- 1Department of Midwifery, College of Medicine & Health Sciences, Dilla University, Dilla, Ethiopia
- 2Department of Nursing, College of Medicine & Health Sciences, Dilla University, Dilla, Ethiopia
- 3School of Public Health, College of Medicine & Health Sciences, Dilla University, Dilla, Ethiopia
Introduction: Necrotizing enterocolitis (NEC) is a serious intestinal condition characterized by ischemic necrosis of the intestinal mucosa, inflammation, and invasion by gas-forming organisms, posing a significant threat to neonatal health. Necrotizing enterocolitis remains a significant cause of neonatal morbidity and mortality, particularly in developing countries. Due to limited research conducted in Ethiopia and the study area, there is a lack of information regarding the risk factors associated with necrotizing enterocolitis. Therefore, the goal of this study is to fill the aforementioned gap.
Objective: This study aims to identify the risk factors of necrotizing enterocolitis among neonates admitted to the neonatal intensive care unit (NICU) at selected general and referral hospitals in southern Ethiopia in the year 2023.
Methods and materials: A facility-based unmatched case–control study was conducted. All neonates admitted to the NICU and diagnosed with necrotizing enterocolitis by the attending physician during the data collection period were considered as cases, whereas neonates admitted to the NICU but not diagnosed with necrotizing enterocolitis during the data collection period were considered as controls. Data were collected through face-to-face interviews and record reviews using the Kobo toolbox platform. The binary logistic regression method was used to determine the relationship between a dependent variable and independent variables. Finally, a p-value of < 0.05 was considered statistically significant.
Results: This study included 111 cases and 332 controls. Normal BMI [AOR = 0.11, 95% CI: (0.02, 0.58)], history of khat chewing [AOR = 4.21, 95% CI: (1.96, 9.06)], term gestation [AOR = 0.06, 95% CI: (0.01, 0.18)], history of cigarette smoking [AOR = 2.86, 95% CI: (1.14, 7.14)], length of hospital stay [AOR = 3.3, 95% CI: (1.43, 7.67)], and premature rupture of membrane [AOR = 3.51, 95% CI: (1.77, 6.98)] were significantly associated with NEC.
Conclusion: The study identified several risk factors for necrotizing enterocolitis, including body mass index, history of khat chewing, gestational age, history of cigarette smoking, length of hospital stays, and premature rupture of membrane. Therefore, healthcare providers should be aware of these risk factors to identify newborns at high risk and implement preventive measures.
Introduction
Necrotizing enterocolitis (NEC) is a serious intestinal condition characterized by ischemic necrosis of the intestinal mucosa, as well as inflammation and enteric gas-forming organism invasion (1, 2). It is one of the most prevalent and fatal neonatal gastrointestinal crises (3, 4). NEC is a leading cause of death and morbidity in neonatal intensive care units, with a mortality rate of 15%–30% that rises with lower birth weight and preterm (5, 6). The worldwide incidence rate of necrotizing enterocolitis is 7% (7). In high-income countries, the incidence of necrotizing enterocolitis varied according to gestational age (GA), birth weight, and country (8). Evidence showed that in developed countries, the incidence rate of NEC among preterm and low birth weight babies was 2%–7% and 5%–22%, respectively (9). The studies conducted in the United States revealed that the incidence rate of NEC was 7%–10.7% (10, 11). Studies revealed that the prevalence rate of NEC was 4.87% in China (12), 25.4% in Addis Ababa (13), and 9.7% in Gurage (14). A study conducted in Indonesia found that 10% of full-term newborns had NEC (15).
Over 50% of infants diagnosed with NEC who require surgery die, and those with poor prognosis often experience neurological problems in their later years (11). The mortality rates for necrotizing enterocolitis affect approximately 25% of all infected newborns, and the survivors frequently experience long-term neurodevelopmental and/or gastrointestinal complications (10). It has a high medical cost for both families and society (16).
Prematurity, low birth weight, enteral feeding, blood transfusion, postnatal hypoxia, sepsis, meconium aspiration syndrome, and other factors have all been associated with NEC, according to numerous studies (16–19). Necrotizing enterocolitis has been associated with maternal factors such as chorioamnionitis, increased BMI, mode of delivery, placental abruption, preeclampsia, and smoking (8, 20).
Mortality and poor neurodevelopmental outcomes of infants with NEC remain high in resource-constrained regions (3). Despite advances in neonatal treatment, the overall mortality rate for necrotizing enterocolitis remains high, particularly in a newborn with advanced disease (8, 21). The Sustainable Development Goals (SDGs) include newborn health as a key component, and by 2030, countries are expected to have reduced neonatal mortality to at least 12 deaths per 1,000 live births (22). Necrotizing enterocolitis (NEC) continues to make a significant contribution to neonatal morbidity and mortality, especially in developing countries (18). Furthermore, a significant proportion of survivors experience neurodevelopmental delay, which lowers the quality of life for the patient and family and increases treatment costs (21). Necrotizing enterocolitis was diagnosed in approximately 4% of neonates admitted to the NICU, with a 46.2% fatality rate (23). Necrotizing enterocolitis was the leading cause of death in Ethiopia for preterm infants born at 28–31 weeks, 32–34 weeks, and 35–36 weeks of gestation, accounting for 22.2%, 55.6%, and 22.2% of deaths, respectively (24).
As a result, necrotizing enterocolitis is a serious condition that primarily affects premature babies and has a high mortality rate in Ethiopia. Preventive measures are required for a fatal disease that is very challenging to treat once it has been identified (25). However, to prevent necrotizing enterocolitis, we must first identify the risk factors. A few studies on the magnitude and associated factors of necrotizing enterocolitis have been conducted in Ethiopia, but all of them were conducted using a cross-sectional and retrospective approach. As a result, the current study differs in terms of study design and the inclusion of some variables that were not addressed in previous studies. Furthermore, there is a lack of data in the study setting and throughout the country that takes into account maternal lifestyle behaviors, pregnancy-related factors, and perinatal and neonatal factors associated with necrotizing enterocolitis, which is useful for designing contextual interventions.
