- 1Neonatal Intensive Care Unit, Hôpital Universitaire de Bruxelles, Université Libre de Bruxelles (ULB), Brussels, Belgium
- 2Center of Human Genetics, Hôpital Universitaire de Bruxelles, Université Libre de Bruxelles (ULB), Brussels, Belgium
Neonatal encephalopathy (NE) is a complex clinical condition with diverse etiologies. Hypoxic-ischemic encephalopathy (HIE) is a major contributor to NE cases. However, distinguishing NE subtypes, such as pontocerebellar hypoplasia type 1E (PCH1E), from HIE can be challenging due to overlapping clinical features. Here, we present a case of PCH1E in a neonate with a homozygous mutation c.72delT p. (Phe24LeufsTer20) in the SLC25A46 gene. The severity of PCH1E associated NE highlighted the significance of early recognition to guide appropriate clinical management.
Introduction
Neonatal encephalopathy (NE) is characterized by disruption of brain function, manifested through altered consciousness, accompanied by seizures, cardiorespiratory compromise, or abnormal tone and reflexes (1).
Hypoxic-ischemic encephalopathy (HIE) accounts for half of the cases of NE and typically arises from acute hypoxic-ischemic insults during the intrapartum perinatal period. The other etiologies of NE include acquired conditions (e.g., congenital infections, anemia, stroke), genetic factors, neurometabolic disorders, and cardiac anomalies (2).
Initial management of suspected HIE imply resuscitation, intensive care, and diagnostic assessments to determine the extent of brain injury. When HIE diagnosis is uncertain or excluded, second line investigations are needed to identify alternative etiologies and tailor appropriate therapeutic strategies.
This report presents a case of pontocerebellar hypoplasia (PCH), a rare disorder linked to NE. PCH is a group of autosomal recessive disorders characterized by neurodegeneration of the cerebellum and brainstem, leading to severe neurodevelopmental delays and early mortality. Once categorized into two clinical subtypes (3), advancements have refined the classification to differentiate 16 subtypes, each correlated with specific genetic anomalies (4). The incidence of each subtype remains undetermined except for the most frequent, PCH2A, which is estimated to be lower than 1/200.000 (5).
Distinct from other PCH forms, PCH type 1 exhibits additional central and peripheral motor dysfunction along with anterior horn cell degeneration similar to spinal muscular atrophy (6). Although cerebellar hypoplasia is a consistent feature in PCH type 1, the involvement of the ventral pons and cerebrum is variable (7).
Presently, PCH type 1 comprises six subcategories (PCH1 A-F), with PCH1E (OMIM 610826) linked to mutations in the SLC25A46 gene, representing the most severe form (8). SLC25A46, a mitochondrial carrier protein belonging to the SLC25 family, has been implicated in neurodegeneration. Animal models have demonstrated the protein's engagement with inner mitochondrial membrane remodeling, with disruptions in mitochondrial dynamics in mutated zebrafish neurons (9, 10). Biallelic mutations in the SLC25A46 gene are also associated with hereditary motor and sensory neuropathy type 6B (HMSN6B), a less severe condition displaying overlapping features with PCH1E (10). Wan et al. determined that mutations leading to a significant reduction in SLC25A46 levels are responsible for PCH1E, the most severe clinical presentation among the spectrum of SLC25A46-related diseases (8).
This report highlights the distinct clinical features of PCH1E, characterized by early-onset severe hypotonia and respiratory insufficiency leading to death within weeks of birth (11). Currently, diagnostic methods for early PCH1E detection remain unavailable. Thus, it is crucial for neonatologists to consider genetic or metabolic causes of neonatal encephalopathy, fostering a proactive approach in investigating potential underlying conditions. While encountering patients with PCH1E may be rare, a broader understanding of various etiologies beyond hypoxia is essential for informed clinical decision-making and accurate diagnosis in cases of neonatal encephalopathy.
Case presentation
We report the case of a term male infant born to second-degree consanguineous Syrian parents, with a history of one sibling's neonatal death of unknown etiology. No antenatal concerns such as reduced fetal movements, contractures or polyhydramnios have been reported, an elective Caesarean section for breech presentation was performed at 38 weeks of gestation in a local hospital.
