Lagae L, Legros B, Weckhuysen S. Recommendations for the treatment of epilepsy in adult and pediatric patients in Belgium: 2020 update. Acta Neurol Belg. (2021) 121(1):241–57. doi: 10.1007/s13760-020-01488-y
16. Geng H, Wang CZ. Efficacy and safety of oxcarbazepine in the treatment of children with epilepsy: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. (2017) 13:685–95. doi: 10.2147/NDT.S130269
17. Zhang LL, Wang CZ, Li W. A meta-analysis of randomized controlled trials on levetiracetam in the treatment of pediatric patients with epilepsy. Neuropsychiatr Dis Treat. (2018) 14:769–79. doi: 10.2147/NDT.S151413
18. Zhao BY, Liao S, Zhong XF, Lou YY, Hong SQ, Chen M, et al. Effectiveness and safety of oxcarbazepine vs. levetiracetam as monotherapy for infantile focal epilepsy: a longitudinal cohort study. Front Neurol. (2022) 13:909191. doi: 10.3389/fneur.2022.909191
19. Shi KL, Guo JX, Zhao HM, Hong H, Yang CZ, Wu YH, et al. The effect of levetiracetam and oxcarbazepine monotherapy on thyroid hormones and bone metabolism in children with epilepsy: a prospective study. Epilepsy Behav. (2020) 113:107555. doi: 10.1016/j.yebeh.2020.107555
20. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 Explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J (2021) 372:n160. doi: 10.1136/bmj.n160
21. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Coppola G, Franzoni E, Verrotti A, Garone C, Sarajlija J, Operto FF, Pascotto A, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open-label, parallel group trial. Brain Dev. (2007) 29:281–4. doi: 10.1016/j.braindev.2006.09.008
24. Suo GH, Zheng YQ, Wu YJ, Tang JH. Effects of levetiracetam and oxcarbazepine monotherapy on intellectual and cognitive development in children with benign epilepsy with centrotemporal spikes. Acta Neurol Belg. (2021) 121:1265–73. doi: 10.1007/s13760-021-01613-5
25. Bai YM, Du KX, Chen H, Jia TM, Gong H, Yu SY, et al. Effect of sodium valproate, oxcarbazepine and levetiracetam on the development of different functional areas in children with epilepsy by griffiths development scales-Chinese edition. Chin Gen Pract. (2023) 26:2918–22. doi: 10.12114/j.issn.1007-9572.2023.0102
26. Jin B, Zheng G, Lu XP, Jin JP. Effects of three antiepileptic drugs on clinical efficacy, electroencephalogram and cognitive function in children with benign epilepsy and electrical status epilepticus during sleep. Acta Univ Med Nanjing (Nat Sci). (2013) 33:1754–56. doi: 10.7655/NYDXBNS20131229
27. Du YK, Chen F, Wang L, Zhao DY, Liu J, Sun SZ. Effect of levetiracetam on electroencephalogram and cognitive function in children with partial seizures. Chin Gen Pract. (2019) 22:1689–95. doi: 10.12114/j.issn.1007-9572.2019.00.027
28. Yılmaz Ü, Yılmaz TS, Akıncı G, Korkmaz HA, Tekgül H. The effect of antiepileptic drugs on thyroid function in children. Seizure. (2014) 23:29–35. doi: 10.1016/j.seizure.2013.09.006
29. Operto FF, Pastorino GMG, Mazza R, Carotenuto M, Roccella M, Marotta R, et al. Effects on executive functions of antiepileptic monotherapy in pediatric age. Epilepsy Behav. (2020) 102:106648. doi: 10.1016/j.ejpn.2021.04.004
30. Aziz RAAA, ELela MA. Is it necessary to measure thyroid hormone levels in children receiving antiepileptic drugs? J Pediatr Epilepsy. (2018) 7:136–41. doi: 10.1055/s-0038-1648242