Methods and materials
Study area, period, and design
The study was conducted from February 1, 2023 to August 31, 2023, among newborns admitted to the neonatal intensive care unit (NICU) in public hospitals of Hawasa and Dilla town, Southern Ethiopia. A facility-based unmatched case-control study was conducted.
Population
The source population consisted of all neonates with index mothers who were admitted to the neonatal intensive care unit in Hawassa and Dilla town public hospitals. During the data collection period, all neonates with their index mothers were admitted to the neonatal intensive care unit in selected public hospitals in Hawassa and Dilla towns. These newborns met the inclusion criteria for both cases and controls, making them suitable for the study population.
Eligibility criteria
Inclusion criteria
All neonates who were admitted to the NICU at the selected public hospitals during the data collection period and neonates who were diagnosed with necrotizing enterocolitis (NEC) by the physician based on a standardized set of clinical and/or radiological criteria (modified bell stage criteria) or neonates who met the definition criteria of NEC and were admitted to the NICU at the selected public hospitals during the data collection period were considered as cases.
All neonates who were admitted to the NICU at the selected public hospitals during the data collection period and did not develop NEC during their stay in the NICU or neonates who did not meet the criteria for NEC and were admitted to NICU at the selected public hospitals during the data collection period were considered as controls.
Exclusion criteria
Cases: Neonates who were initially diagnosed with NEC but subsequently recovered and had other health issues throughout the data collection period were excluded from the study. Controls: During the data collection period, neonates diagnosed with NEC and other neonatal diseases were excluded from the study. Neonates admitted to the NICU with major congenital anomalies or genetic disorders, neonates whose mothers were absent, deaf, or unable to communicate, and neonates with incomplete card information were excluded from the study as both cases and controls.
Sample size determination
The sample size for an unmatched case–control study was calculated using the OpenEPI Info version 7 software and the double population proportion calculation, taking into account the variable of failure to breathe or be resuscitated from the previous study conducted in Addis Ababa (13), as it provided the largest sample size. The proportion of newborns who failed to breathe/were resuscitated among the control group is 21.3%, with a minimum detectable odds ratio (OR) of 2.1. This study assumed a precision of 5%, a power of 80%, and a case-to-control allocation ratio of 1:3. After accounting for a 10% non-response rate, the final sample size was 443 (111 cases and 332 controls).
Sampling techniques and procedures
Three public hospitals [Adare General Hospital (AGH), Dilla University General Hospital (DUGH), and Hawassa University Comprehensive & Specialized Hospital (HUCSH)] were selected for this study. The sample size was allocated to the selected hospitals in proportion to their neonatal admission from the 6-month record of the previous year. Case–control incidence density sampling was used to select the study participants. Incidence density sampling involved selecting controls based on the diagnoses of the cases. For each diagnosed case, three controls were selected from the cohort who did not have the same diagnosis at that time. Due to the rarity of the case, each instance was included in the study until the required sample size was reached. Controls were selected on the day when the case was identified every k interval (k = 4), using a systematic sampling technique.
Study variables
Dependent variable
The dependent variable of the study was the necrotizing enterocolitis.
Independent variables
The independent variables of the study were the sociodemographic factors (maternal age, sex of neonate, neonatal age, monthly income, maternal educational, and occupational status), pregnancy-related factors (parity, body mass index, preeclampsia, prolonged rupture of membrane, antenatal care (ANC) follow-up, maternal diabetes mellitus, maternal urinary infection, isoimmunization, placenta complication, prolonged labor, chorioamnionitis, use of steroid), maternal lifestyle behavior (drinking alcohol, smoking, khat chewing), and neonatal-related variables (gestational age, birth weight, neonatal sepsis, neonatal asphyxia, multiple gestations, mode of delivery, length of hospital stay, meconium aspiration syndrome, enteral feeding).
Operational and term definitions
Necrotizing enterocolitis is a surgical disorder of the gastrointestinal tract that manifests clinically as abdominal distention, abdominal wall erythema, feeding intolerance, vomiting, and blood in the stool in neonates. Radiologically, it is identified by the presence of dilated bowel loops, bowel wall edema, pneumatosis intestinalis, portal venous gas, and pneumoperitoneum (14, 26).
Criteria for diagnosing NEC
Necrotizing enterocolitis is commonly diagnosed by evaluating a combination of clinical signs and radiological findings, and it is also classified according to modified Bell's staging system (27).
Data collection tools and procedures
After reviewing previous related literature, the investigators prepared a structured interview-administered questionnaire and checklist to review medical records. The medical record checklist includes maternal sociodemographic, pregnancy-related, and perinatal and neonatal variables. An exit face-to-face interview and record review were used to collect data. Six midwives with BSc degrees were employed to collect the data, while two supervisors with MSc degrees were recruited for supervisory duties. The researchers provided theoretical and practical training to data collectors and supervisors regarding data collection instruments, interviewing methodologies, information confidentiality, and the purpose and significance of the study. The data collector's Android phone was equipped with the Kobo Collect version 3.1 program, and the blank form was obtained from the Kobo toolbox server.