At birth, the newborn unexpectedly presented with profound hypotonia, bradycardia (HR <60 beats/minute), and absent spontaneous respiratory efforts, with APGAR scores of 1, 3, and 5 at 1, 5, and 10 min respectively. Umbilical arterial blood gas analysis showed mixed acidosis (pH 7.10, pCO2 64 mmHg, pO2 59 mmHg, lactate 5.5 mmol/L, base excess −11 mmol/L). Physical examination revealed no dysmorphic features, a birth weight of 3,610 g (P50-90), length of 52 cm (P90), and head circumference of 34 cm (P 90) according to Fenton's growth charts (12).
Prompt resuscitation was performed according to the newborn life support guidelines (13), involving positive pressure ventilation, chest compressions, and endotracheal intubation. Physical examination showed lethargy, severe hypotonia, absent Moro's and sucking reflexes, and weak grasping ability. Irregular respiratory patterns with apnea and hyperventilation were observed. Given of the discordance between the neurological status of the patient and the lack of severe metabolic acidosis combined with the absence of sentinel event cooling was not started. The neonate then was referred and transferred to our level 3 neonatal intensive care unit (NICU) with stable vital parameters. Clinical improvement was observed upon arrival, efficient respiratory efforts prompted transition to high-flow nasal cannula at 6 L/min. Laboratory investigations revealed no evidence of multiorgan involvement. Capillary lactate levels normalized from birth to 6 h of life. In view of improving conditions the decision of normothermia was maintained. However, mechanical ventilation was restarted at eight hours of life due to recurrent apneas and worsening hypotonia.
First line assessments included cranial ultrasound indicating bilateral mild periventricular hyperechogenicity, and continuous video- electroencephalography (EEG) of 24 h. The trace was moderately discontinuous, mainly synchronous, labile and reactive. An abnormal low voltage background likely suggested global cortical dysfunction. Several dysmature patterns were noted: no cycle distinction between wakefulness and sleep, persistent delta brushes, and discontinuous pattern all along the trace, with 5–10 s of interburst interval as expected at 34 weeks. Bursts were composed by multirythmic activities and frequent frontal rhythmic delta activities. No epileptiform discharges. No seizure was recorded. These features are indicative of mild to moderate encephalopathy. Absence of seizures was noted. Antibiotics were stopped following negative blood culture and C-reactive protein results after 48 h.
Absence of neurological improvement prompted extended metabolic and endocrine evaluations: the levels of serum amino acids, sialotransferrin, TSH, free T4, cortisol, ammonia, and 17 OH progesterone were found to be within normal range. Urine amino acid analysis detected mild elevation (11 mmol/mol creatinine) in excretion of 3-methylglutaconic acid, implying possible mitochondrial dysfunction (14). Brain MRI on day 6 showed cerebellar hypoplasia with symmetric involvement of the vermis and hemispheres, without dysplasia (Figure 1) and a potential ischemic thalamic lesion (Figure 2).
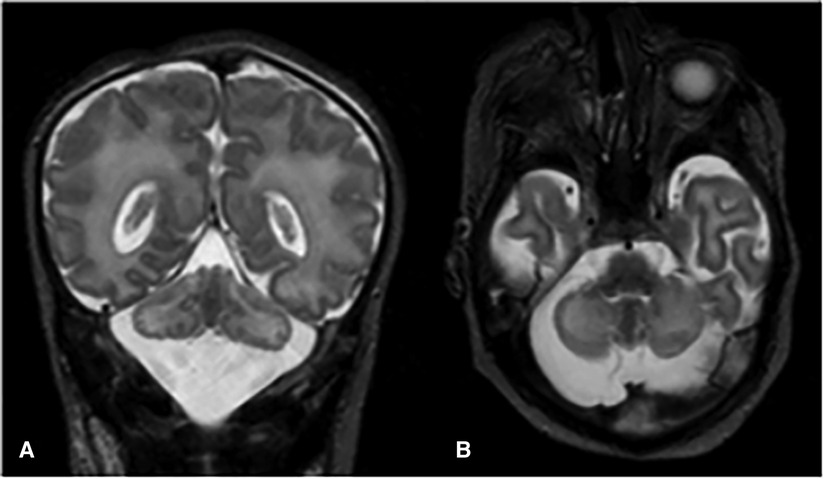
Figure 1. Coronal (A) and axial (B) T2-weighted images show global cerebellar hypoplasia with symmetric involvement of both hemispheres and vermis, along with a very large cisterna magna. There was no obvious abnormality of the brainstem. The cerebellar folia and fourth ventricle were not dilated, indicating there was no volume loss. The cerebellar foliation pattern was normal and there were no heterotopic nodules of gray matter, indicating there was no dysplasia.
