- 1Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, China
- 2Department of Cardiothoracic Surgery, Hunan Children's Hospital, Changsha, China
- 3Department of Clinical Pharmacology, Xiangya School of Pharmacy, Central South University, Changsha, China
Objective: To evaluate the prevalence and associated factors of undernutrition among children with congenital heart disease (CHD) who have not undergone surgeries in China.
Methods: This cross-sectional study included 734 CHD children along with their parents. The outcome of interest was undernutrition, including underweight, wasting, and stunting, defined as Z-scores (i.e., weight-for-age, weight-for-height, and height-for-age) ≤−2, according to the World Health Organization (WHO) growth standard. Exposures of interest, containing demographics, obstetric factors, maternal dietary factors, parents' life behaviors and habits, birth-related factors, cardiac-related factors, and preoperative factors, were analyzed using a multivariate logistic regression model to test their associations with undernutrition in CHD children.
Results: Overall, 36.1%, 29.7%, and 21.3% of cases were underweight, wasted, and stunted, respectively. Multivariate logistic regression indicated that underweight was associated with demographic factors (including parents' occupational status, family income, and maternal body mass index pre-pregnancy), low birth weight (OR = 4.60, 2.76–7.70), pulmonary hypertension (OR = 4.46, 3.09–6.43), and pneumonia (OR = 1.88, 1.28–2.76). Artificially-fed children were 2.34 (1.36–4.01) times more likely to be underweight. Occupied mothers (OR = 0.62, 0.44–0.88) and fathers (OR = 0.49, 0.26–0.92) served as protective factors, while mothers having gestational complications (OR = 1.56, 1.11–2.18) and exposed to noisy environment (OR = 1.64, 1.11–2.42) during this pregnancy, and pulmonary hypertension (OR = 3.21, 2.30–4.49) increased the chance of wasting in offspring. The odds of being stunted were greater in families with >2 children (OR = 1.88, 1.13–3.14), placental abruption during this pregnancy (OR = 25.15, 2.55–247.89), preterm births (OR = 1.84, 1.02–3.31), low birth weight (OR = 3.78, 2.16–6.62), pulmonary hypertension (OR = 2.35, 1.56–3.53) and pneumonia (OR = 1.93, 1.28–2.90). In subgroup analyses, the associations differed between patients with different feeding patterns (breastfeeding vs. non-breastfeeding), CHD classifications (cyanotic vs. acyanotic), and prematurity (preterm vs. non-preterm).
Conclusion: Undernutrition is common in preoperative CHD children. Familial demographics, maternal factors (including having gestational complications and exposure to noisy environment during pregnancy), and patient-related factors (encompassing preterm births, low birth weight, pulmonary hypertension, pneumonia, and feeding pattern) were found to contribute to undernutrition in CHD cases. However, associated factors among the three subgroups of distinct feeding patterns, CHD categorization, and prematurity exhibited varied outcomes, suggesting the necessity for targeted interventions.
1 Introduction
Across the globe, congenital heart disease (CHD) is the most prevalent birth defect and ranks the 7th leading cause of infant mortality (1), with the prevalence rising from 4.5 per 1,000 births in 1970–1974 to 9.4 per 1,000 births in 2010–2017 (2). Despite the fact that disability-adjusted life years (DALY) of CHD children aged under 10 years decreased by 41.6% from 1990 to 2019 (3), which is mainly attributed to the advancement and wide application of neonatal surgical interventions, as well as perioperative care, preoperative undernutrition is emerging as a global problem among CHD children, affecting their long-term outcomes.
According to World Health Organization (WHO), undernutrition refers to deficiencies in a child's intake of energy and/or nutrients, affecting 15%–64% of children with cardiac anomalies, and the proportion is higher in developing countries (4, 5). A case-control study in Nigeria indicated that preoperative CHD children were at higher risk of undernutrition than healthy counterparts (90.4% vs. 21.1%) (6). Based on our previous meta-analysis published in 2022, 27.4%, 24.4%, and 24.8% of preoperative CHD children globally suffered from underweight, wasting and stunting, respectively (7). Apart from general causes of undernutrition such as insufficient caloric intake and growing energy demand, several disease-related causes may be explanatory, including hypoxia (primary concern for cyanotic CHD), congestive heart failure (commonly seen in acyanotic CHD), pulmonary hypertension, and gastrointestinal dysfunction as a result of complications (5, 8, 9). A number of articles have sufficiently narrated that undernutrition in CHD children is associated with worse hospital outcomes, higher infection rates, increased morbidity, mortality and complications (10, 11), impaired neurodevelopment, and poor social and academic performance (12, 13), which exert long-term negative impact on the overall development until adulthood.
Previous investigators have demonstrated associated factors of undernourishment among CHD children who had not performed cardiac surgeries, ranging from patients' demographics (including age, gender, residence, family size and income, etc.), to their cardiac factors (containing cyanotic heart disease, pulmonary hypertension, heart failure, anemia, etc.) (14–16). Maternal-related factors, which are comprised of elder age, low education level, unemployment, high anxiety and depression level, were also reported to be associated with offspring's growth retardation (17, 18). In addition, a few studies explored father's demographic characteristics as associated factors (15, 16, 19). Noticeably, Vaidyanathan et al. performed 24-h dietary recall on mothers and found that fat intake below 50 g per day was associated with underweight and stunting in CHD children (16), implying that maternal unbalanced dietary pattern adversely impacts nutritional status in the offspring. However, there is a lack of research documenting the relationship between maternal periconceptional dietary factors and their CHD offspring's undernourishment, as well as obstetric factors (i.e., history of pregnancy outcomes and gestational complications during this pregnancy), life behaviors and habits and exposure to environmental hazards during pregnancy. Considering that only 38.7% of syndromic CHD infants were breastfed reaching 6 months mainly due to sucking inactivity (20), probing predictors of undernutrition independently among breastfed and non-breastfed cases were of vital significance. Furthermore, preceding publications reached heterogenous results, particularly on influencing factors of undernutrition in cyanotic and acyanotic cases (15, 21). Attention should also be paid to nutritional status of preterm CHD births, defined as born <37 weeks of gestation, as they exhibit different growth patterns with full-term infants (22). Therefore, we conducted a cross-sectional study to investigate the associated factors of underweight, wasting and stunting among preoperative CHD children in China, and analyzed these factors separately based on feeding patterns, cyanosis, and prematurity, so as to lay foundation for targeted interventions to mitigate preoperative undernutrition.
2 Materials and methods
2.1 Ethics compliance
This study was approved by the Ethics Committee of Xiangya School of Public Health, Central South University. We have obtained written informed consent from all participants, and this research has been registered in the Chinese Clinical Trial Registration Center (Registration Number: ChiCTR1800016635).