Data quality management
A language expert translated the questionnaire into the local language to ensure data quality. The data collectors and supervisors were trained for 2 days to become familiar with different types of data, tools, and data collection procedures and objectives, as well as a 1 day training session on Kobo Collect practice. The questionnaire was pre-tested 3 weeks prior to the actual data collection at Yirgalem General Hospital, which shares a similar status with the study area. To ensure data quality, the supervisors reviewed the completed questionnaires for critical items before uploading them from the Android mobile phone to the Kobo toolbox server. All data were collected on-site using Android mobile devices and transmitted to the Kobo server on a weekly basis using Kobo collect version 3.1. The principal investigator verified the files submitted by each data collector regularly for consistency and completeness. Finally, suitable data coding and categorization were performed to verify data quality.
Data processing and analysis
The data from the Kobo server were downloaded as an Excel file and imported into StataSE V.17 software for cleaning, coding, confirming completeness and accuracy, and further analysis. The outcome variable was divided into two categories: cases and controls (1 = cases, 0 = controls). A descriptive analysis was performed to describe the pertinent characteristics of the study participants. A binary logistic regression was used to examine the relationship between each independent variable and the outcome variable. The Hosmer and Lemeshow test was used to determine the goodness of fit. To control for any possible confounding variables, the variables from the bivariate analysis with a 95% confidence interval and a P-value < 0.25 were included in the multivariable logistic regression analysis. Furthermore, the factors that were found to be significant in previous studies and relevant from a contextual perspective were included in the final model, regardless of whether they satisfied the aforementioned criteria. The independent effect was found using the forward logistic regression model. Colinearity diagnostic statistics were used to screen for multicollinearity using variance inflation factors and a tolerance test. P-values less than 0.05 were considered statistically significant, as were adjusted odds ratios with a 95% confidence interval. Finally, data were presented in the form of tables and texts.
Results
Sociodemographic, economic, and lifestyle behavioral characteristics of the study participants
This study included 111 cases and 332 controls, with a 100% response rate. The mean age of the mothers was 28.33 years, with a standard deviation of 4.66 years. The majority of the case mothers (61, 54.95%) and control mothers (224, 67.47%) were between the ages of 25 and 34 years. In terms of residence, 43 (38.74%) of the case mothers and 123 (37.05%) of the control mothers lived in rural areas. Approximately 87 (78.38%) of the cases and 287 (86.45%) of controls were married. A total of 57 (51.35%) cases and 149 (44.88%) controls had primary education levels in terms of educational status. Of the respondents, 10 (9.01%) cases and 10 (3.01%) controls had a higher body mass index. During their pregnancy, 10 (10.01%) cases and 44 (13.25%) controls had a history of alcohol consumption. Regarding the sex of newborns, 85 cases (76.58%) and 148 controls (44.58%) were male (Table 1).
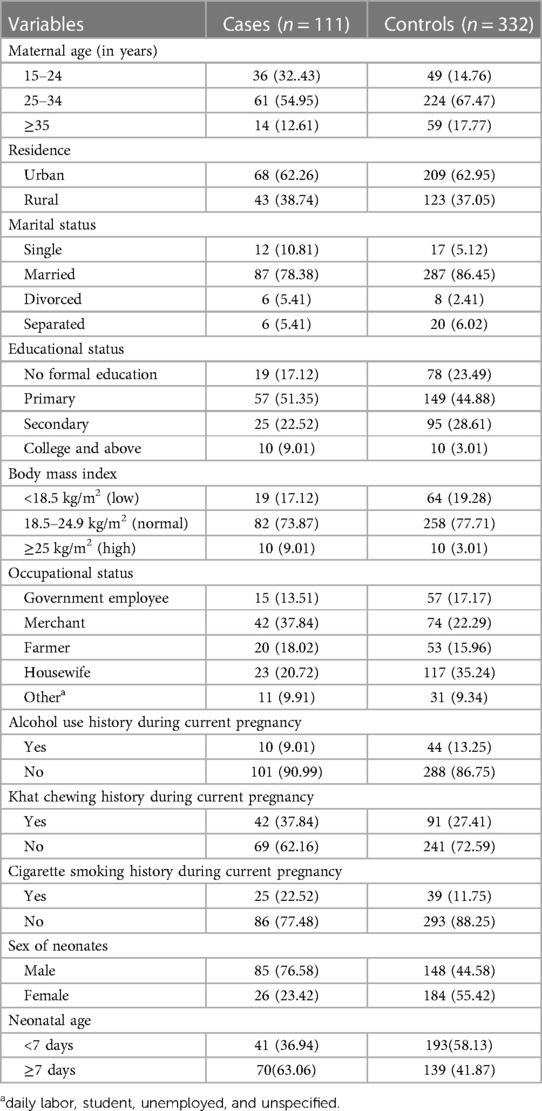
Table 1. Sociodemographic, economic, and lifestyle behavioral characteristics of the study participants, southern Ethiopia, 2023 (N = 443).
Pregnancy-related characteristics of the study participants
Out of the participants in the study, 94 (84.68%) of the cases and 241 (72.59%) of controls were identified as multiparous mothers. During their pregnancy, approximately 83 (74.77%) of the cases and 224 (67.47%) of controls received antenatal care. Gestational hypertension was found in 11 (9.91%) of the cases and 29 (8.73%) of controls. Eighteen (16.22%) of the case mothers and 140 (42.17%) of controls had their labors prolonged. Premature rupture of membrane (PROM) was found in 84 (75.68%) of the case mothers and 108 (32.53%) of control mothers (Table 2).
