- 1Department of Pediatrics, Academic Teaching Hospital, Landeskrankenhaus Feldkirch, Feldkirch, Austria
- 2Department of Psychiatry and Psychotherapy, Medical University of Innsbruck, Innsbruck, Austria
Aim: To determine short-term morbidity and mortality rates in the first state-wide Austrian neonatal cohort and comparison to (inter)national data.
Methods: Observational, population-based cohort study, analyzing data of preterm infants (<32 + 0 weeks of gestation) born between 2007 and 2020 (n = 501) in an Austrian state who were admitted to the neonatal intensive care unit. Outcome criteria were mortality, neonatal morbidities: bronchopulmonary dysplasia (BPD), severe necrotizing enterocolitis (NEC), severe intraventricular hemorrhage (IVH grades III–IV), severe retinopathy of prematurity (ROP grades III–V) and survival-free of major complications.
Results: Overall survival rate was 95%, survival free of major complications was 79%. Prevalence for BPD was 11.2%, surgical NEC 4.0%, severe IVH 4.6%, and for severe ROP 2.6%, respectively. In the extremely low gestational age neonates (ELGAN) born <28 weeks of gestation (n = 158), survival was 88% and survival free of major complications 58.8%. Over time, mortality decreased significantly, predominantly driven by the improvement of infants born <28 week of gestation and survival free of major complications improved.
Conclusions: This study demonstrates a very low mortality rate that decreases over time. Short-term morbidities and survival free of major complications do not differ from (inter)national data in a similar group of very preterm infants. Standard operating procedures, simulation trainings and accordance to international trials may improve patient care and surpass center case loads.
Highlights
• First state-wide Austrian study including 501 very preterm infants shows a low mortality, low morbidity and encouraging survival free of major complication rates
• Parameters do not differ from (inter)national data and improve over time, especially in extremely low birth gestational age neonates
• Good results can be reached in a medium-provider volume neonatal intensive care unit with a well organized network
Introduction
Preterm infants with very and extremely low birth weights are at a high risk for morbidity and even mortality. Cognitive and motor development as well as behavioral outcomes are determining factors for lifetime well-being. Changes in pre- and postnatal decision-making, modified and improved strategies in delivery room management, high-end neonatal and intensive care medicine, and progressing post-discharge management lead to improved outcome (1, 2). In Austria, the current border of viability decreased and now lies at 23 weeks of gestational age (GA), with an individual recommendation for the postnatal management of infants born at the border of viability of 23 + 0–23 + 6 GA (1, 3).
In Austria, the proportion of very preterm infants born before 32 weeks of GA is approximately 1% of all live births (2). Since 2007, we have prospectively entered anonymized data on all very preterm infants in a local register. National guidance for setting up standardized neonatal data was established in 2012 by a working group of neonatologists and pediatric intensivists (4, 5), and data were stored in a national quality assessment program named “Österreichisches Frühgeborenen Outcome Register (ÖFGOR)”. Data submission to this register is aimed for all institutions caring for very low birth weight infants, but only neurodevelopmental outcome examination is mandatory by law (3). Recently, national data for this population were published by the Austrian Preterm Outcome Study Group in 2019 (4).
Aim
This study reports the first state-wide outcome rates for survival, short-term morbidities, and survival free of major complications in very preterm infants over a 14-year period in a regional center of an Austrian neonatal intensive care unit (NICU). We evaluate the changes over time, discuss the results in comparison with national and international data and offer tools to surpass smaller center caseloads.
Material and methods
In this population-based registry study, we analysed data on all very preterm infants between 23 + 0 and 31 + 6 weeks of GA, delivered from January 1, 2007, to December 31, 2020, in the state of Vorarlberg, Austria, who were admitted to the NICU. We included all infants with congenital malformations and excluded all stillbirths and patients who died in the delivery room. In addition, we calculated data of ELGAN, who were born before 28 + 0 weeks of GA (6). We divided the period into two equal time frames (P1 from 2007 to 2013 and P2 from 2014 to 2020). The study reflects information from three units, two neonatal intermediate units (NIMCU), and one combined NICU. All preterm infants (n = 501, mean 36 very preterm infants per year) are either born at Feldkirch Academic Teaching Hospital, or are transferred to the NICU there within their first day of life. Our concept of regionalization provides and supports antenatal transport. To our knowledge, only a small number of very preterm infants (n = 49, 8.9%) were delivered in others regions of Austria for no medical reasons (7). The infrastructural requirements, which we fulfill, are defined in the Austrian Structural Health Care Plan (Österreichischer Strukturplan Gesundheit 2017) (3) and include the coverage of essential staff (neonatologist, anesthesiologist and nursing staff) over 24/7 days. Since 2007, neonatal data have been prospectively collected, anonymized, and stored electronically in an internal register. A secure interface protected confidentiality and privacy of data. A local study coordinator (K.K.), as the one who has access to the register by a secure password, was responsible for data collection and quality control. Sociodemographic and clinical data were extracted from the register and included the patients' GA, birth weight, sex, administration of antenatal steroids, multiple births, mode of delivery, and APGAR scores (2, 5).