2.2 Study population
This was a cross-sectional study conducted in the Department of Cardiothoracic Surgery of Hunan Children's Hospital between November 2017 and January 2021, which was part of a larger case-control study, thus the procedure and related details have been previously described (23).
All non-syndromic CHD children aged 0–5 years, of Han Chinese descent, who had not undergone cardiac surgery, and their parents were enrolled in this study. These patients had been diagnosed with CHD after screening for symptoms, physical examination, and echocardiography, according to the 10th version of the International Classification of Diseases (ICD-10). The exclusion criteria were: (1) cases who underwent emergency surgeries; or (2) with severely impaired liver and kidney function; or (3) diabetics; or (4) patients and/or his or her parents who lack relevant information. Worthy of mentioning, children who have congenital metabolic disorders, lesions in other organ systems apart from the cardiovascular system, genetic syndromes or chromosomal aberrations (e.g., Down's syndrome, catarrhal syndrome, Noonan syndrome, Holt–Oram syndrome, etc.) were not taken into consideration.
2.3 Sample size calculation
The sample size calculation was based on the prevalence rates of underweight (26.2%), wasted (24.6%), and stunted (22.3%) CHD children observed in a previous study (24). Additionally, we used the formula for calculating sample size in a cross-sectional study. Assuming a margin of error (d) of 0.15*p (p stands for prevalence), we calculated the required sample sizes for underweight, wasted, and stunted children as 481, 524, and 595, respectively. To ensure adequate statistical power, we selected the largest sample size of 595 as the final sample size for our study. After considering a non-response rate of 5%, the ultimate sample size was determined to be 625.
2.4 Exposures of interest
At the time of hospital admission, eligible parents were asked to complete a self-designed questionnaire during face-to-face interviews conducted by health professionals trained in the survey and supervised by the principal investigator to minimize investigation bias. The questionnaire contained items on parents' demographic characteristics, life behaviors and habits, maternal history of adverse pregnancy outcomes, family history of congenital malformation, gestational complications during this pregnancy, periconceptional dietary factors, intake of folic acid, and history of exposure to environmental hazards, along with CHD children's demographic characteristics, birth-related factors, cardiac-related factors, preoperative factors, and feeding pattern. Additionally, CHD subtypes determined by echocardiography results, diagnosis of cardiac complications were retrieved from medical records.
The following maternal features were collected, including (1) demographic characteristics (i.e., age at this pregnancy, residence, education level, occupational status, family annual income, number of children, and body mass index before this pregnancy), (2) history of adverse pregnancy outcomes which is the sum of history of adverse birth outcomes (i.e., history of spontaneous abortion, history of stillborn fetus, history of premature birth, history of low birth weight, and history of intrauterine growth arrest) and history of gestational complications (i.e., history of gestational diabetes mellitus, history of gestational hypertension, history of placenta previa, history of premature rupture of membrane, history of antepartum/postpartum hemorrhage, history of anemia during pregnancy, and history of ectopic gestation), (3) family history of congenital malformation, (4) gestational complications during this pregnancy (i.e., gestational diabetes mellitus, gestational hypertension, placenta previa, placental abruption, premature rupture of membrane, antepartum/postpartum hemorrhage, and anemia during pregnancy), (5) periconceptional dietary factors (i.e., eating pickles, eating preserved eggs, eating salted eggs, eating smoked food, eating fried food, eating dairy products, eating eggs, eating vegetables, eating fruits, eating beans, eating meat, and eating seafood), (6) life behaviors and habits before or during early stage of pregnancy (i.e., smoking, passive smoking, drinking, drinking tea, drinking coffee, using computers, using cellphones, and experiencing negative events), (7) intake of folic acid, and (8) history of exposure to environmental hazards (i.e., exposure to air pollution, exposure to a noisy environment, exposure to newly renovated houses, and exposure to radiation).
In addition, the father's demographic characteristics (i.e., education level and occupational status) and life behaviors (i.e., smoking, drinking, drinking tea, drinking coffee, using computers, and using cellphones) were considered as well.
For CHD children, we collected demographic characteristics (i.e., age, gender), birth-related factors (i.e., mode of delivery, premature birth, low birth weight, and multiple births), CHD subtypes and complications (i.e., cyanosis, hemodynamics, pulmonary hypertension, pneumonia), and feeding pattern. Detailed definitions of exposures of interest listed above are shown in Supplementary Table S1.
Furthermore, a perinatal health care handbook (PHCH) is distributed to each Chinese pregnant woman, recording basic demographic characteristics, lifestyle behaviors, family history of congenital malformations (patients' first and second degree relatives), history of various diseases (including history of previous and current gestational complications), diverse medical examinations during pregnancy, and folic acid supplementation. Relevant information from PHCH and medical records were extracted to confirm the corresponding results, which dramatically reduced recall bias.
2.5 Outcomes of interest
The outcome of interest was undernutrition, classified into underweight, wasting, and stunting. At the time of hospital admission, measurements of weight and height were carried out on each patient. Anthropometric measurements and subsequent calculation of three indicators were performed according to 2006 WHO child growth standards (available at: https://www.who.int/toolkits/child-growth-standards).
After zeroing the weight scale, children were weighed 2 h after eating or in fasted state, with clothes and socks removed until minimal clothes. Weight readings were recorded after stabilization, to the nearest 0.1 kg. Upon weighing, infants and children within 2 years old had their lengths measured in the supine position, the body kept straight, with the top of head and soles of feet touching two edges of the measuring board. Heights of children over 2 years old were measured when standing, eyes straight ahead, chest slightly upright, abdomen slightly retracted, arms naturally hanging down, heels closing together, toes 60 degrees apart, scapulae, hips and heels touching the column of the stadiometer (sensitivity of 0.1 cm).
Weight for age Z-score (WAZ), weight for height Z-score (WHZ), and height for age Z-score (HAZ), were then calculated via WHO Anthro software (access at: http://www.who.int/childgrowth/software/en/), based on the following formula.
Underweight, wasting, and stunting were defined if WAZ ≤−2, WHZ ≤−2, HAZ ≤−2, respectively. Of note, to facilitate comparison with other publications, we merged severe undernutrition (i.e., severe underweight, severe wasting, and severe stunting), which was defined as Z-scores ≤−3, into undernutrition.
2.6 Statistical analysis
Non-normally distributed values including age were presented as medians and interquartile range (IQR), and categorical variables containing exposures and outcomes were expressed as absolute and percentage distribution. Missing values were interpolated using means or medians. Chi-squared tests or Fisher's exact tests were used to examine the differences of unordered categorial variables between two groups. For variates of significance, Odds ratios (ORs) and 95% confidence intervals (CIs), along with P-values were later computed by multivariable logistic regression analysis. Statistical analysis was performed using SPSS software. Two-sided P < 0.05 was considered statistically significant.
