- Department of Pharmacy, Children’s Hospital of Hebei Province, Shijiazhuang, Hebei, China
Background: The aim of this study was to explore the current status of vitamin D2 (VD2) deficiency in hospitalized children in a region of China.
Methods: The instances of detection of vitamin D (VD) and VD2 in children who visited the hospital from January 2022 to May 2023 were analyzed retrospectively. Additionally, the relationships between VD2 level and gender and age were further analyzed. Furthermore, for departments with a high frequency of VD detection, the VD2 deficiencies in children with different diseases were further analyzed.
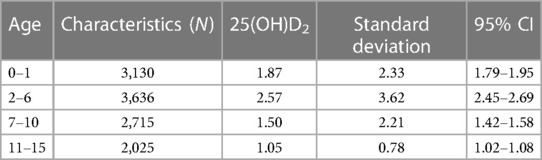
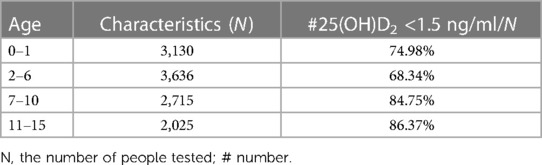
Results: Among the different age groups, children aged 11–15 years exhibited the most severe VD2 deficiency, followed by those aged 7–10 years, 0–1 years, and 2–6 years. Moreover, 25(OH)D2 levels were significantly lower in children aged 7–10 years and 11–15 years compared with 2–6 years. Gender did not have an impact on the level of 25(OH)D2. When analyzing the orthopedics, dermatology, thoracic surgery, and nephroimmunology departments’ data on children's levels of 25(OH)D2, it was found that an average of approximately 76.56% had levels below <1.5 ng/ml compared to individuals with levels between >15 ng/ml and 100 ng/ml. The average ratio between individuals with <1.5 ng/ml vs. those with <15 ng/ml was found to be 91.22%.
Conclusions: Children who came to the hospital were severely deficient in VD2. The degree of deficiency was related to age, but there was no gender difference. The phenomenon of VD2 deficiency was reflected in children with both skeletal and non-skeletal diseases.
Introduction
Vitamin D (VD) is a steroid hormone that plays a crucial role in the growth and development of children. Within the VD family, the major members are ergocalciferol (vitamin D2, VD2) and cholecalciferol (vitamin D3, VD3). VD2 primarily exists in fungi and some plants, while VD3 is synthesized from 7-dehydrocholesterol in human or animal skin through ultraviolet light photochemical action, serving as the primary source of vitamin D in the human body. Upon entering the liver, both VD2 and VD3 undergo conversion to 25-hydroxyvitamin D [25(OH)D] by 25-hydroxylase within hepatocyte microsomes. Subsequently, hydroxylation of 25(OH)D leads to its active form known as 1,25-dihydroxyvitamin D [1,25-(OH)2-D], which exerts physiological effects. VD has many metabolites, but there are no recognized metabolites for measuring the content of VD currently. The serum concentration of 25(OH)D is used as an indicator for assessing the level of VD due to a longer half-life (1). VD deficiency not only leads to skeletal diseases but also affects the occurrence and progression of various non-skeletal health outcomes, including autism, systemic lupus erythematosus, kidney disease, cardiovascular disease, immune system disorders, metabolic disorders, and nervous system disorders (2). Individuals with VD deficiency are more susceptible to these diseases compared to those with normal VD levels. In recent years, there have been many studies about VD and VD3, but research on VD2 is relatively insufficient. This study aimed to analyze the current status of VD2 deficiency in children visiting a hospital and lay the foundation for future research on VD2.
Methods
Subjects
Experienced doctors would empirically assess the possibility of VD deficiency in children based on their daily VD supplementation status and the presence of clinical symptoms such as constipation, sleep disorders, and calcium deficiency and arrange for VD testing. The study was conducted by a retrospective analysis of 11,506 children who underwent VD and VD2 testing in our hospital from January 2022 to May 2023, including 5,923 boys and 5,583 girls, with an average age of (4.82 ± 4.29) years. The children were divided into four groups according to age: 0–1 years, 2–6 years, 7–10 years, and 11–15 years.