31. Yılmaz U, Yılmaz TS, Dizdarer G, Akıncı G, Güzel O, Tekgül H. Efficacy and tolerability of the first antiepileptic drug in children with newly diagnosed idiopathic epilepsy. Seizure. (2014) 23:252–9. doi: 10.1016/j.seizure.2013.12.001
32. Aygün F, Ekici B, Aydinli N, Aydin BK, Baş F, Tatli B. Thyroid hormones in children on antiepileptic therapy. Int J Neurosci. (2012) 122:69–73. doi: 10.3109/00207454.2011.627486
33. Bertsche A, Neininger MP, Dahse AJ, Syrbe S, Bernhard MK, Frontini R, et al. Initial anticonvulsant monotherapy in routine care of children and adolescents: levetiracetam fails more frequently than valproate and oxcarbazepine due to a lack of effectiveness. Eur J Pediatr. (2014) 173:87–92. doi: 10.1007/s00431-013-2125-1
34. Chen T, Guo QL, Yang YL. Efficacy comparison of levetiracetam and oxcarbazepine monotherapy on children epilepsy. Chongqing Med. (2013) 42:1826–7. doi: 10.3969/j.issn.1671-8348.2013.16.011
35. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
36. Arya R, Glauser TA. Pharmacotherapy of focal epilepsy in children: a systematic review of approved agents. CNS Drugs. (2013) 27:273–86. doi: 10.1007/s40263-013-0048-z
37. Guerreiro MM, Vigonius U, Pohlmann H, de Manreza ML, Fejerman N, Antoniuk SA, et al. A double-blind controlled clinical trial of oxcarbazepine vs. phenytoin in children and adolescents with epilepsy. Epilepsy Res. (1997) 27:205–13. doi: 10.1016/s0920-1211(97)00025-9
38. Wang Y, Chen YB, Zhang YQ, Luo R, Wang H, Lv JL, et al. Oxcarbazepine oral suspension in pediatric patients with partial seizures and/or generalized tonic-clonic seizures: a multi-center, single arm, observational study in China. World J Pediatr. (2017) 13:551–9. doi: 10.1007/s12519-017-0045-2
39. Kowski AB, Weissinger F, Gaus V, Fidzinski P, Losch F, Holtkamp M. Specific adverse effects of antiepileptic drugs–A true-to-life monotherapy study. Epilepsy Behav. (2016) 54:150–7. doi: 10.1016/j.yebeh.2015.11.009
40. Rochtus AM, Herijgers D, Jansen K, Decallonne B. Antiseizure and thyroid hormone homeostasis: literature review and practical recommendations. Epilepsia. (2022) 63:259–70. doi: 10.1111/epi.17117
41. Cansu A. Antiepileptic drugs and hormones in children. Epilepsy Res. (2010) 89:89–95. doi: 10.1016/j.eplepsyres.2009.09.008
42. Zhang YX, Shen CH, Lai QL, Fang GL, Ming WJ, Lu RY, et al. Effects of antiepileptic drug on thyroid hormones in patients with epilepsy: a meta-analysis. Seizure. (2016) 35:72–9. doi: 10.1016/j.seizure.2016.01.010
Keywords: levetiracetam, oxcarbazepine, monotherapy, children with epilepsy, meta-analysis
Citation: Liu Y, Wang Y, Li X and Wu X (2024) Efficacy and safety of levetiracetam vs. oxcarbazepine in the treatment of children with epilepsy: a systematic review and meta-analysis. Front. Pediatr. 12:1336744. doi: 10.3389/fped.2024.1336744
Received: 11 November 2023; Accepted: 4 April 2024;
Published: 22 April 2024.
Edited by:
Carlotta Spagnoli, Santa Maria Nuova Hospital, ItalyReviewed by:
Francesca Felicia Operto, University of Salerno, ItalySilvia Vincentiis, University of São Paulo, Brazil
© 2024 Liu, Wang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingzhou Li bHh6aHdoZDA0NTRAMTI2LmNvbQ== Xiaomin Wu d3htNTgwNUAxMjYuY29t
†These authors have contributed equally to this work
 Yuanyuan Liu
Yuanyuan Liu Yanxu Wang2
Yanxu Wang2