2.7 Subgroup analyses
Subgroup analyses were performed between breastfed and non-breastfed children, cyanotic and acyanotic children, and children born with or without prematurity. To facilitate calculation, artificially feeding and mixed feeding children were combined into non-breastfed group. Comprehensive definitions were provided in Supplementary Table S1. The statistical analysis followed the same protocol as primary analysis, as described in Section 2.6.
3 Results
3.1 Prevalence of underweight, wasting, and stunting
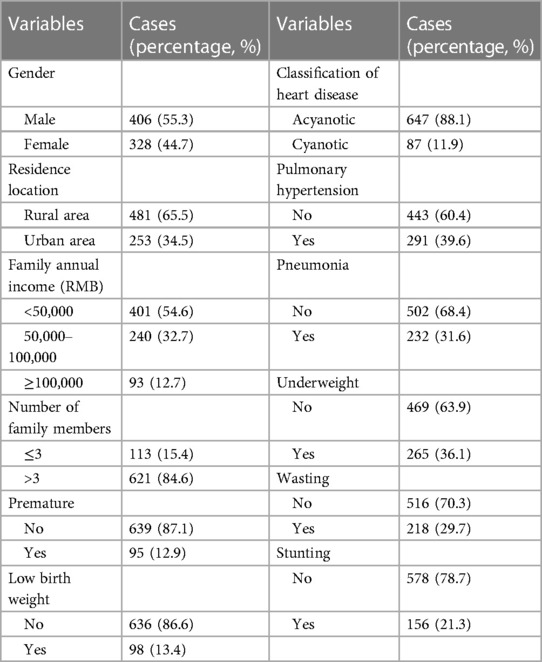
From November 2017 to January 2021, 753 CHD children meeting inclusion criteria, along with their parents, were recruited for this study. 19 of them met exclusion criteria (10 cases had genetic syndromes or chromosomal aberrations, 4 cases had liver or kidney failure, 5 cases had single parent and lack relevant information) and were excluded. Thus, 734 children and their parents were eligible for following analysis. Over half of the cases (55.3%) were male, and the median age was 9 months (IQR: 4–24). 40.2% of cases (n = 295) were admitted to the hospital before 6 months of age, and the distribution of age at admission was shown in Supplementary Figure S1. The majority of children lived in rural areas (65.5%), with more than two other family members (84.6%), who altogether earned <50,000 RMB annually (54.6%). Of the total, 12.9% (n = 95) participants were born prematurely and 13.4% (n = 98) individuals were of low birth weight. There was a similar proportion of children born by spontaneous vaginal delivery (n = 383, 52.2%) and caesarean section (n = 351, 47.8%). The most common malformations among CHD patients at admission were: ventricular septal defect (69.3%), patent foramen oval (62.4%) patent ductus arteriosus (44.1%), and atrial septal defect (23.8%). These CHD subtypes were mostly acyanotic heart disease (88.1%). Pulmonary hypertension and pneumonia were documented in 39.6% and 31.6% of the patients, respectively.
The overall prevalence of undernutrition was 48.1% (n = 353, 95% CI: 44.5%–51.7%) of the 734 CHD children, and 6.4% (n = 47) of whom showed all the abnormal parameters, containing WAZ, WHZ, HAZ <−2 simultaneously. The prevalence of children underweight, wasted, and stunted was 36.1% (n = 265, 95% CI: 32.6%–39.6%), 29.7% (n = 218, 26.4%–33.0%), and 21.3% (n = 158, 18.3%–24.2%), respectively. Baseline characteristics are shown in Table 1.
3.2 Univariate analysis of associated factors of undernutrition in CHD children
Results of the association between undernutrition and maternal factors, paternal factors, and CHD children's factors were displayed in Supplementary Tables S2–S5.
Being underweight was significantly associated with maternal occupational status (unemployed), family annual income (<50,000 RMB), number of children (>2), body mass index before this pregnancy (underweight), history of intrauterine growth arrest, gestational complications during this pregnancy, premature rupture of the membrane during this pregnancy, never eating seafood. Additionally, the following influence factors were also considered explanatory, including paternal educational level (high school and below) and occupational status (unemployed), born prematurely, of low birth weight, multiple births, cyanotic heart disease, pulmonary hypertension, pneumonia and feeding pattern (artificial feeding).
About wasting in CHD children, parents’ occupational status (unemployed), paternal education level (high school and below), family annual income (<50,000 RMB), history of intrauterine growth arrest, gestational complications during this pregnancy, maternal exposure to a noisy environment were significant predictors. On top of that, pulmonary hypertension, pneumonia and feeding pattern (artificial feeding) emerged as significant risk factors.
With respect to stunted CHD children, the significant associate factors included maternal number of children (>2), gestational hypertension during this pregnancy, placental abruption during this pregnancy, and premature rupture of membrane during this pregnancy. Moreover, mode of delivery (caesarean section), born prematurely, of low birth weight, multiple births, pulmonary hypertension, pneumonia, and feeding pattern (artificial feeding) were significantly associated with stunting.
3.3 Multivariable logistic regression analysis for associated factors of undernutrition in CHD children
Multivariate logistic regression analysis was performed by forward stepwise procedure, using significant variables from univariate analysis, and the results of underweight, wasting and stunting are shown in Tables 2–4 respectively.
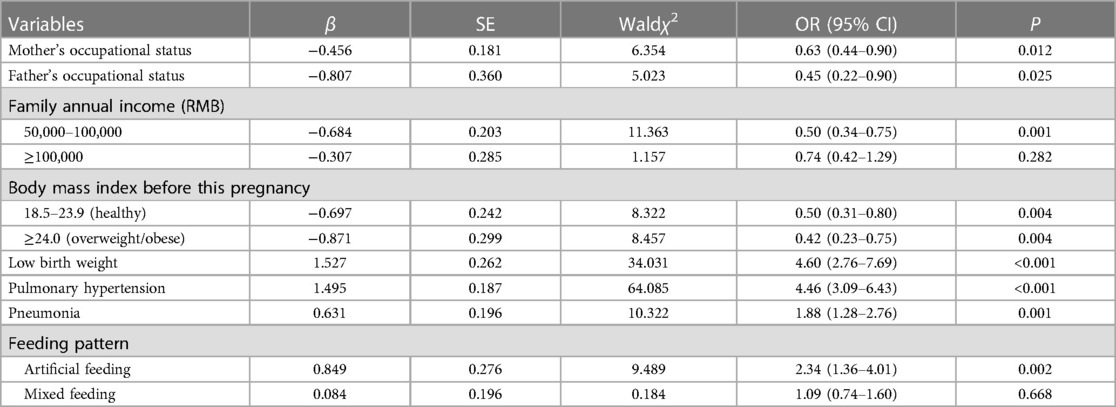
Table 2. Associated factors of underweight in CHD children based on multiple logistic regression analysis.

Table 3. Associated factors of wasting in CHD children based on multiple logistic regression analysis.