Definition of the normal range of VD
According to the standards of the clinical practice guidelines of the Endocrine Society of America and the expert consensus on the clinical application of VD in China (3), the nutritional status of children with VD was defined as follows: The normal range of 25(OH)D is 15 ng/ml to 100 ng/ml. The 25(OH)D level can reflect the normal VD nutritional status of children. The normal range of 25(OH)D2 is 1.5 ng/ml to 30 ng/ml. The 25(OH)D2 level indicates the VD2 nutritional status of children.
Detection method of VD
Laboratory tests for VD were performed by personnel from the pharmacological laboratory of the Department of Pharmacy. Researchers collected 2 ml venous blood from children, centrifuged for 6 h to obtain serum, and stored frozen in a refrigerator at −20°C for subsequent test. During the detection process, 25(OH)D and 25(OH)D2 were detected by liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). Under the condition of mass spectrometry, the electrospray source positive ion mode was used for the operation. The ion source temperature was set at 450°C and the spray voltage at 5,500 V. The MRM mode was used for data acquisition and detection. Based on the difference in molecular weight and mass charge ratio, HPLC-MS/MS could distinguish VD2 and VD3 (4).
Data analysis
SPSS 21.0 software was used to analyze the data. Measurement data that conformed to normal distribution were expressed as mean ± standard deviation. The differences between groups were compared via t-test; if the data were non-normally distributed, the Mann–Whitney U test was used. One-way ANOVA test analysis was used to compare the VD2 levels of children of different ages. The difference was considered as statistically significance with P < 0.05.
Role of the funding source
The funding agencies did not participate in the study design, data collection, data analysis, or manuscript writing. The corresponding authors were responsible for all aspects of the study to ensure that issues related to the accuracy or integrity of any part of the work were properly investigated and resolved. The final version was approved by all authors.
Results
25(OH)D2 concentration in all age groups
The results presented in Table 1 demonstrated variations in the concentration of 25(OH)D2 among children across different age groups. The highest levels of 25(OH)D2 were observed in children aged 2–6 years, while a gradual decrease was observed in children aged 0–1 years, 7–10 years, and 11–15 years. Multiple comparisons revealed that the levels of 25(OH)D2 in children aged 7–10 years and 11–15 years were significantly lower than those in children aged 2–6 years (P = 0.042).
25(OH)D2 deficiency in different age groups
The deficiencies of 25(OH)D2 were severe in children across all age groups. As depicted in Table 2, the severity was highest among children aged 11–15 years, followed by those aged 7–10 years, 0–1 years, and 2–6 years. The proportion of individuals with a level of 25(OH)D2 lower than 1.5 ng/ml to the total population exhibited the highest value of 86.37%, lowest value of 68.34%, and average value of 78.61%.
Effect of gender on 25(OH)D2 deficiency
This study analyzed the prevalence of 25(OH)D2 deficiency in boys and girls across different age groups. The results indicated that there was no significant difference in 25(OH)D2 levels between boys and girls (P = 0.28).