Neonatal data
The primary outcome measure was overall survival, which was defined as the number of infants admitted who were discharged alive. The secondary outcome measures were the four major adverse short-term morbidities: bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), intraventricular haemorrhage (IVH), retinopathy of prematurity (ROP), and survival-free of major complications.
Overall mortality included all deaths that occurred during the first admission to our NICU until discharge from the hospital. All stillbirths and patients who died in the delivery room were excluded, and infants with congenital malformations were included in our calculations.
In accordance with the definition given by Jobe & Bancalari (8), BPD was defined as the requirement for supplemental oxygen at 36 weeks of postconceptional age or discharge to home, whichever came first. BPD was not graded according to severity (8). As we took part in the NIPPV (9) and SAIL (10) trials the definition of BPD changed in the second period of the study to respiratory support regardless of FiO2 to maintain oxygen saturation ≥90% and to perform an oxygen reduction test (11–13). A completed course of antenatal steroids was considered as two doses with a 24-h interval, with the last dose administered more than 24 h before birth. NEC was defined according to Bell's criteria (14), where severe NEC requires surgical intervention. IVH was classified according to Papile et al. (15) and severe IVH was graded as IVH ≥grade III. ROP was graded in conformity with the international classification, and grades III–V were classified as severe ROP (16). Adverse short-term outcomes were defined as the development of any of the following adverse morbidities: BPD, severe NEC, severe IVH (grades III and IV), or severe ROP (grades III–V). The percentage of the cohort who survived without any of the four major short-term morbidities was summed up as survival free of major complications.
Ethics
The nationwide registry was approved by the Ethics Committee of the Medical University of Vienna (EK 1828/2019). The study was approved by the Ethics Committee of the State of Vorarlberg (EK-2-7/2020), conducted in accordance with the Declaration of Helsinki and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies. The authors disclose any potential conflicts of interest.
Statistical analysis
Data analyses were performed using SPSS software, version 21.0 for Windows (IBM; Armonk, New York, USA). Descriptive statistics are provided as percentages unless otherwise stated. Group differences were analysed using the chi-square test (χ2). Statistical significance was set at p < 0.05.
Results
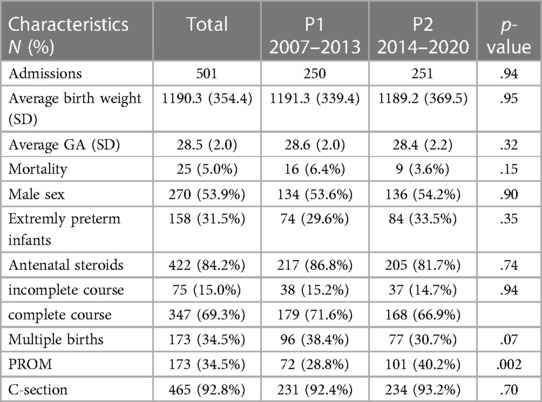
In total, 501 infants (250 in P1, 251 in P2) with a mean birth weight of 1190 (+/−354) grams and a mean GA of 28.5 (+/−2.0) weeks were included. Delivery room deaths were excluded from the study. There were no missing data on sex or mortality. For mode of delivery and single vs. multiple births, missing data were low, for BPD, NEC, IVH, and ROP, with a maximum of 1.2% for NEC. For antenatal steroids, missing data were 8%.
Neonatal characteristics were summarized using GA (Table 1). Male sex (53.9%), mode of delivery (C-section 92.8%), multiple births (34.5%), mean birth weight, and mean GA remained similar over time (Table 2).
Mortality
Overall, 25 very-preterm infants died (5.0%). For ELGAN, the mortality rate was 12%, and for those >28 weeks of GA, it was 1.7% (Table 2). The death rates in infants at 23, 24, 25, 26, and 27 weeks of GA were 20%, 26.1%, 26.7%, 4.9%, and 3.4%, respectively. The corresponding death rates for infants at 28, 29, 30, and 31 weeks of GA were 1.6%, 4.0%, 1.2%, and 0.8%, respectively (Table 1, Figure 1). Mortality decreased from 6.4% in P1 to 3.6% in P2 (p = .14) (Table 2).