Table 4. Associated factors of stunting in CHD children based on multiple logistic regression analysis.
CHD children, raised by working mother (OR = 0.63, 95% CI: 0.44–0.90) or father (OR = 0.45, 0.22–0.90), had lower odds of underweight than those nurtured by jobless parents. Compared to CHD children whose family annual income was lower than 50,000 RMB, those with household income ranging 50,000–100,000 RMB per year were 50.4% (33.9%–75.1%) times less likely to be underweight. Normal body mass index and being overweight or obese before this pregnancy emerged as protective factors, as the chance of underweight decreased by 50.2% (20.0%–69.0%) and 58.1% (24.7%–76.7%), respectively. On the other hand, the possibility of being underweight increased 4.60 (2.76–7.69) times, 4.46 (3.09–6.43) times, 1.88 (1.28–2.76) times, 2.34 (1.36–4.01) times in CHD children of low birth weight, with pulmonary hypertension, with pneumonia, fed artificially, respectively, as is described in Table 2.
Maternal history of gestational complications during this pregnancy, and their exposure to a noisy environment raised by 55.6% (11.2%–117.7%), 63.7% (10.6%–42.3%) the chance of wasting in CHD children, respectively. Patients with pulmonary hypertension were 3.21 (2.30–4.49) times more likely to be wasted. Nevertheless, mothers and fathers who were office workers reduced the risk of wasting by 37.6% (12.4%–55.6%), 51.0% (7.6%–74.0%), respectively, based on Table 3.
The number of children was positively associated with stunting in CHD children, as those who had siblings were 1.88 (1.13–3.14) times more likely to be stunted. The odds of stunting dramatically rose when mothers had placental abruption during this pregnancy (OR = 25.15, 2.55–247.89). Additionally, cases who were born prematurely, were of low birth weight, had pulmonary hypertension and pneumonia were 1.84 (1.02–3.31) times, 3.78 (2.16–6.62) times, 2.35 (1.56–3.53) times, 1.93 (1.28–2.90) times more risky to be stunted, respectively. Importantly, placental abruption during this pregnancy had the strongest association. Table 4 showed the results.
3.4 Subgroup analyses
Subgroup analyses were conducted on breastfed and non-breastfed children, cyanotic and acyanotic children, preterm and non-preterm children, to assess whether feeding pattern, disease severity, and prematurity play a role. The results were shown in Tables 5–7, respectively.
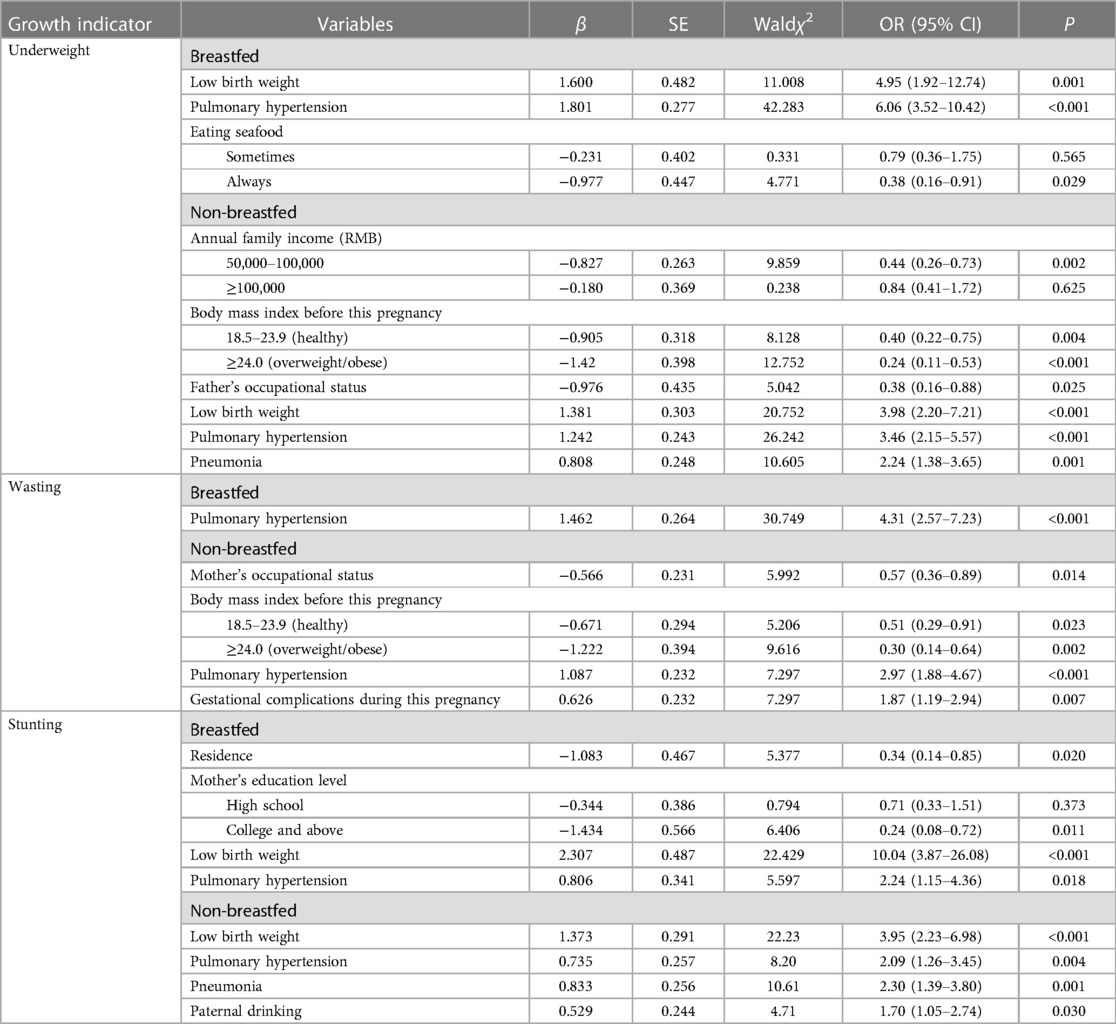
Table 5. Associated factors of underweight, wasting, and stunting in breastfed and non-breastfed CHD children based on multiple logistic regression analysis.