25(OH)D2 deficiency in departments with higher detection frequencies
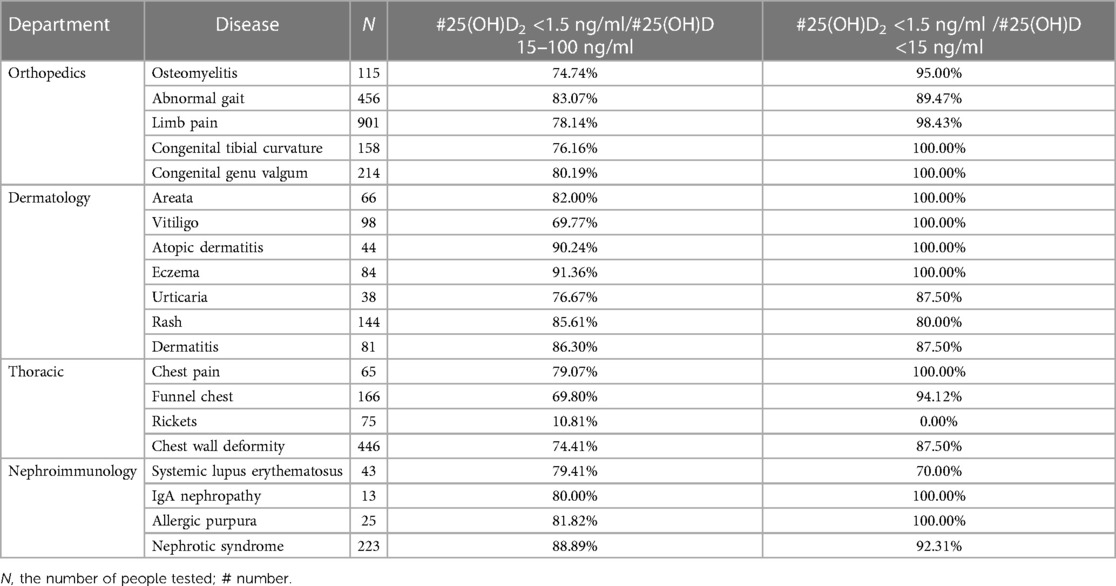
By analyzing the data from the orthopedics, dermatology, thoracic surgery, and nephroimmunology departments, the findings revealed that the average proportion of individuals with 25(OH)D2 levels below 1.5 ng/ml to those with 25(OH)D levels between 15 and 100 ng/ml was 76.56%. Moreover, the average ratio of individuals with 25(OH)D2 levels less than 1.5 ng/ml to those with 25(OH)D levels lower than 15 ng/ml was found to be approximately 91.22%. The 25(OH)D2 deficiencies among children in each department are summarized in Table 3.
Discussion
As a fat-soluble vitamin, VD is an essential nutritional element for maintaining human health. Its primary functions include promoting the absorption of calcium and phosphorus by small intestinal mucosal cells, stimulating the growth and differentiation of skin cells, and regulating immunity. The hormone–receptor complex formed when 1,25-(OH)2-D binds to the VD receptor in the nucleus acts on specific DNA sequences within target genes, thereby influencing various aspects (5). Research has confirmed that many diseases have low levels of 25(OH)D in patients, such as diabetes (6), hypertension (7), cardiovascular disease (8), and cancer (9).
Although VD plays a crucial role in the human body, VD deficiency remains a global public health issue (10), particularly among children. Due to their rapid growth and high nutrient demands, such as VD, children are more susceptible to nutritional deficiencies. Studies conducted in various countries have reported a high prevalence of VD deficiency across different age groups, similar to other populations worldwide (11, 12). However, many of these studies failed to differentiate VD types and defaulted to considering only VD3 while disregarding the presence of VD2. Specifically, the side chain of VD2 contains a double bond between carbons 22 and 23, with a methyl group attached at carbon 24, whereas VD3 lacks both groups. Moreover, VD2 also has the characteristic of fast metabolism, which may be related to the low level of VD2 in the body. Although it exists in low quantities among humans, VD2 plays an essential physiological role. Yang found that combining Shenqi Jianer decoction with VD2 injection effectively improved serum calcium and phosphorus levels while reducing bone alkaline phosphatase levels in children with rickets (13). Su et al. found that intramuscular injection of VD2 significantly improved the status of VD deficiency in patients with IgA nephropathy and reduced proteinuria (14). Li et al. found that high-dose intramuscular administration of VD2 was beneficial for type 2 diabetic patients with distal symmetric polyneuropathy (15).
In this study, HPLC-MS/MS was utilized for assessing 25(OH)D and 25(OH)D2 levels due to its high sensitivity and specificity, providing certain methodological advantages (16).The investigation aimed to analyze the levels of VD2 in pediatric patients attending the hospital. This study found that there were differences in the levels of VD2 among children of different age groups, but there was no significant difference in VD2 levels between boys and girls. The reasons for the differences in the degree of VD2 deficiency among children of different age groups may include different individual needs for VD2, different exposure times to sunlight, and different levels of VD2 intake. VD2 can only be obtained from external sources. Notably, Simon et al. found that mushrooms are good sources of VD2 (17). Mushrooms contain ergosterol, a precursor that enables them to synthesize VD2 when exposed to sunlight or ultraviolet light. Bisporus mushrooms are rich in high nutritional value and have numerous therapeutic benefits. Therefore, they are widely used in countries around the world.