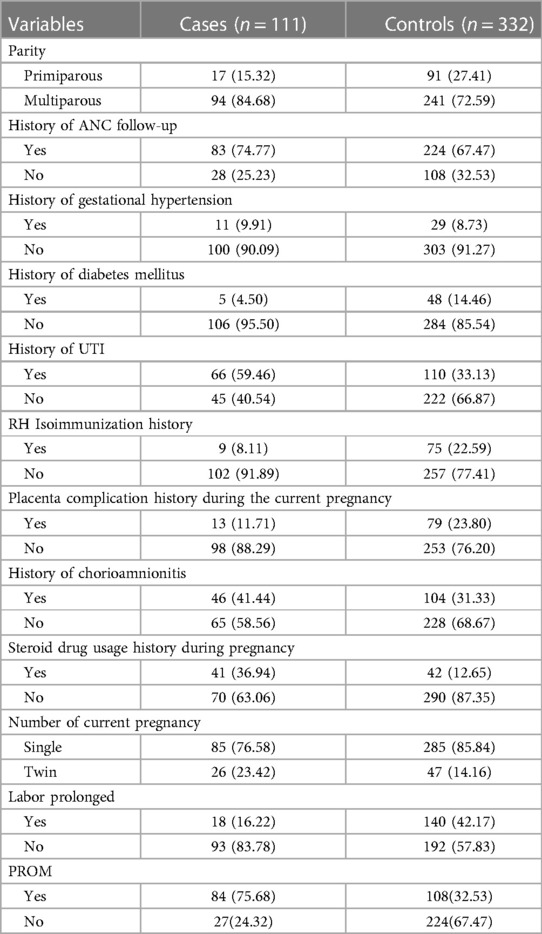
Table 2. Pregnancy-related characteristics of the study participants, southern Ethiopia, 2023 (N = 443).
Perinatal and neonatal-related characteristics of the study participants
Among the study participants, 93 (83.78%) of the cases and 125 (37.65%) of control newborns were delivered at a preterm gestational age, and 76 (68.47%) of the cases and 88 (26.51%) of control newborns were delivered with less than 2,500 g birth weight. It was reported that 23 of the cases (20.72%) and 137 of controls (41.27%) had a history of neonatal sepsis. Regarding the mode of delivery, 89 of the cases (80.18%) and 156 of controls (46.99%) had spontaneous vaginal deliveries. Trophic feeding was given to approximately 66 (59.46%) of the cases and 220 (66.27%) of controls. Regarding the type of initial milk feeding, breast milk was provided first to 71 (63.96%) of the cases and 230 (69.28%) of controls (Table 3).
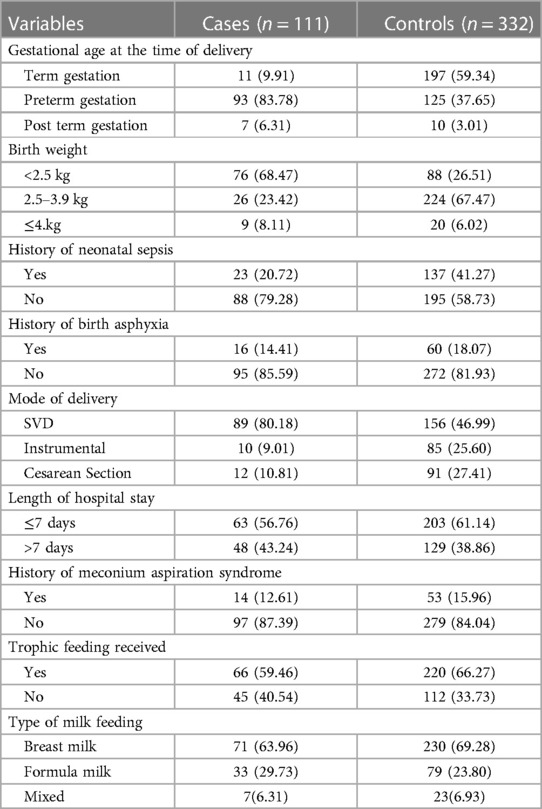
Table 3. Perinatal and neonatal-related characteristics of the study participants, southern Ethiopia, 2022 (N = 443).
Risk factors of necrotizing enterocolitis
To determine the risk factors of NEC, the variables with a p-value of less than 0.25 in the bivariate analysis, significant associations in prior studies, and biological plausibility were considered for the multivariable model in this study. In the binary logistic regression analysis, maternal age, history of khat chewing, antenatal care follow-up, gestational age of newborn, birth weight, gestational hypertension, history of cigarette smoking, length of hospital stay, and PROM were identified as candidate variables for the final multivariable logistics regression analysis model. Finally, body mass index, khat chewing history, gestational age, history of cigarette smoking, length of hospital stay, and PROM were all found to be significantly associated with necrotizing enterocolitis in the multivariable analysis.
Neonates born to mothers with a normal body mass index had an 89% lower likelihood of developing NEC compared with neonates with mothers who had their body mass index of ≥ 25 kg/m2 [AOR = 0.11, 95% CI: (0.02, 0.58)]. Neonates whose mothers chewed khat during their pregnancy were 4.21 times more likely to develop necrotizing enterocolitis than neonates whose mothers did not chew khat during their pregnancy [AOR = 4.21, 95% CI: (1.96, 9.06)]. Newborns who had delivered at a term gestation were 94% less likely to have necrotizing enterocolitis than those newborns delivered at a preterm gestation [AOR = 0.06, 95% CI: (0.01, 0.18)].
Neonates whose mothers had a history of cigarette smoking were 2.86 times more likely to have necrotizing enterocolitis than neonates whose mothers without a history of cigarette smoking [AOR = 2.86, 95% CI: (1.14, 7.14)]. The odds of having necrotizing enterocolitis were 3.3 times more likely in neonates who stay at the hospital for more than 7 days than newborns who stay at the hospital ≤ 7 days [AOR = 3.3, 95% CI: (1.43, 7.67)]. Neonates born with PROM were 3.51 times more likely to have necrotizing enterocolitis than neonates without PROM [AOR = 3.51, 95% CI: (1.77, 6.98)] (Table 4).