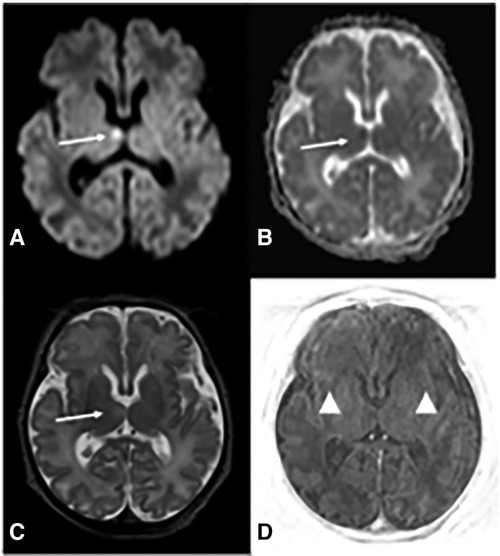
Figure 2. Brain MRI at d6 demonstrates deep gray and white matter hypoxia. Axial diffusion-weighted (DWI) image (A) reveals increased b1000 signal in the right ventro-medial thalamus and corresponding decreased apparent diffusion coefficient (ADC) (B) (arrows in A and B) indicating restricted diffusion. There is associated pathological T2 prolongation (arrow in C). Axial TI|R-weighted image (D) shows lack of the physiological T1 hyperintensity of posterior limb of internal capsules (PLIC) (arrowheads), whereas PLIC is usually hyperintense on T1 with respect to the thalami and basal ganglia.
At two weeks of life, electrophysiological studies (sensory and motor nerve conduction and electromyography) revealed severe sensorimotor neuropathy. The newborn was managed with supportive care, and died to respiratory insufficiency on day 18. Post-mortem examination was declined in accordance with parental wishes.
Subsequent exome sequencing unveiled a homozygous mutation c.72delT p. (Phe24LeufsTer20) in the SLC25A46 gene, with both parents identified as heterozygous carriers.
Discussion
PCH1E is marked by a grave clinical course, with most patients dying within the first month of life at neonatal intensive care units (3, 9–11, 15–20). Prompt and accurate diagnosis remains challenging, as the definitive confirmation through exome sequencing often arrives after life-sustaining decisions have been made.
Recognizing neuromuscular diseases such as PCH1E within the broad spectrum of NE is imperative for facilitating management and appropriate palliative care decisions. Impaired postnatal adaptation, as evident in inefficient respiration and severe hypotonia, compares with typical neonatal encephalopathy caused by perinatal asphyxia (10, 11, 17, 18). An important decision emerged in the management of the presented case, as the medical team opted not to initiate cooling therapy in the face of neonatal encephalopathy of unknown origin. This decision, while supported by certain clinical indicators such as the discrepancy between the severity of clinical presentation at birth and absence of fetal distress or sentinel events, coupled with the lack of severe acidosis at birth, remains subject to controversy. Conventionally, when faced with neonatal encephalopathy and uncertainty regarding a hypoxic event, the initiation of therapeutic hypothermia is often recommended. The presence of a thalamic lesion in the presented case indicates potential perinatal hypoxic brain damage in addition to PCH1E. Although cooling would not have changed the outcome for our patient, it could prove beneficial in situations where NE is caused by factors other than HIE and is the cause rather than the consequence of asphyxic brain damage.
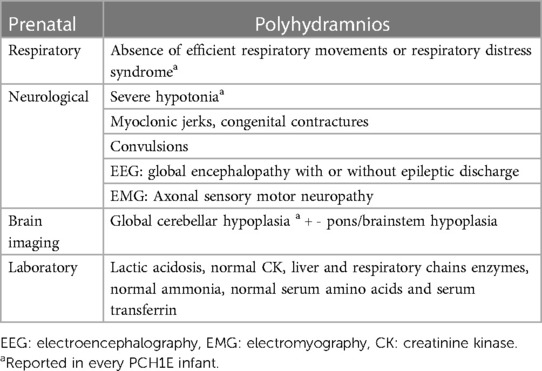
The diagnostic journey in NE involves tailored approaches based on clinical presentation. In this case, the association of a history of neonatal death or consanguinity, and the lack of metabolic aberrations, coupled with neuroimaging and neuromuscular findings, prompted pursuit of a neuromuscular etiology. Understanding the nuanced clinical presentation of PCH1E orientates neonatologists in diagnosis and informed decision-making. The clinical observations reported in PCH1E are summarized in Table 1, the association of respiratory failure, cerebellar pons hypoplasia and sensory-motor neuropathy being the key features.