Short-term morbidities
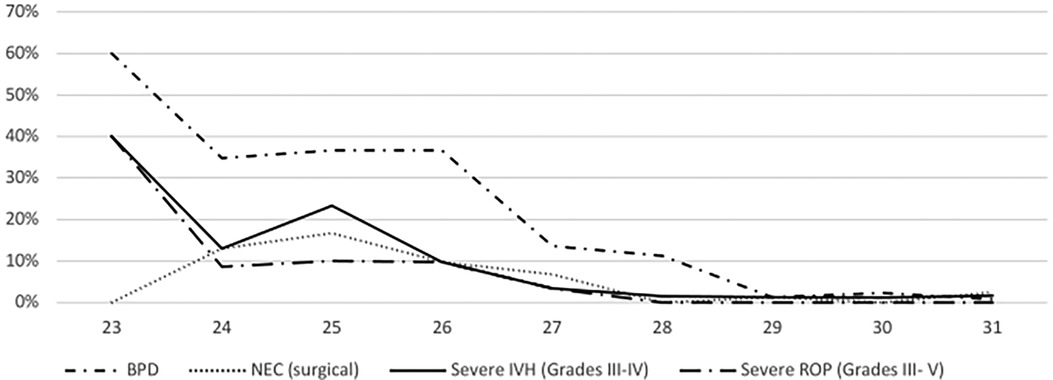
Overall, the incidence rates were 11.2% for BPD, 4.0% for surgical NEC, 4.6% for severe IVH, and 2.6% for severe ROP. BPD rates significantly increased from P1 to P2 (7.2% to 15.1%, p = .04), whereas other morbidities showed non-significant changes from P1 (2.4% for NEC, 4.8% for IVH, 1.6% for ROP) to P2 (5.6% for NEC, 4.4% for IVH, 3.6% for ROP). For detailed information, see Figure 2.
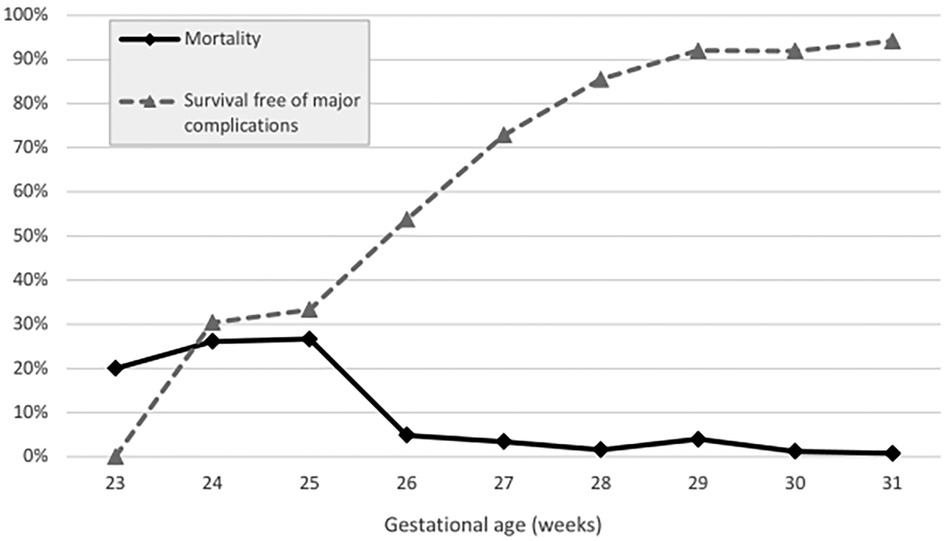
Figure 2. Mortality vs. survival free of major complications (in percent) stratified across gestational age groups (weeks).
Adverse short-term outcome
The risk to be discharged with one or more of the predefined morbidities was 10.6% for one, 3.3% for two and 0.8% for three morbidities. No infants had all of the four morbidities at discharge. In ELGAN, the risk for at least one short-term morbidity ranged from 69.6% with 24 weeks of GA to 14.5% in those with 28 weeks and 5.8% in those with 31 weeks of GA.
Survival free of major complications
Overall, the survival free of major complications rate was 79.0%. It ranged from 30.4% in infants with 24 weeks of GA to 94.2% in those with 31 weeks of GA (Figure 1), and showed no significant difference over time (82.4% for P1 to 75.7% for P2, p = .15).