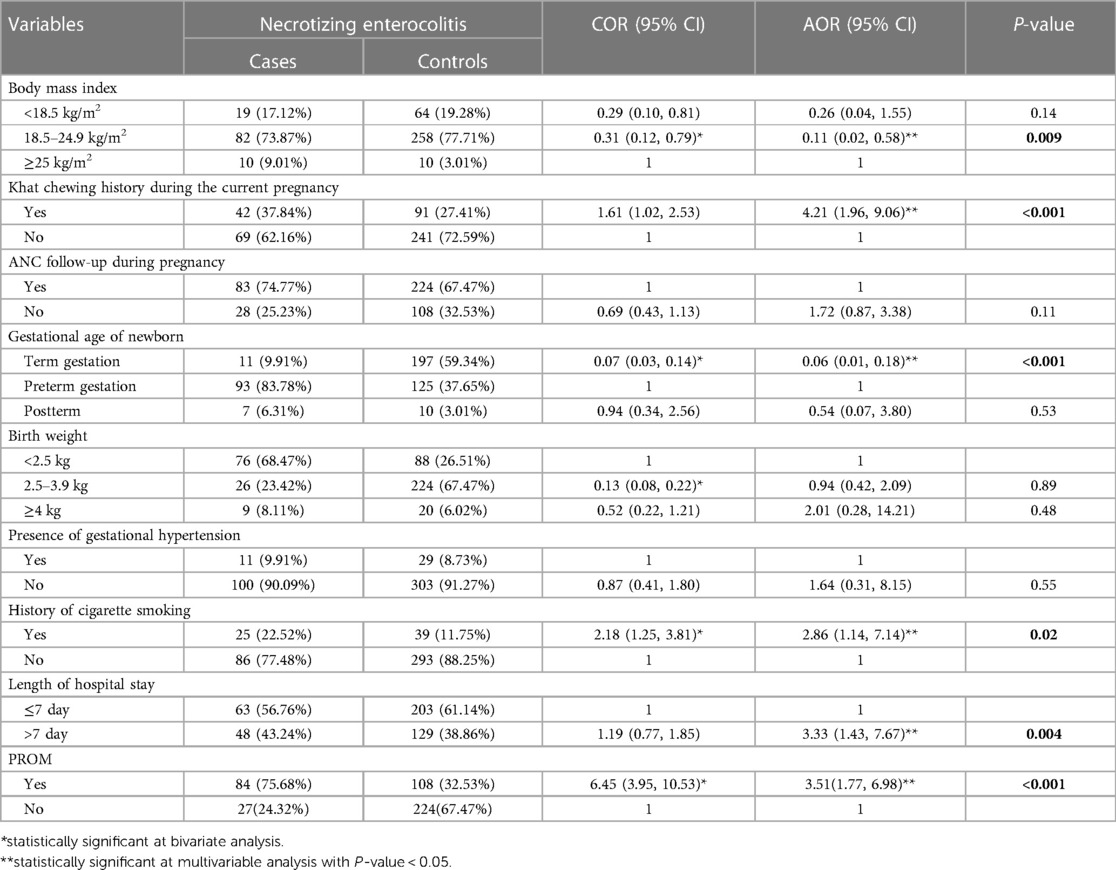
Table 4. Bivariate and multivariable logistic regression analysis of factors associated with necrotizing enterocolitis among the study participants, southern Ethiopia, 2023 (N = 443).
Discussion
Necrotizing enterocolitis is one of the most common gastrointestinal emergencies in newborns (28). It is a potentially fatal gastrointestinal disease frequently observed in NICUs worldwide. NEC causes ischemia and necrosis in some areas of the infant's gastrointestinal system and has a reported mortality rate of up to 25% (29).
Although the exact cause of NEC is still unknown, several risk factors have been identified. Thus, determining the risk factors associated with necrotizing enterocolitis is also beneficial in developing guidelines that aim to prevent contributing factors, lower the burden on the healthcare system, and promote community health. This study indicated that necrotizing enterocolitis was significantly associated with body mass index, history of khat chewing, gestational age, history of cigarette smoking, length of hospital stays, and premature rupture of membrane.
Maternal BMI has been found as a possible risk factor for necrotizing enterocolitis. This study found that neonates born to mothers with a normal BMI were 89% less likely to have NEC than neonates born to mothers with a higher BMI. The finding of this study is consistent with the previous study conducted in Canada (8). The findings of this study contradict those of a Swedish study (19). The mechanism by which maternal BMI increases the risk of necrotizing enterocolitis is not yet understood. The possible reason may be that obesity in mothers may change the composition of breast milk, which may affect the newborn's gut microbiota and increase the risk of necrotizing enterocolitis. Inflammation and oxidative stress, which are both recognized to be increased in obese individuals, are other plausible causes. These factors can increase the risk of NEC by inducing intestinal inflammation and damage. However, further research is needed to fully understand these relationships.
The risk of necrotizing enterocolitis was 4.21 times higher in mothers who chewed khat while pregnant. This could be explained by the fact that chewing khat during pregnancy may lead to a variety of obstetric complications, including preterm birth, low birth weight, and premature membrane rupture (30–33), secondary to which the newborn may increase the risk of necrotizing enterocolitis, according to the critical review results conducted by the WHO Expert Committee on Drug Dependence (ECDD) (34) and other studies. Another explanation might be the vasoconstrictive effects of the component khat cathinone, which can lower blood flow to the digestive system and increase the risk of NEC. NEC is a severe gastrointestinal disease that is believed to be influenced by the gut microbiota (35). Therefore, it is plausible to suggest that changes in the oral microbiota induced by khat chewing could potentially influence the gut microbiota, consequently affecting the risk of developing NEC (36). In conclusion, while khat chewing has been associated with modifications in the periodontal microbiota as a risk factor for NEC, it is complex and likely involves multiple factors. Further research is needed to fully understand these relationships.