Prenatal diagnosis can be challenging; while congenital contractures have occasionally been described at birth, they were not reported during the prenatal care of PCH1E cases (1, 7–9, 11, 15–17, 19, 20). In PCH2, ultrasonography is unreliable for early diagnosis, as characteristic findings of pontocerebellar dysgenesis typically develop after 30 weeks of gestation (21). To our knowledge, anomalies of the posterior fossa, such as a large cisterna magna or a reduction in cerebellar transverse diameter, were not reported during the pregnancy of patients with PCH1E. Although not strongly supported by evidence, this observation suggests a late presentation of abnormalities in PCH1E, akin to PCH2.
Our patient's variant, c.72delT in SLC25A46, is novel. No biochemical experiments were conducted to determine the functional impact of the mutation. However, the correlation of our case with the severe clinical phenotype found in the loss-of-function mutations aligns with a class IV variant causing PCH1E. While therapeutic options are currently limited, emerging gene therapy interventions hold promise, as demonstrated in murine models (22).
Conclusion
This report delineates a novel pathogenic variant c.72delT p. (Phe24LeufsTer20) in the SLC25A46 gene, expanding the spectrum of genetic alterations linked to PCH1E. Early recognition of the distinct clinical manifestations of PCH1E assists neonatologists in diagnosing and making informed decisions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was not obtained from the minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article because This article was written a few month after patient’s death and we lost contact with the parents. However, all identifying information about the child and their family has been removed or anonymized to the point where they cannot be identified. The case report no longer poses a significant risk to the child’s privacy.
Author contributions
AG: Writing – original draft. VS: Writing – review & editing. GS: Writing – review & editing. DK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum Dev. (2010) 86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010
2. Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F346–58. doi: 10.1136/archdischild-2015-309639
3. Barth PG. Pontocerebellar hypoplasias. An overview of a group of inherited neurodegenerative disorders with fetal onset. Brain Dev. (1993) 15:411–22. doi: 10.1016/0387-7604(93)90080-r
4. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University. Online Mendelian Inheritance in Man, OMIM®. Available online at: https://omim.org/ (Accessed May 2, 2022)
5. Sánchez-Albisua I, Frölich S, Barth PG, Steinlin M, Krägeloh-Mann I. Natural course of pontocerebellar hypoplasia type 2A. Orphanet J Rare Dis. (2014) 9:70. doi: 10.1186/1750-1172-9-70
6. van Dijk T, Baas F, Barth PG, Poll-The BT. What’s new in pontocerebellar hypoplasia? An update on genes and subtypes. Orphanet J Rare Dis. (2018) 13:92. doi: 10.1186/s13023-018-0826-2
7. Namavar Y, Barth PG, Poll-The BT, Baas F. Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet J Rare Dis. (2011) 6:50. doi: 10.1186/1750-1172-6-50
8. van Dijk T, Rudnik-Schöneborn S, Senderek J, Hajmousa G, Mei H, Dusl M, et al. Pontocerebellar hypoplasia with spinal muscular atrophy (PCH1): identification of SLC25A46 mutations in the original Dutch PCH1 family. Brain. (2017) 140:e46–e46. doi: 10.1093/brain/awx147
9. Wan J, Steffen J, Yourshaw M, Mamsa H, Andersen E, Rudnik-Schöneborn S, et al. Loss of function of SLC25A46 causes lethal congenital pontocerebellar hypoplasia. Brain. (2016) 139:2877–90. doi: 10.1093/brain/aww212