Extremely low gestational age neonates (ELGAN)
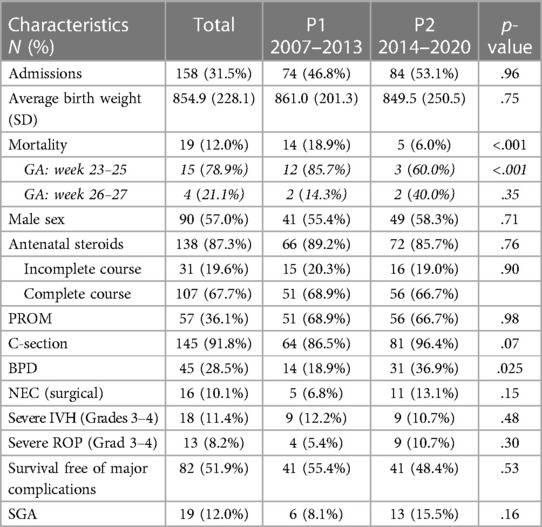
In this group of infants born below 28 + 0 weeks of GA, mean birth weight was 854.9 (+/−228.1) grams. The mortality rate was 12.0% and decreased significantly from 19% in P1 to 6.0% in P2 (p = .012) (Table 3). The incidence of short-term morbidities was 28.5% for BPD, with a significant increase over time (18.9% in P1 vs. 36.9% in P2, p = .025), 10.1% for surgical NEC, 11.4% for severe IVH, and 8.2% for severe ROP, with no changes over time. The overall survival free of major complications in this group was 51.9%, with no significant change over time (Table 3).
Discussion
The main result of this population-based, state-wide register study is a very low overall mortality rate of 5%. Furthermore, mortality was nearly halved over time in favor of the most immature infants below 28 weeks of GA. The low mortality rate is remarkable compared with recent nationwide Austrian (4) and Swiss (17) studies, with mortality rates of 8.4% from all live births and 9.9% of all infants admitted to the NICU, respectively. Mortality is also lower when compared to former national data from 1999 to 2001 (18). Our results are also comparable with international data from eight countries, which show an overall mortality rate of 10%, ranging from 5% in Japan to 17% in Spain (19). This study investigates infants over a similar time frame with the same neonatal demographic characteristics, mainly the same birth weight, gestational age, sex, rate of completed antenatal steroids, C-sections, and rate of multiple births like the studies cited above. In this study, we also report more than simple outcome parameters, such as mortality and morbidities, but also show a fair incidence of survival free of major complications of 73%, which is one of the most valuable concern in counseling parents.
Second, our short-term morbidity rates do not significantly differ from national data (4), despite a slightly higher incidence with an increasing change over time for BPD, whereas other morbidities showed non-significant changes over time. For BPD, countries with higher mortality rates show relatively low rates and vice versa (19, 20) which underlines our finding. National analyses in Austria (4, 18), Switzerland (17) and Germany (21) show BPD rates of 10%, 9.5% and 19.2%, respectively, international data ranges from 15% for Israel and Spain to 32% for the United Kingdom (19) and is considered a critical parameter for long-term outcomes and early death (8, 11, 22). A recent review (23) states that use of the NIH 2018 criteria identified a greater proportion of patients with BPD than did the NIH 2001 definitions (8). Numerous studies (9–11) offer approaches to factors influencing BDP, like sustained inflation, mechanical ventilation vs. CPAP, less invasive surfactant application, oxygen saturation limits or even nutrition, but incidences and treatment do not show significant changes over time. In our study, we find a decrease in the administration of antenatal steroids over time, and interpret this as an additional potential cause of our rising BPD rates. This decrease does not correspond to an expected negative impact on the IVH rate (24, 25) which might be due to the fact of the too small sample size or that other parameters may outweigh this effect e.g., hemodynamic management. Furthermore, we participated in two studies [NIPPV (9) and SAIL stud y10], in which BPD is diagnosed using an oxygen reduction test in all infants treated with any respiratory support (mechanical ventilation, nCPAP, nasal cannula) regardless of FiO2 to maintain an oxygen saturation of ≥90%. In our opinion, this definition (13), improved survival over time, an oxygen saturation target of 90%, and greater awareness may have led to more infants being eligible for this test and subsequent BPD diagnosis, especially in the ELGAN.
Third, the ELGAN show encouraging results, with a low and significantly decreasing mortality over time and a fair rate of survival free of major complications. However, when comparing P1–P2 we see doublings in all morbidities except IVH. We attribute this result to the decreasing mortality and the composition of the group. In P2, we see distinctly more infants younger than 25 weeks of gestational age.