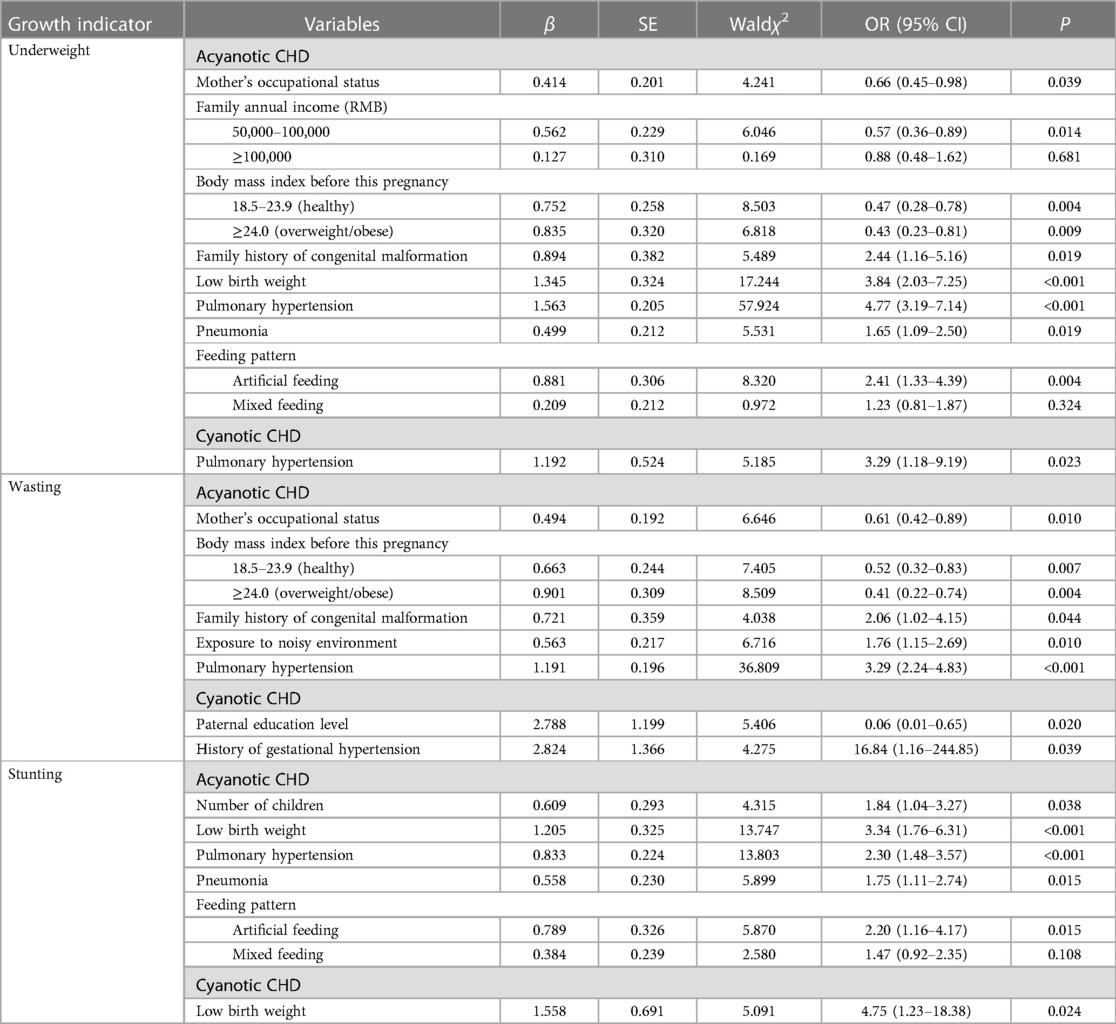
Table 6. Associated factors of underweight, wasting, and stunting in cyanotic and acyanotic CHD children based on multiple logistic regression analysis.
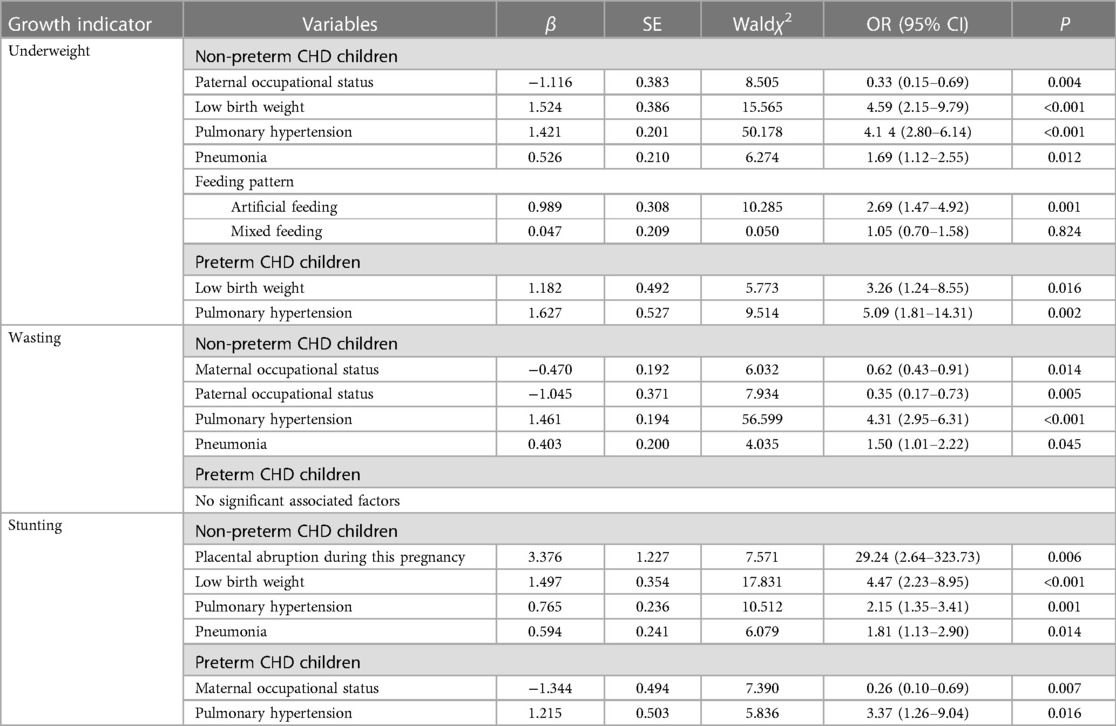
Table 7. Associated factors of underweight, wasting, and stunting in preterm and non-preterm CHD children based on multiple logistic regression analysis.
Pulmonary hypertension was the mutual predictive factor of undernutrition in both breastfed and non-breastfed children. Children of low birth weight were prone to be underweight and stunted, regardless of their feeding patterns. It is worth mentioning that mothers eating seafood ≥3 times per week dramatically decreased the chance of being underweight in breastfed children (OR = 0.38, 0.16–0.91). Mothers who were occupied and whose body mass index was ≥18.5 lowered the possibility of being wasted for their non-breastfed children, while having gestational complications during this pregnancy increased the hazard. Living in urban areas, mothers receiving higher education served as protective factors for stunting in breastfed children. Non-breastfed children who were diagnosed with pneumonia and whose fathers drank alcohols had higher odds of being stunted.