In addition, by analyzing the VD2 levels of children in departments with a high frequency of VD detection, the findings revealed that not only children with orthopedic and thoracic surgery-related diseases exhibited VD2 deficiency but also those with non-skeletal-related conditions such as skin and kidney diseases. Further data analysis indicated that 76.56% of the children had VD2 deficiency despite their VD values falling within the normal range. Moreover, among children with severely deficient VD levels, 89.19% exhibited insufficient VD2 levels. For specific conditions such as congenital tibial bending and curvature of the tibia in orthopedics, areata, vitiligo, atopic dermatitis, and eczema in dermatology, chest pain in thoracic surgery, and IgA nephropathy and allergic purpura in nephroimmunology, if there were VD deficiencies, these children would have 100% rates of VD2 deficiency. Another study suggested that populations undergoing VD2 testing were 4.2 times more likely to check out VD deficiency compared to those without VD2 testing (18). In conclusion, assessing the level of VD2 was highly significant for evaluating an individual's VD status.
Therefore, it was recommended that the detection of VD2 should be conducted simultaneously with VD detection in order to more accurately assess the nutritional status of children. Furthermore, considering a limitation of this study was that samples from hospitals were utilized, the generalizability of the findings may be impacted. Additionally, other factors such as seasonal variations, daily dietary intake, and individual sun protection behavior that could potentially affect VD content need to be further taken into account. Hence, more comprehensive research is warranted to investigate the VD2 deficiency and the associations between VD2 and specific diseases in order to provide better guidance for clinical practice.
Data availability statement
The datasets presented in this article are not readily available because patient privacy concerns. Requests to access the datasets should be directed tobGpicmF2ZTFAMTYzLmNvbQ==.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Children's Hospital of Hebei Province approved the study (NO: 202136). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZA: Methodology, Validation, Writing – review & editing, Data curation, Formal Analysis, Investigation, Resources. NA: Methodology, Validation, Writing – review & editing, Data curation, Investigation. YZ: Conceptualization, Data curation, Investigation, Methodology. GZ: Conceptualization, Data curation, Investigation, Methodology. DZ: Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jorde R, Grimnes G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Med Hypotheses. (2018) 111:61–5. doi: 10.1111/ner.13065
2. Argano C, Mirarchi L, Amodeo S, Orlando V, Torres A, Corrao S. The role of vitamin D and its molecular bases in insulin resistance, diabetes, metabolic syndrome, and cardiovascular disease: state of the art. Int J Mol Sci. (2023) 24(20):15485. doi: 10.3390/ijms242015485
3. Children’s Health Branch of the Chinese Preventive Medicine Association. Expert consensus on clinical application of vitamin A and vitamin D in Chinese children. Chin J Child Health Care. (2021) 29(01):116–22. doi: 10.11852/zgetbjzz2020-2118
4. Lyu H, Wang S, Jin Y, Shen R, Chen J, Zhu C, et al. Simultaneous determination of VD2, VD3, 25(OH)D2, and 25(OH)D3 in human plasma using electrospray LC-MS/MS as well as its application to evaluate VD plasma levels in depressive, schizophrenic patients and healthy individuals. Biomed Chromatogr. (2020) 34(11):e4932. doi: 10.1002/bmc.4932