In addition, our data for other short-term morbidities, severe NEC, ROP and IVH show favorable results with moderate incidences which do not change over time and can be compared to (inter)national results (4, 17, 19, 21). Caution is required in interpreting these data due to variations in case of definition (e.g., for NEC) or missing data [e.g., ROP (17, 21)].
In general, it is difficult to compare our results because of absent other national data (1, 4), different outcome parameters reported, time periods observed, and inclusion of infants in this age group. Based on the small sample size only a descriptive analysis is adequate. However, our data reflect the efforts in advancing delivery room management (9, 10, 26), but also show possibilities for improvement (26–28).
Strength and limitations
Our study has some limitations. According to our register (ÖFGOR), all stillbirths and delivery room (DR) deaths are excluded which certainly influence the results, namely survival by underestimate of the actual mortality rate. When introducing the register in 2012 (5) we decided on a minimal dataset which did not include this parameter—as well as periventricular leucomalacia (PVL) and sepsis—because entering data is voluntary and not funded (2, 4, 5). Retrospectively, delivery room death can not be determined because live-born infants below 32 weeks of GA are not registered in national (29) or regional data sets (7). However, other studies (30, 31) also exclude DR deaths and a recent meta-analysis of 65 studies shows that this refers mainly to the ELGAN at the limit of viability (30): for infants of 25 weeks GA and above the percentage of survival of all live births and survival of infants admitted at the NICU does not show any notable differences. On the other hand, infants, where therapy was withheld after admission in the further course to the NICU, were also included in our death rates (4).
We did not adjust for risk factors because of the limited sample size. However, analyses conducted by ÖFGOR show that statistical risk predictors for death or adverse short-term outcome are not only low GA and low birth weight, but also missing or incomplete course of antenatal steroids, male sex, and multiple births (4).
To reduce the bias of different inclusion criteria, time periods, or countries with different cultures and health care systems (19, 32, 33) we describe our outcome with respect to national (4, 18), as well as to our neighboring countries Switzerland (17) and Germany (16, 21) and international data with similar timeframes and health care systems (19, 32–34). The long time period of our study may contribute to bias because patient care has changed. Certainly, the strategy in ventilatory support has undergone the biggest changes by new non-invasive measures like CPAP, NIPPV, less invasive surfactant application (LISA), or higher rates in non-invasive ventilation. Others remain constant like human milk feeding, or no probiotic supplementation. However, our study was not designed to quantify these effects and the number of included patients is far too limited to give any evidence. Of concern is the higher NEC rate in the ELGAN, when compared to others (16), which lead to several measures like an early implementation of human milk-based nutritional products in the latter time period of the study.
Finally, this study investigated outcome data at discharge but did not define long-term outcomes which are of great concern for patients, clinicians, and families, and affect the quality of life (12, 35).
The strength of this study is its prospective, population-based design over a long period of 14 years in a defined area. Considering the fact that data entry was based on voluntariness and no funding was available for individual centres (4), the inclusion rate seems adequate and is comparable to that of other studies: missing data in other population studies ranged from 3% in the Israel Neonatal Network to up to more than 40% in the Neonatal Research Network of Japan (19).
This is the first and only investigation of short-term outcomes in a regional setting in Austria. In this manuscript, we disclose our data and results to the public concerning benchmarks for very preterm infants and patient volumes which are strongly and repetitively discussed in health and politics (36, 37): some might argue that our group might not be representative. However, in our register, the mean birth weight, GA, sex or rate of completed antenatal steroids are the same as in the national studies for Austria and Switzerland (4, 17). In addition, we have a low rate of missing values, which reflects a good adherence to the register. Therefore, we are convinced that our cohort and subsequently our results are comparable to nationwide as well as international data.