In acyanotic children, whose mothers unoccupied, with body mass index less than 18.5 before this pregnancy, with family history of congenital malformation were the risk factors for being underweight and wasted. Artificial and mixed feeding adversely impacted children's growth, and elevated the rate of underweight and stunting. However, for children who presented cyanosis, we only found associations between pulmonary hypertension and underweight (OR = 3.29, 1.18–9.19), paternal education level (OR = 0.06, 0.01–0.65), history of gestational hypertension (OR = 16.84, 1.16–244.85) and wasting, low birth weight and stunting (OR = 4.75, 1.23–18.38).
In both preterm and non-preterm children, the presence of pulmonary hypertension increased the likelihood of being underweight and stunted. Additionally, pneumonia was found to be correlated with undernutrition in full-term children (OR = 1.69, 1.12–2.55), but this association was not observed among preterm births. Importantly, low birth weight was found to be associated with both preterm (OR = 3.26, 1.24–8.55) and non-preterm children (OR = 4.59, 2.15–9.79), suggesting a potential interaction between prematurity and low birth weight.
4 Discussion
The study was designed to extend our understanding of the relationship between preoperative undernutrition in CHD patients and associated factors in China context. We provided new evidence that gestational complications during this history, maternal exposure to a noisy environment, and artificial feeding increased the odds of undernutrition in their offspring. Additionally, our subgroup analyses have revealed the necessity of implementing distinct interventions tailored to CHD children across diverse categories, such as prematurity, breastfeeding status, as well as distinguishing between those with cyanotic CHD and acyanotic CHD.
In our study, the overall prevalence of underweight, wasting and stunting among CHD children preoperatively reached 36.1%, 29.7%, and 21.3%, respectively. Similar results were shown in previous studies among Chinese and Thai CHD children (15, 17, 25). Qin et al. reported that 25.3%, 25.3%, and 28.6% of Chinese CHD cases were underweight, wasted, and stunted, respectively (17). In this study, children aged 2–4 years with simple repair (SR) CHD, and their mothers were included, while preterm were excluded. We observed a higher proportion of underweight in CHD children because patients with severe CHD subtypes, preterm, and infants who were vulnerable to undernutrition were included in our study. In contrast, parameters of insufficient growth documented in other developing countries were higher than our results (14, 19, 21, 26). For instance, Batte et al. illustrated that, in Uganda, the proportion of underweight, wasting, and stunting in children aged under 5 years was 44.8%, 31.5%, and 48.3%, respectively (14). Studies in Nigeria (21), Egypt (26), and Ethiopia (27), showed 42.9%, 61.9%, and 49.1% of the subjects suffered underweight, wasted and stunted, respectively. Higher rates may be explained by differences in the economy level, availability of medical resources, and inclusion criteria of participants. Differ from our observation, lower prevalence was documented by the research in France, as underweight and wasted children accounted for 14% and 17% of CHD infants (28), which may be attributed to different standards for outcomes.
Contradicting to the former study that working mothers tend to have children with impaired linear growth due to inadequate childcare time (29), we found that employed parents were more likely to raise healthy CHD children. A probable explanation is that employed parents, with stable income and greater financial resources, are more likely to possess a heightened understanding of CHD, ensuring that their children receive timely medical treatment and maintain proper attention to nutritional status. Compared to the only child, a higher proportion of children with siblings were observed to be stunted, supported by Vaidyanathan et al. (16), indicating that caregivers ought to pay more attention to the children's feeding and chronic nutritional status, especially when they have more than one child to attend. Cases, with maternal BMI <18.5 before pregnancy were more likely to be underweight. Likewise, a cohort study in Japan found maternal pre-conception underweight increased the odds of adverse outcomes in neonates, including preterm birth, low birth weight, and small-for-gestational-age (30). Our study furthered evidence about potential long-term effect of maternal low BMI on growth inadequacy among CHD children.
Obstetric factors were evaluated to be potential predictors of growth retardation. A handful of studies have articulated that adverse pregnancy outcomes elevated the odds of perinatal death and morbidity, particularly growth restriction (31–33). Downes et al. performed a systematic review, finding the association between placental abruption and low birth weight, intrauterine growth restriction, and mortality (31). A Danish cohort study proved that premature rupture of membrane mediated spontaneous preterm birth in CHD neonates (32). However, it remains unclear about the relationship between adverse pregnancy outcomes and undernourishment in children with cardiac malformations. We initially reported relevant evidence showing a significant correlation between chronic undernutrition, indicated by stunting, and placental abruption during pregnancy, using a multivariate logistic regression model. Additionally, newborns were more likely to suffer wasting if theirs had history of gestational complications during this conception. Univariate analysis showed that gestational hypertension and premature rupture of membrane during this gestation played a part in faltering growth. Dysregulation of utero circulation and inadequate vessel supply, resulting in fetal hypoxia, acidosis, and subsequent immature growth of gastrointestinal tract, might be explanatory for malabsorption and delayed growth. It is interesting to note the relationship between history of intrauterine growth arrest before this pregnancy and undernutrition in the offspring. Nevertheless, there is lack of literature on the underlying mechanisms, highlighting the need for future research in this area.
To our knowledge, this is the first study represents the first attempt to the possible association of maternal dietary factors, life behaviors and habits, exposure to environmental hazards exert effect, and nutritional status of preoperative CHD children. We found children's nutrition was positively associated with maternal consumption of healthy food, containing eggs, vegetables, fruits, beans, but the association did not reach statistical significance. Children, whose mothers never eat seafood (e.g., fish), defined as less than once per week, had higher odds of being underweight, while mothers eating seafood ≥3 times per week served as a protective factor of underweight in breastfed children. Generally shaped by sociocultural settings, family dietary patterns, particularly maternal feeding behaviors, influence children's food preferences and overall growth (34). In terms of parent's life behaviors and habits during periconceptional period, our former study demonstrated that maternal active and passive smoking, whether before pregnancy or during early phase of pregnancy, were risk factors for CHD in offspring (35). Whereas, no significant association between parental smoking, along with other behaviors and undernutrition in children was observed in our study. Importantly, we acquired that a greater proportion of subjects experienced wasting with mothers exposed to a noisy environment during gestation. As also evidenced by a British study that exposure-response relationship existed between traffic noise and low birth weight, suggesting that noise-induced activation of sympathetic nervous system and successive reduction in blood flow through uterus posed threat to infants (36, 37), and may exert lasting influence on children's nourishment post birth.
It was observed that low birth weight was the risk factor for underweight and stunting, in line with earlier studies among CHD subjects (16, 28, 38). Our subgroup analysis on children born with or without prematurity suggested a potential interaction between preterm birth and low birth weight in relation to children's undernutrition. Interestingly, newborns delivered via caesarean section had an increased susceptibility to be stunted, although our analysis did not reveal a statistically significant association in multivariate logistic model. This mode of delivery disturbs colonization of microbes which are naturally transmitted from mothers through vaginal delivery, thereby this altered composition of gut microbiota might enhance the chance of opportunistic infection and diminish absorption of nutrients, leading to long-term damage to somatic development (39, 40).