The gestational age was found to be a risk factor for NEC in this study. Necrotizing enterocolitis was 94% less likely in neonates delivered at full-term gestation than in newborns delivered during preterm gestation. This finding is supported by the studies conducted in Ethiopia (13, 37), China (38), Korea (39), the Netherlands (40), Taiwan (41), Indonesia (42), and Canada (8). Because the intestines of a preterm newborn are not fully matured, they are more susceptible to infections and inflammation. The most common cause of NEC is prematurity. This can be explained by ischemia mucosal damage in the underdeveloped gut of the preterm newborns.
Neonates whose mothers had a history of cigarette smoking were 2.86 times more likely to have necrotizing enterocolitis than neonates whose mothers did not have a history of cigarette smoking. Other studies conducted in Louisville (43) and Canada (8) also revealed that smoking cigarettes during their pregnancy was significantly associated with necrotizing enterocolitis. Pieces of evidence revealed that smoking during pregnancy is a well-established risk factor that increases the likelihood of adverse pregnancy outcomes, including preterm birth and low birth weight (44, 45). These factors, in turn, increase the risk of developing NEC. Premature newborns have underdeveloped bowels, rendering them more susceptible to developing NEC compared with full-term babies. This finding is inconsistent with a study conducted in Sweden (19).
The odds of having necrotizing enterocolitis were 3.3 times more likely in neonates who stay at the hospital for more than 7 days than in newborns who stay at the hospital for ≤ 7 days. A similar finding was also reported in Ethiopia (14) and Iran (46). This could be related to the possibility of developing nosocomial infection while staying in the hospital for an extended period.
The finding of this study also found that premature rupture of the membranes could raise the likelihood of developing NEC. Neonates born with PROM were 3.51 times more likely to have necrotizing enterocolitis than neonates without PROM. This finding is consistent with the studies conducted in Ethiopia (37), China (38), Atlanta (47), and the Netherlands (40). The possible explanation could be that the fetus is more exposed to the bacteria in the vaginal canal as a result of the premature rupture of the membrane. The gastrointestinal tract may then become infected and inflamed as a result of this bacterial colonization. This may make the newborn more vulnerable to necrotizing enterocolitis following birth. In contrast, studies conducted in Sweden and China found no statistically significant association between premature membrane rupture and necrotizing enterocolitis (19, 48). This discrepancy may be caused by differences in the study design and sample size. Our study has some limitations that need to be acknowledged. First, we did not include variables such as gut dysbiosis and perinatal antibiotic exposure, which may have risk factors for NEC. Future studies should consider these variables and their potential interactions with other risk factors. Second, our study was conducted in a hospital setting and limited to a single region in Ethiopia. Therefore, our findings may not be generalizable to other populations or settings. Further research is needed to validate our results and explore the epidemiology and risk factors of NEC in different contexts.
Conclusion and recommendation
This study identified several risk factors for necrotizing enterocolitis, including body mass index, history of khat chewing, gestational age, history of cigarette smoking, length of hospital stays, and premature rupture of the membrane. Therefore, healthcare providers should be aware of these risk factors to identify high-risk newborns and implement preventive measures.
Data availability statement
To protect participant confidentiality, the datasets generated and/or analyzed for this study are not publicly available. However, they are available from the corresponding author upon reasonable request.
Ethics statement
Ethical clearance was obtained from the institutional ethical review board, College of Medicine and Health Science, Dilla University with project grant code duirb/024/23-04. Support letters from each hospital's chief executive officer were obtained before data collection began. After explaining the purpose and procedures of the research written informed consent was obtained from study participants. Any data collected from respondents were kept private and anonymous. To maintain confidentiality, respondents' names were replaced with code numbers. No one else has access to the data, which was kept strictly confidential, except the investigators. During data collection, possible COVID-19 prevention measures were implemented. Every relevant procedure was completed in compliance with the institutional and Helsinki Declaration guidelines.
Author contributions
MA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. TT: Formal Analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – review & editing. GM: Investigation, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The research work was funded by Dilla University under project grant code duirb/024/23-04, which covered the cost of stationery supplies and data collection. The funders had no involvement in the study design, data collection and analysis, publication decisions, or manuscript preparation.
Acknowledgment
We extend our heartfelt appreciation to Dilla University, data collectors, and study participants for their invaluable contributions to this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Duchon J, Barbian ME, Denning PW. Necrotizing enterocolitis. Clin Perinatol. (2021) 48(2):229–50. doi: 10.1016/j.clp.2021.03.002
3. Satardien M, Van Wyk L, Sidler D, Van Zyl J. Outcomes of neonates requiring neonatal intensive care admission for necrotizing enterocolitis in a resource-restricted hospital in Cape Town, South Africa. J Trop Pediatr. (2021) 67(1):fmaa130. doi: 10.1093/tropej/fmaa130
4. Arnold M, Moore SW, Nadler EP. Necrotizing enterocolitis. Pediatr Surg. (2020):727–45. doi: 10.1007/978-3-030-41724-6_70
5. Berman L, Moss RL. Necrotizing enterocolitis: an update. Seminars in fetal and neonatal Medicine. New Haven, CT: Elsevier (2011).