Others might state that our patient volume is too low and the outcome might be better in a larger center (36–42). The effect of case load may be underestimated, if the results are not adjusted for the systematic referral of infants to the larger tertiary centers (40) which is not the case in our population. A recommended minimum provider volume does not exist (43) in Austria (3) nor in Germany (36, 42). The benchmark of a minimum of 30 preterm infants per year with <1,250 g birth weight was suspended by the Federal Social Court in 2015 in Germany (36) pending a final decision (42). Regionalization policies of neonatal care has been endorsed to improve neonatal outcome and geography plays a major role in how the neonatal care networks are set up and function (43). Although significant investments in infrastructure and other nationwide improvements were made in the European Union, Portugal is a country to demonstrate a significant decline in neonatal mortality coincident with closing smaller centers arguing strongly for the effect of regionalization on this outcome (41). While the positive effects of volume seem clear, the optimal threshold for discrimination low-volume from high-volume centers remains unclear (38, 41, 42). A study from Switzerland demonstrated that center variability in survival rates cannot be explained by baseline demographics or center case load (44). Providing slightly above 35 very preterm infants per year, we demonstrate one of the lowest mortality rates published in equal cohorts over an equal timeframe. Our results can be partially explained by the fact that all hospitals in our state are affiliated within a formal network with the same standard operating procedures, such as guidelines for antenatal maternal transfers or newborn resuscitation algorithms and the low rate of severe IVH may also reflect this good standard of care (36). Another explanation might be the mandatory regular attendance at simulation training programs for every team member and participation in the Video Apgar (22–26) and other trials (9, 10), which improved our DR management. Participating in such clinical studies means that we transparently expose our efforts to critical discussion within our institution and at other centers which may lead to standardization and improvement of our work.
Further implications
Reducing morbidities among vulnerable infants remains a challenge for clinicians and investigators. Participation in (inter)national quality activities, multicenter registers, and clinical trials improves the quality of care and is increasingly considered an index of quality assurance. Precise data collection is an important component of guiding strategies for better outcomes. However, neither a continuous reduction in the viability limit nor an increase in survival rates, but rather the quality of life, should be the most important parameter in perinatal medicine.
Conclusion
In this prospective, population-based, state-wide study, we show that the overall mortality in our cohort is low and decreases over time, mainly in the ELGAN born before 28 weeks of GA. Short-term morbidities and survival free of major complications do not differ as compared to nationwide Austrian and international data in a similar age group, despite lower patient numbers. The limited size does not permit any risk adjustment and so it is not possible to deviate how much of the very good outcome are due to patient characteristics. Participation in international trials is essential for improving areas of difficulty. Respiratory support strategies, hemodynamic management, and antenatal care must be targeted to improve BPD and NEC rates in our population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Medical University of Vienna (EK 1828/2019). Ethics Committee of the State of Vorarlberg (EK-2-7/2020). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KK: Conceptualization, Investigation, Data curation, Methodology, Writing – original draft. DR: Data curation, Methodology, Formal Analysis, Writing – review & editing. AB: Data curation, Writing – review & editing. SG: Conceptualization, Investigation, Writing – review & editing. BS: Conceptualization, Investigation, Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berger A, Kiechl-Kohlendorfer U, Berger J, Dilch A, Kletecka-Pulker M, Urlesberger B, et al. Management of premature infants at the border of viability: joint guidelines of the working group for neonatology and pediatric intensive care medicine of the Austrian society of pediatrics and adolescent medicine (ÖGKJ), the working group on ethics in pediatrics and adolescent medicine of the ÖGKJ and the institute for ethics and law in medicine at the university of Vienna (IERM). Monatsschr Kinderheilkd. (2017) 165:139–47. doi: 10.1007/s00112-016-0149-0
2. Konzett K, Kiechl-Kohlendorfer U, Simma B. für den Fachbeirat des Österreichischen Frühgeborenen-outcome-registers (ÖFGOR). Frühgeborenennachsorge in osterreich: etablierung eines nationalen registers. Monatsschr Kinderheilkd. (2022) 170:1–5. doi: 10.1007/s00112-021-01387-3
3. Bundesministerium für Arbeit, Soziales, Gesundheit und Konsumentenschutz (BMASGK). Wien: Österreichischer Strukturplan Gesundheit (ÖSG) (2017). Available at: https://www.sozialministerium.at/dam/jcr:6102a229-7b92-44fd-af1f-3aa691900296/220406_The-Austrian_Health-Care-System_EN_pdfUA.pdf (Accessed June 20, 2023).
4. Kiechl-Kohlendorfer U, Simma B, Urlesberger B, Maurer-Fellbaum U, Wald M, Wald M, et al. Low mortality and short-term morbidity in very preterm infants in Austria 2011–2016. Acta Paediatr. (2019) 108:1419–26. doi: 10.1111/apa.14767
5. Kiechl-Kohlendorfer U, Brandstetter B, Fuiko R, Maurer-Fellbaum U, Wiesinger-Eidenberger G, Dietz W, et al. Standardisierte entwicklungsneurologische Nachuntersuchung von Frühgeborenen mit weniger als 32 Schwangerschaftswochen: Konsensuspapier der Österreichischen Gesellschaft für Kinder- und Jugendheilkunde (ÖGKJ). Monatsschr Kinderheilkd. (2012) 160:681–3. doi: 10.1007/s00112-012-2681-x
6. March of Dimes, PMNCH, Save the Children, WHO. Born too soon: the global action report on preterm birth. In: Howson CP, Kinney MV, Lawn JE. Geneva: World Health Organization (2012). p. 8–15.