10. Abrams AJ, Hufnagel RB, Rebelo A, Zanna C, Patel N, Gonzalez MA, et al. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat Genet. (2015) 47:926–32. doi: 10.1038/ng.3354
11. Braunisch MC, Gallwitz H, Abicht A, Diebold I, Holinski-Feder E, Van Maldergem L, et al. Extension of the phenotype of biallelic loss-of-function mutations in SLC25A46 to the severe form of pontocerebellar hypoplasia type I: bRAUNISCH et al. Clin Genet. (2018) 93:255–65. doi: 10.1111/cge.13084
12. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
13. Madar J, Roehr CC, Ainsworth S, Ersdal H, Morley C, Rüdiger M, et al. European resuscitation council guidelines 2021: newborn resuscitation and support of transition of infants at birth. Resuscitation. (2021) 161:291–326. doi: 10.1016/j.resuscitation.2021.02.014
14. Wortmann SB, Kluijtmans LAJ, Rodenburg RJ, Sass JO, Nouws J, van Kaauwen EP, et al. 3-methylglutaconic aciduria—lessons from 50 genes and 977 patients. J Inherit Metab Dis. (2013) 36:913–21. doi: 10.1007/s10545-012-9579-6
15. Charlesworth G, Balint B, Mencacci NE, Carr L, Wood NW, Bhatia KP. SLC25A46 mutations underlie progressive myoclonic ataxia with optic atrophy and neuropathy. Mov Disord. (2016) 31:1249–51. doi: 10.1002/mds.26716
16. Hammer MB, Ding J, Mochel F, Eleuch-Fayache G, Charles P, Coutelier M, et al. SLC25A46 mutations associated with autosomal recessive cerebellar ataxia in north African families. Neurodegener Dis. (2017) 17:208–12. doi: 10.1159/000464445
17. Janer A, Prudent J, Paupe V, Fahiminiya S, Majewski J, Sgarioto N, et al. SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for leigh syndrome. EMBO Mol Med. (2016) 8:1019–38. doi: 10.15252/emmm.201506159
18. Nguyen M, Boesten I, Hellebrekers DMEI, Mulder-den Hartog NM, de Coo IFM, Smeets HJM, et al. Novel pathogenic SLC25A46 splice-site mutation causes an optic atrophy spectrum disorder. Clin Genet. (2017) 91:121–5. doi: 10.1111/cge.12774
19. Abrams AJ, Fontanesi F, Tan NBL, Buglo E, Campeanu IJ, Rebelo AP, et al. Insights into the genotype-phenotype correlation and molecular function of SLC25A46. Hum Mutat. (2018) 39:1995–2007. doi: 10.1002/humu.23639
20. Sulaiman RA, Patel N, Alsharif H, Arold ST, Alkuraya FS. A novel mutation in SLC25A46 causes optic atrophy and progressive limb spasticity, with no cerebellar atrophy or axonal neuropathy. Clin Genet. (2017) 92:230–1. doi: 10.1111/cge.12963
21. Graham JM, Spencer AH, Grinberg I, Niesen CE, Platt LD, Maya M, et al. Molecular and neuroimaging findings in pontocerebellar hypoplasia type 2 (PCH2): is prenatal diagnosis possible? American J of Med Genetics Pt A. (2010) 152A:2268–76. doi: 10.1002/ajmg.a.33579
Keywords: neonatal encephalopathy, pontocerebellar hypoplasia type 1E, SLC25A46, clinical diagnosis, therapeutic strategies
Citation: Guillaume A, Stejskal V, Smits G and Kelen D (2024) A novel homozygous variant in SLC25A46 gene associated with pontocerebellar hypoplasia type 1E: a case report. Front. Pediatr. 12:1303772. doi: 10.3389/fped.2024.1303772
Received: 28 September 2023; Accepted: 15 February 2024;
Published: 23 February 2024.
Edited by:
Paolo Montaldo, Imperial College London, United KingdomReviewed by:
Gusztav Belteki, Cambridge University Hospitals NHS Foundation Trust, United KingdomSimona Puzone, University of Campania Luigi Vanvitelli, Italy
© 2024 Guillaume, Stejskal, Smits and Kelen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adrien Guillaume YWRyaWVuLmd1aWxsYXVtZUBodWJydXhlbGxlcy5iZQ==
†ORCID Adrien Guillaume orcid.org/0000-0002-7732-9993
 Adrien Guillaume
Adrien Guillaume Vojtech Stejskal
Vojtech Stejskal Dorottya Kelen
Dorottya Kelen