Furthermore, we found that children with cyanotic heart disease bore a greater risk of growth deficiency, yet the association was not proved to be statistically significant in multivariate logistic regression, which was in accordance with current studies (14, 41). On the contrary, a Chinese study among postoperative children reported that acyanotic CHD patients tended to be underweight and cyanotic ones were prone to be stunted (20). Opposing this finding, Hassan et al. stated that acyanotic heart disease was linked with stunting in Egyptian CHD children (26). With regard to the discrepancy among published papers, diverse study populations (have or have not undergone surgeries) and classifications of cyanotic heart disease cannot be ruled out. Convincing systematic review and meta-analysis concerning differences in the correlation did not occur to date. In our subgroup analysis, huge heterogeneity of factors associated with undernutrition was observed between cyanotic and acyanotic CHD cases, implying that interventions should be implemented in line with CHD phenotypes and severity.
It is worth noting that pulmonary hypertension was the predictor of all three growth parameters, in keeping with a few studies (19, 26, 42) that advocate for heightened attention towards CHD cases with pulmonary hypertension. Despite this, pneumonia was associated with growth insufficiency. In contrast to our result, Batte et al. illustrated preoperative factors such as the history of pneumonia was not statistically related to undernutrition among children post-operation (14). One likely explanation is the corrective surgery triggered catch-up growth in patients (43). Poor feeding elevates the risk of infections, such as pneumonia, which conversely worsens children's nutritional condition, forming a vicious cycle. For one thing, children diagnosed with pneumonia often manifest high fever and tachypnoea, resulting in increased metabolic demands, while diminishing appetites, and causing hypoxia (44). For another, antibiotics, which served as treatments for infections, bring about changes in the diversity and richness of gastrointestinal microbiota, giving rise to gut dysfunction and lack of absorption (45).
It is also important to stress that artificial feeding was a contributary factor of undernutrition, compared with breastfeeding and mixed feeding. Davis et al. articulated the prominence of breastfeeding, including unparalleled properties of appropriate concentration of protein, antibodies, and digestive enzymes, in comparison with formula milk (46). In Haiti, children adhering to breastfeeding practices were less likely to be wasted (47). All the evidence highlights the significance of promoting breast-feeding to mothers with CHD children. Thus, we conducted a subgroup analysis for breastfed and non-breastfed children. Results demonstrated that associated factors of undernutrition differed strikingly between the two groups, indicating that suggestions should be specifically offered to children with diverse feeding patterns. Furthermore, a randomized-controlled trial indicated that CHD patients fed with human milk and energy supplementation/fortifier gained higher energy than counterparts only fed with breast milk (48). Considering the elevated energy consumption for children with cardiac defects, personalized feeding patterns should be further studied.
Our study carries the following limitations. Firstly, participants were selected from a single tertiary hospital, affecting the study population's representativeness, and thus, extrapolation may be restricted. Secondly, due to the inherent drawback of cross-sectional studies, we were unable to verify the causality of the associated factors and growing deficit in CHD children. Moreover, these data must be interpreted with caution because of recall bias and investigation bias, although we implemented measures including checking medical records and information in PHCH, and training investigators. Thirdly, our study did not assess potential influence factors such as dietary factors of patients and their nutritional status post-operation. Since 40.2% of cases aged before 6 months in our survey and infants at this age were mostly breastfed, it was difficult to investigate their dietary factors. Instead, we recorded their feeding patterns. Fourthly, our findings could be impacted by intricate interactions between the variables we examined, such as the potential impact of demographic traits on cardiac factors, possibly mediated by hospital presentations and management. Therefore, multi-centered prospective cohort study with larger sample size should be conducted in the future, so that prominent and modifiable factors contributing to undernourishment in children with CHD can be detected.
5 Conclusion
Our study reveals a high prevalence of undernutrition among preoperative CHD children in China. Various factors have been identified to be linked to undernourishment, including parents' occupational status, family income, number of children, maternal body mass index before pregnancy, gestational complications (e.g., placental abruption), and maternal exposure to noisy environment. Additionally, we have found correlations between undernutrition and preterm birth, low birth weight, pulmonary hypertension, pneumonia, and feeding patterns. It is worth noting that factors associated with the nutritional status of breastfed and non-breastfed, cyanotic and acyanotic, preterm and non-preterm children differ significantly, underscoring the need for tailored interventions. Confirming these associations and explore additional potential factors, such as patients' dietary factors, necessitates further research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xiangya School of Public Health, Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
XR participated in conceptualization, methodology, statistical analysis, and writing of the manuscript. JO participated in statistical analysis of the manuscript. YC reviewed the data and revised the manuscript. JD designed the study. PH supervised the performance of research. XS, JW, and MS reviewed and revised the manuscript. HS participated in statistical analysis of the manuscript. LL, JT, and HL participated in data collection. JQ designed the study, revised and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation Program of China (82073653 and 81803313), Hunan Outstanding Youth Fund Project (2022JJ10087), National Key Research and Development Project (2018YFE0114500), China Postdoctoral Science Foundation (2020M682644), Hunan Provincial Science and Technology Talent Support Project (2020TJ-N07), Hunan Provincial Key Research and Development Program (2018SK2063), Open Project from NHC Key Laboratory of Birth Defect for Research and Prevention (KF2020006), Natural Science Foundation of Hunan Province (2018JJ2551 and 2022JJ40207), Science and Technology Planning Project of Guangdong Province (2020A1414010152), Changsha Municipal Natural Science Foundation (kq2202470) and the Fundamental Research Funds for the Central Universities of Central South University (2022ZZTS0968).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1167460/full#supplementary-material
References
1. GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc Health. (2020) 4:185–200. doi: 10.1016/S2352-4642(19)30402-X
2. Liu Y, Chen S, Zühlke L, Black GC, Choy M, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48:455–63. doi: 10.1093/ije/dyz009
3. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
4. World Health Organization. Malnutrition: key facts. (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/malnutrition (Cited 2023 Dec 12).