7. Amt der Vorarlberger Landesregierung, Landesgesundheitsbericht Vorarlberg 2022. Wie xsund ist Vorarlberg. (2022). Available at: https://vorarlberg.at/documents/302033/24397990/Landesgesundheitsbericht+Vorarlberg+2022.pdf/188d06e8-16ab-9b86-9215-62b089f8cc66?t=1685014161683 (Accessed Juli 16, 2023).
8. Jobe AH, Bancalari E. NICHD/NHLBI/ORD workshop summary. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
9. Kirpalani H, Millar D, Lemrye B, Yoder BA, Chiu A, Roberts RS, for the NIPPV Study Group. A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med. (2013) 369:611–20. doi: 10.1056/NEJMoa1214533
10. Kirpalani H, Ratcliffe SJ, Keszler M, Davis PG, Foglia EE, te Pas A, et al. Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants. The SAIL randomized clinical trial. JAMA. (2019) 321:1165–75. doi: 10.1001/jama.2019.1660
11. Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensmamn A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. (2004) 114:1305–11. doi: 10.1542/peds.2004-0204
12. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. (2018) 197:300–8. doi: 10.1016/j.jpeds.2018.01.043
13. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200:751–9. doi: 10.1164/rccm.201812-2348OC
14. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187:1–7. doi: 10.1097/00000658-197801000-00001
15. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
16. International classification of retinopathy of prematurity revisited: international committee for the classification of retinopathy of prematurity. Arch Ophthalmol. (2005) 123:991–9. doi: 10.1001/archopht.123.7.991
17. Chen F, Bajwa NM, Rimensberger P, Posfay-Barbe KM, Pfister RE, Swiss Neonatal Network. Thirteen-year-mortality and morbidity in preterm infants in Switzerland. Arch Dis Child Fetal Neonatal Ed. (2016) 101:F377–88. doi: 10.1136/archdischild-2015-308579
18. Weber C, Weninger M, Klebermass K, Reiter G, Wiesinger-Eidenberger G, Brandauer M, et al. Mortality and morbidity in extremely preterm infants (22 to 26 weeks of gestation): Austria 1999–2001. Wien Klin Wochenschr. (2005) 117:740–6. doi: 10.1007/s00508-005-0468-y
19. Shah PS, Lui K, Sjörs G, Mirea L, Reichman B, Adams M, et al. Neonatal outcomes of very low birth weight and very preterm neonates: an international comparison. J Pediatr. (2016) 177:144–52. doi: 10.1016/j.jpeds.2016.04.083
20. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AB, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. (2010) 126:443–56. doi: 10.1542/peds.2009-2959
21. Humberg A, Härtel C, Rausch TK, Stichtenoth G, Jung P, Wieg C, et al. Active perinatal care of preterm infants in the German neonatal network. Arch Dis Child Fetal Neonatal Ed. (2020) 105:F190–5. doi: 10.1136/archdischild-2018-316770
22. Pomar EG, Concina VA, Samadi A, Westgate PM, Bada HS. Bronchopulmonary dysplasia: comparison between the two most used diagnostic criteria. Front Pediatr. (2018) 6:1–6. doi: 10.3389/fped.2018.00001
23. Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. Br Med J. (2021) 375:1–17. doi: 10.1136/bmj.n1974
24. McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. (2020) 25:CD004454. doi: 10.1002/14651858.CD004454.pub4
25. Fortmann I, Mertens L, Boeckel H, Grüttner B, Humberg A, Astiz M, et al. A timely administration of antenatal steroids is highly protective against intraventricular hemorrhage: an observational multicenter cohort study of very low birth weight infants. Front Pediatr. (2022) 10:721355. doi: 10.3389/fped.2022.721355
26. Simma B, Walter S, Konstantelos D, van Vonderen J, te Pas A, Rüdiger M, et al. Delivery room management of infants with very low birth weight in 3 European countries—the video Apgar study. J Pediatr. (2020) 222:106–11. doi: 10.1016/j.jpeds.2020.03.035
27. Express Group, Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. (2022) 21:435–6. doi: 10.1001/jama.2009.771
28. Ancel PY, Goffinet F, EPIPAGE-2 Writing Group, Kuhn P, Langer B, Matis J, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011. Results of the EPIPAGE-2 cohort study. JAMA Pediatr. (2015) 169:230–8. doi: 10.1001/jamapediatrics.2014.3351