6. Kordasz M, Racine M, Szavay P, Lehner M, Krebs T, Luckert C, et al. Risk factors for mortality in preterm infants with necrotizing enterocolitis: a retrospective multicenter analysis. Eur J Pediatr. (2022) 181:1–7. doi: 10.1007/s00431-021-04266-x
7. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20(1):1–15. doi: 10.1186/s12887-020-02231-5
8. Alganabi M, Lee C, Bindi E, Li B, Pierro A. Recent advances in understanding necrotizing enterocolitis. F1000Res. (2019) 8:1–9. doi: 10.12688/f1000research.17228.1
9. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F182–F9. doi: 10.1136/archdischild-2017-313880
10. Jammeh ML, Adibe OO, Tracy ET, Rice HE, Clark RH, Smith PB, et al. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J Perinatol. (2018) 38(10):1386–90. doi: 10.1038/s41372-018-0184-x
11. DeRienzo C, Smith PB, Tanaka D, Bandarenko N, Campbell ML, Herman A, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. (2014) 90(5):237–40. doi: 10.1016/j.earlhumdev.2014.02.003
12. Zhang L-p, Lei X-p, Luo L-j, Dong W-b. Risk factors for necrotizing enterocolitis in very preterm infants: a case–control study in southwest China. J Matern Fetal Neonatal Med. (2019) 32(6):896–901. doi: 10.1080/14767058.2017.1395011
13. Mekonnen SM, Bekele DM, Fenta FA, Wake AD. The prevalence of necrotizing enterocolitis and associated factors among enteral fed preterm and low birth weight neonates admitted in selected public hospitals in Addis Ababa, Ethiopia: a cross-sectional study. Glob Pediatr Health. (2021) 8:2333794X211019695. doi: 10.1177/2333794X211019695
14. Temere BC, Mewahegn AA, Zewudie BT, GebreEyesus FA, Kassaw A, Walle BG, et al. Necrotizing enterocolitis and its predictors among preterm neonates admitted in neonatal intensive care units of Gurage zone public hospitals, Southwest Ethiopia, 2021. Pediatric Health Med Ther. (2022) 13:95. doi: 10.2147/PHMT.S353663
15. Nita R, Matulatan F. Necrotizing enterocolitis in preterm newborn with a history of maternal COVID-19: a case report. Radiol Case Rep. (2022) 17(8):2630–4. doi: 10.1016/j.radcr.2022.04.056
16. Lu Q, Cheng S, Zhou M, Yu J. Risk factors for necrotizing enterocolitis in neonates: a retrospective case–control study. Pediatr Neonatol. (2017) 58(2):165–70. doi: 10.1016/j.pedneo.2016.04.002
17. Berkhout DJ, Klaassen P, Niemarkt HJ, de Boode WP, Cossey V, van Goudoever JB, et al. Risk factors for necrotizing enterocolitis: a prospective multicenter case–control study. Neonatology. (2018) 114:277–84. doi: 10.1159/000489677
18. Angelika D, Etika R, Kusumawardani NN, Mithra S, Ugrasena IDG. Observational study on necrotizing enterocolitis in neonates born to SARS-CoV-2-positive mothers. Ann Med Surg. (2022) 78:103711. doi: 10.1016/j.amsu.2022.103711
19. Ahle M, Drott P, Elfvin A, Andersson RE. Maternal, fetal and perinatal factors associated with necrotizing enterocolitis in Sweden. A national case–control study. PloS One. (2018) 13(3):e0194352. doi: 10.1371/journal.pone.0194352
20. Perger L, Mukhopadhyay D, Komidar L, Wiggins-Dohlvik K, Uddin MN, Beeram M. Maternal pre-eclampsia as a risk factor for necrotizing enterocolitis. J Matern Fetal Neonatal Med. (2016) 29(13):2098–103. doi: 10.3109/14767058.2015.1076386
21. Zani A, Pierro A. Necrotizing enterocolitis: controversies and challenges. F1000Res. (2015) 4:1–11. doi: 10.12688/f1000research.6888.1
23. Kokeb M, Desta T. Institution based prospective cross-sectional study on patterns of neonatal morbidity at Gondar University Hospital neonatal unit, North-West Ethiopia. Ethiop J Health Sci. (2016) 26(1):73–9. doi: 10.4314/ejhs.v26i1.12
24. Muhe LM, McClure EM, Nigussie AK, Mekasha A, Worku B, Worku A, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. (2019) 7(8):e1130–e8. doi: 10.1016/S2214-109X(19)30220-7
25. Bazacliu C, Neu J. Pathophysiology of necrotizing enterocolitis: an update. Curr Pediatr Rev. (2019) 15(2):68–87. doi: 10.2174/1573396314666181102123030
26. Marcdante K, Kliegman RM. Nelson Essentials of Pediatrics E-book. Zambia: Elsevier Health Sciences (2014).
27. Patel RM, Ferguson J, McElroy SJ, Khashu M, Caplan MS. Defining necrotizing enterocolitis: current difficulties and future opportunities. Pediatr Res. (2020) 88(Suppl 1):10–5. doi: 10.1038/s41390-020-1074-4
28. Refat NH, AboEl-Gheit AM, Hasanien SS. Risk factors of necrotizing enterocolitis in neonatal intensive care unit of Assiut University Children Hospital. J Curr Med Res Pract. (2020) 5(4):423–8. doi: 10.4103/JCMRP.JCMRP_168_19
29. Tarracchini C, Milani C, Longhi G, Fontana F, Mancabelli L, Pintus R, et al. Unraveling the microbiome of necrotizing enterocolitis: insights in novel microbial and metabolomic biomarkers. Microbiol Spectr. (2021) 9(2):e01176–21. doi: 10.1128/Spectrum.01176-21
30. Yitayih Y, Vanderplasschen W, Vandewalle S, Rita VD, Gilbert L. The effects of khat use during pregnancy on perinatal and maternal outcomes: a meta-analysis. Arch Women’s Ment Health. (2023) 26(1):11–27. doi: 10.1007/s00737-023-01293-5
31. Roble AK, Gundappa R, Sheik Abdirahman F, Abdi AM. Determinants of adverse birth outcomes in public hospitals of the Somali region, Eastern Ethiopia: a multicenter unmatched case–control study. Clin Med Insights. (2023) 17:11795565231195253. doi: 10.1177/11795565231195253
32. Wogayehu B, Demissie T, Wolka E, Alemayehu M, Daka K. The epidemiology of khat (Catha edulis) chewing and alcohol consumption among pregnant women in Ethiopia: a systematic review and meta-analysis. PLOS Glob Public Health. (2023) 3(9):e0002248. doi: 10.1371/journal.pgph.0002248
33. Abdel-Aleem M. Khat chewing during pregnancy: an insight on an ancient problem. Impact of chewing Khat on maternal and fetal outcome among Yemeni pregnant women. J Gynecol Neonatal Biol. (2015) 1(2):1–04. doi: 10.15436/2380-5595.15.004
34. Meeting WECoDD, Organization WH. WHO Expert Committee on Drug Dependence: Thirty-Seventh Report: World Health Organization; (2016).