5. Fathi N, Ahmadian E, Shahi S, Roshangar L, Khan H, Kouhsoltani M, et al. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed Pharmacother. (2019) 09:391–401. doi: 10.1016/j.biopha.2018.10.102
6. Wang M, Zhou T, Li X, Ma H, Liang Z, Fonseca V, et al. Baseline vitamin D status, sleep patterns, and the risk of incident type 2 diabetes in data from the UK biobank study. Diabetes Care. (2020) 43(11):2776–84. doi: 10.2337/dc20-1109
7. Cai M, Wang Y, Liu T, Huang Y. Interaction between vitamin D status and calcium intake in association with blood pressure and hypertension. J Nutr Sci Vitaminol. (2023) 69(2):81–9. doi: 10.3177/jnsv.69.81
8. Cortese F, Costantino MF, Luzi G, Di Marino S, Giordano P, Monitillo F. Vitamin D and cardiovascular disease risk. A literature overview. Mol Biol Rep. (2022) 49(9):8925–42. doi: 10.1007/s11033-022-07373-6
9. Li Z, Shi J, Wang Z, Chen H, Liu Y. Nutrient status of vitamin D among cancer patients. Zhongguo Fei Ai Za Zhi. (2021) 24(5):345–50. doi: 10.3779/j.issn.1009-3419.2021.101.10
10. Cediel G, Pacheco-Acosta J, CastiUo-Durdn C. Vitamin D deficiency in pediatric clinical practice. Arch Argent Pediatr. (2018) 116(1):e75–81. doi: 10.5546/aap.2018.eng.e75
11. Pereira-Santos M, Santos JYGD, Carvalho GQ, Santos DBD, Oliveira AM. Epidemiology of vitamin D insufficiency and deficiency in a population in a sunny country: geospatial meta analysis in Brazil. Crit Rev Food Sci Nutr. (2019) 59(13):2102–9. doi: 10.1080/10408398.2018.1437711
12. Leal ACGB, Corrêa MP, Holick MF, Melo EV, Lazaretti-Castro M. Sun-induced production of vitamin D3 throughout 1 year in tropical and subtropical regions: relationship with latitude, cloudiness, UV-B exposure and solar zenith angle. Photochem Photobiol Sci. (2021) 20(2):265–74. doi: 10.1007/s43630-021-00015-z
13. Yang M. Clinical observation on the treatment of infantile rickets with shenqi jian'er decoction combined with vitamin D2 injection. J Pract Tradit Chin Med. (2021) 37(11):1840–2. doi: 10.3969/j.issn.1004-2814.2021.11.syzyyzz202111022
14. Su CQ, Luo MH, You YH, Liang M. Clinical observation of intramuscular vitamin D2 in treatment of vitamin D deficiency in patients with IgA nephropathy. CJITWN. (2022) 23(3):212–5. doi: 10.3969/j.issn.1009-587X.2022.03.007
15. Li RG, Wang CL, Liu DX. Decision tree study on the efficacy of vitamin D2 in the treatment of diabetes peripheral neuropathy. J Nanyang Inst Tech. (2020) 12(4):113–8. doi: 10.16827/j.cnki.41-1404/z.2020.04.021
16. Jenkinson C, Desai R, McLeod M, Wolf Mueller J, Hewison M, Handelsman DJ. Circulating conjugated and unconjugated vitamin D metabolite measurements by liquid chromatography mass spectrometry. J Clin Endocr Metab. (2022) 107(2):435–49. doi: 10.1210/clinem/dgab708
17. Joshi I, Singh S. Button mushroom—potential source of vitamin D2 and possibilities of value addition. Curr Nutr Food Sci. (2023) 19(2):114–24. doi: 10.2174/1573401318666220614110008
Keywords: vitamin D, vitamin D2, clinical diseases, investigation, deficiency
Citation: Liu J, An Z, An N, Zhao Y, Zhang G and Zhao D (2024) Current status of vitamin D2 deficiency among children in a region of China. Front. Pediatr. 12:1333769. doi: 10.3389/fped.2024.1333769
Received: 6 November 2023; Accepted: 15 January 2024;
Published: 26 January 2024.
Edited by:
Wenquan Niu, Capital Institute of Pediatrics, ChinaReviewed by:
Guenter Klaus, KfH-Kuratorium für Dialyse und Nierentransplantation, GermanyGiovanni Mario Pes, University of Sassari, Italy
© 2024 Liu, An, An, Zhao, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deyun Zhao bGpicmF2ZTFAMTYzLmNvbQ==
 Jia Liu
Jia Liu Zhihua An
Zhihua An