29. Available at: https://www.statistik.at (Accessed December 17th, 2023).
30. Myrhaug HT, Brurberg KG, Hov L, Markestad T. Survival and impairment of extremely premature infants: a meta-analysis. Pediatrics. (2019) 143(2). doi: 10.1542/peds.2018-0933
31. Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004–2007 and 2014–2016. JAMA. (2019) 321:12. doi: 10.1001/jama.2019.2021
32. Rüegger C, Hegglin M, Adams M, Bucher HU, Swiss Neoanatel Network. Population based trends in mortality, morbidity and treatment for very preterm- and very low birth weight infants over 12 years. BMC Pediatr. (2012) 12:17. doi: 10.1186/1471-2431-12-17
33. Hirata K, Kimura T, Hirano S, Wada K, Kusuda S, Fujimura M, Neonatal Research Network of Japan. Outcomes of outborn very-low-birth-weight infants in Japan. Arch Dis Child Fetal Neonatal Ed. (2021) 106:F131–6. doi: 10.1136/archdischild-2019-318594
34. Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol. (2016) 33:318–28. doi: 10.1055/s-0035-1571202
35. Adams-Chapman I, Heyne RJ, DeMauro SB, Duncan AF, Hintz SR, Pappas A, et al. Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics. (2018) 141. doi: 10.1542/peds.2017-3091
36. Obladen M. Mindestmengen in der Versorgung sehr untergewichtiger Frühgeborener: Eine Literaturübersicht. Minimum patient volume in the care for very low birthweight infants. Z Geburtsh Neonatolog. (2007) 211:110–7. doi: 10.1055/s-2007-960745
37. Bartels DB, Wypij D, Wenzlaff P, Dammann O, Poets CF. Hospital volume and neonatal mortality among very low birth weight infants. Pediatrics. (2006) 117:2206–14. doi: 10.1542/peds.2005-1624
38. Rogowski JA, Horbar JD, Staiger DO, Kenny M, Carpenter J, Geppert J. Indirect vs. direct hospital quality indicators for very low-birth-weight infants. J Am Med Assoc. (2004) 291:202–9. doi: 10.1001/jama.291.2.202
39. Shah PS, Mirea L, Ng E, Solimano A, Lee SK, Canadian Neonatal Network. Association of unit size, resource utilization and occupancy with outcomes of preterm infants. J Perinatol. (2015) 35:522–9. doi: 10.1038/jp.2015.4
40. Ray KN, Lorch SA. Hospitalization of rural and urban infants during the first year of life. Pediatrics. (2015) 130:1084–93. doi: 10.1542/peds.2012-0020
41. Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med. (2007) 356:2165–75. doi: 10.1056/NEJMsa065029
42. Heller G, Schnell R, Rossi R, Thomas T, Maier RF. What is the optimal minimum provider volume in the provision of care for preterm infants with a birth weight below 1250 g in Germany. Z Geburtsh Neonatol. (2020) 224:289–96. doi: 10.1055/a-1259-2689
43. Kunz SN, Phibbs CS, Profit J. The changing landscape of perinatal regionalization. Semin Perinatol. (2020) 44. doi: 10.1016/j.semperi.2020.151241
44. Berger TM, Steurer MA, Woerner A, Meyer-Schiffer P, Adams M, Swiss Neonatal Network. Trends and centre-to-centre variability in survival rates of very preterm infants (<32 weeks) over a 10-year-period in Switzerland. Arch Dis Child Fetal Neonatal Ed. (2012) 97:F323–8. doi: 10.1136/fetalneonatal-2011-301008
Keywords: morbidities, mortality, regional outcome, quality assessment, preterm infants
Citation: Konzett K, Riedl D, Blassnig-Ezeh A, Gang S and Simma B (2024) Outcome in very preterm infants: a population-based study from a regional center in Austria. Front. Pediatr. 12:1336469. doi: 10.3389/fped.2024.1336469
Received: 10 November 2023; Accepted: 17 January 2024;
Published: 2 February 2024.
Edited by:
Hudson Santos, University of Miami, United StatesReviewed by:
Mats Ingmar Fortmann, University of Lübeck, GermanyBlanka Zlatohlávková, 1st Medical Faculty of Charles University and General Faculty Hospital in Prague, Czechia
© 2024 Konzett, Riedl, Blassnig-Ezeh, Gang and Simma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Burkhard Simma YnVya2hhcmQuc2ltbWFAbGtoZi5hdA==
 Karin Konzett1
Karin Konzett1 Burkhard Simma
Burkhard Simma