35. Cassir N, Benamar S, Khalil JB, Croce O, Saint-Faust M, Jacquot A, et al. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates. Clin Infect Dis. (2015) 61(7):1107–15. doi: 10.1093/cid/civ468
36. Al-Alimi A, Taiyeb-Ali T, Jaafar N, Noor Al-hebshi N. Qat chewing and periodontal pathogens in health and disease: further evidence for a prebiotic-like effect. BioMed Res Int. (2015) 2015:1–7. doi: 10.1155/2015/291305
37. Alene T, Feleke MG, Yeshambel A, Amare AT, Tigabu A, Birlie TA, et al. Time to occurrence of necrotizing enterocolitis and its predictors among low birth weight neonates admitted at neonatal intensive care unit of Felege Hiwot Compressive Specialized Hospital Bahir Dar, Ethiopia, 2021: a retrospective follow-up study. Front Pediatr. (2022) 10:959631. doi: 10.3389/fped.2022.959631
38. Su Y, Xu R-H, Guo L-Y, Chen X-Q, Han W-X, Ma J-J, et al. Risk factors for necrotizing enterocolitis in neonates: a meta-analysis. Front Pediatr. (2023) 10:1079894. doi: 10.3389/fped.2022.1079894
39. Cho H, Lee EH, Lee K-S, Heo JS. Machine learning-based risk factor analysis of necrotizing enterocolitis in very low birth weight infants. Sci Rep. (2022) 12(1):21407. doi: 10.1038/s41598-022-25746-6
40. Samuels N, van de Graaf RA, de Jonge RC, Reiss IK, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. (2017) 17(1):1–9. doi: 10.1186/s12887-017-0847-3
41. Yang C-C, Tang P-L, Liu P-Y, Huang W-C, Chen Y-Y, Wang H-P, et al. Maternal pregnancy-induced hypertension increases subsequent neonatal necrotizing enterocolitis risk: a nationwide population-based retrospective cohort study in Taiwan. Medicine (Baltimore). (2018) 97(31):1–6. doi: 10.1097/MD.0000000000011739
42. Izzatazkia LK, Ranuh IGMRG. Newborn risk factors of necrotizing enterocolitis in developing countries: a literature review. World J Adv Res Rev. (2023) 19(3):225–9. doi: 10.30574/wjarr.2023.19.3.1794
43. Downard CD, Grant SN, Maki AC, Krupski MC, Matheson PJ, Bendon RW, et al. Maternal cigarette smoking and the development of necrotizing enterocolitis. Pediatrics. (2012) 130(1):78–82. doi: 10.1542/peds.2011-3808
44. Delcroix M-H, Delcroix-Gomez C, Marquet P, Gauthier T, Thomas D, Aubard Y. Active or passive maternal smoking increases the risk of low birth weight or preterm delivery: benefits of cessation and tobacco control policies. Tob Induc Dis. (2023) 21:21–72. doi: 10.18332/tid/156854
45. Tarasi B, Cornuz J, Clair C, Baud D. Cigarette smoking during pregnancy and adverse perinatal outcomes: a cross-sectional study over 10 years. BMC Public Health. (2022) 22(1):2403. doi: 10.1186/s12889-022-14881-4
46. Sallakh-Niknezhad A, Bashar-Hashemi F, Satarzadeh N, Ghojazadeh M, Sahnazarli G. Early versus late trophic feeding in very low birth weight preterm infants. Iran J Pediatr. (2012) 22(2):171.23056882
47. Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Seminars in fetal and neonatal medicine. Atlanta, GA: Elsevier (2018).
Keywords: risk factors, necrotizing enterocolitis, neonates, Ethiopia, newborns
Citation: Abebe M, Ayehu M, Tebeje TM and Melaku G (2024) Risk factors of necrotizing enterocolitis among neonates admitted to the neonatal intensive care unit at the selected public hospitals in southern Ethiopia, 2023. Front. Pediatr. 12:1326765. doi: 10.3389/fped.2024.1326765
Received: 23 October 2023; Accepted: 9 January 2024;
Published: 31 January 2024.
Edited by:
Bernard La Scola, Aix-Marseille Université, FranceReviewed by:
Massimo Di Maio, Centre Hospitalier Universitaire de Nîmes, FranceNadim Cassir, Aix-Marseille Université, France
© 2024 Abebe, Ayehu, Tebeje and Melaku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mesfin Abebe bWVzZmlhYXVAZ21haWwuY29t
Abbreviations ADH, Adare General Hospital; ANC, antenatal care; DUGH, Dilla University General Hospital; GA, gestational age; HUCSH, Hawassa University Comprehensive & Specialized Hospital; IUGR, intrauterine growth retardation; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; SDG, Sustainable Development Goal; VLBW, very low birth weight.
 Mesfin Abebe
Mesfin Abebe Mequanint Ayehu2
Mequanint Ayehu2