5. El-Koofy N, Mahmoud AM, Fattouh AM. Nutritional rehabilitation for children with congenital heart disease with left to right shunt. Turk J Pediatr. (2017) 59:442–51. doi: 10.24953/turkjped.2017.04.011
6. Okoromah CAN, Ekure EN, Lesi FEA, Okunowo WO, Tijani BO, Okeiyi JC. Prevalence, profile and predictors of malnutrition in children with congenital heart defects: a case-control observational study. Arch Dis Child. (2011) 96:354–60. doi: 10.1136/adc.2009.176644
7. Diao J, Chen L, Wei J, Shu J, Li Y, Li J, et al. Prevalence of malnutrition in children with congenital heart disease: a systematic review and meta-analysis. J Pediatr. (2022) 242:39–47.e4. doi: 10.1016/j.jpeds.2021.10.065
8. Varan B, Tokel K, Yilmaz G. Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Arch Dis Child. (1999) 81:49–52. doi: 10.1136/adc.81.1.49
9. Argent AC, Balachandran R, Vaidyanathan B, Khan A, Kumar RK. Management of undernutrition and failure to thrive in children with congenital heart disease in low- and middle-income countries. Cardiol Young. (2017) 27:S22–30. doi: 10.1017/S104795111700258X
10. Wallace MC, Jaggers J, Li JS, Jacobs ML, Jacobs JP, Benjamin DK, et al. Center variation in patient age and weight at Fontan operation and impact on postoperative outcomes. Ann Thorac Surg. (2011) 91:1445–52. doi: 10.1016/j.athoracsur.2010.11.064
11. Lim JYJ, Wee RWB, Gandhi M, Lim YP, Tan LNM, Quek SC, et al. The associations between preoperative anthropometry and postoperative outcomes in infants undergoing congenital heart surgery. Front Cardiovasc Med. (2022) 9:812680. doi: 10.3389/fcvm.2022.812680
12. Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, et al. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. (2013) 162:250–6.e2. doi: 10.1016/j.jpeds.2012.07.048
13. Medoff-Cooper B, Ravishankar C. Nutrition and growth in congenital heart disease: a challenge in children. Curr Opin Cardiol. (2013) 28:122–9. doi: 10.1097/HCO.0b013e32835dd005
14. Batte A, Lwabi P, Lubega S, Kiguli S, Otwombe K, Chimoyi L, et al. Wasting, underweight and stunting among children with congenital heart disease presenting at Mulago Hospital, Uganda. BMC Pediatr. (2017) 17:10. doi: 10.1186/s12887-017-0779-y
15. Zhang M, Wang L, Huang R, Sun C, Bao N, Xu Z. Risk factors of malnutrition in Chinese children with congenital heart defect. BMC Pediatr. (2020) 20:213. doi: 10.1186/s12887-020-02124-7
16. Vaidyanathan B, Nair SB, Sundaram KR, Babu UK, Shivaprakasha K, Rao SG, et al. Malnutrition in children with congenital heart disease (CHD) determinants and short term impact of corrective intervention. Indian Pediatr. (2008) 45:541–6. 18695271.18695271
17. Qin C, Li Y, Wang D, Shi Z, Yao R, Wang D, et al. Maternal factors and preoperative nutrition in children with mild cases of congenital heart disease. Jpn J Nurs Sci. (2019) 16:37–46. doi: 10.1111/jjns.12211
18. Murni IK, Patmasari L, Wirawan MT, Arafuri N, Nurani N, Sativa ER, et al. Outcome and factors associated with undernutrition among children with congenital heart disease. PLoS One. (2023) 18:e0281753. doi: 10.1371/journal.pone.0281753
19. Tsega T, Tesfaye T, Dessie A, Teshome T. Nutritional assessment and associated factors in children with congenital heart disease-Ethiopia. PLoS One. (2022) 17:e0269518. doi: 10.1371/journal.pone.0269518
20. de Oliveira AC, Poloni S, Barbiero SM, Vian I. Prevalence of breastfeeding in children with congenital heart diseases and down syndrome. Clin Nutr ESPEN. (2021) 44:458–62. doi: 10.1016/j.clnesp.2021.03.023
21. Chinawa AT, Chinawa JM, Duru CO, Chukwu BF, Obumneme-Anyim I. Assessment of nutritional status of children with congenital heart disease: a comparative study. Front Nutr. (2021) 8:644030. doi: 10.3389/fnut.2021.644030
22. Goldberg DL, Becker PJ, Brigham K, Carlson S, Fleck L, Gollins L, et al. Identifying malnutrition in preterm and neonatal populations: recommended indicators. J Acad Nutr Diet. (2018) 118:1571–82. doi: 10.1016/j.jand.2017.10.006
23. Qin J, Li J, Li F, Sun M, Wang T, Diao J, et al. Association of maternal folate use and reduced folate carrier gene polymorphisms with the risk of congenital heart disease in offspring. Eur J Pediatr. (2021) 180:3181–90. doi: 10.1007/s00431-021-04087-y
24. Mu L. Evaluation of preoperative nutritional status and effect on clinical outcome in children with congenital heart disease. Hebei: Hebei Medical University (2017). doi: 10.7666/d.D0120264
25. Sethasathien S, Silvilairat S, Sittiwangkul R, Makonkawkeyoon K, Kittisakmontri K, Pongprot Y. Prevalence and predictive factors of malnutrition in Thai children with congenital heart disease and short-term postoperative growth outcomes. Nutr Health. (2023) 29:549–55. doi: 10.1177/02601060221082382
26. Hassan BA, Albanna EA, Morsy SM, Siam AG, Al Shafie MM, Elsaadany HF, et al. Nutritional status in children with un-operated congenital heart disease: an Egyptian center experience. Front Pediatr. (2015) 3:53. doi: 10.3389/fped.2015.00053
27. Tsintoni A, Dimitriou G, Karatza AA. Nutrition of neonates with congenital heart disease: existing evidence, conflicts and concerns. J Matern Fetal Neonatal Med. (2020) 33:2487–92. doi: 10.1080/14767058.2018.1548602
28. Brief F, Guimber D, Baudelet J-B, Houeijeh A, Piéchaud J-F, Richard A, et al. Prevalence and associated factors of long-term growth failure in infants with congenital heart disease who underwent cardiac surgery before the age of one. Pediatr Cardiol. (2022) 43:1681–7. doi: 10.1007/s00246-022-02933-w
29. Amugsi DA, Dimbuene ZT, Kimani-Murage EW. Socio-demographic factors associated with normal linear growth among pre-school children living in better-off households: a multi-country analysis of nationally representative data. PLoS One. (2020) 15:e0224118. doi: 10.1371/journal.pone.0224118
30. Nakanishi K, Saijo Y, Yoshioka E, Sato Y, Kato Y, Nagaya K, et al. Severity of low pre-pregnancy body mass index and perinatal outcomes: the Japan environment and children’s study. BMC Pregnancy Childbirth. (2022) 22:121. doi: 10.1186/s12884-022-04418-3
31. Downes KL, Grantz KL, Shenassa ED. Maternal, labor, delivery, and perinatal outcomes associated with placental abruption: a systematic review. Am J Perinatol. (2017) 34:935–57. doi: 10.1055/s-0037-1599149
32. Matthiesen NB, Østergaard JR, Hjortdal VE, Henriksen TB. Congenital heart defects and the risk of spontaneous preterm birth. J Pediatr. (2021) 229:168–74.e5. doi: 10.1016/j.jpeds.2020.09.059
33. Rocha de Moura MD, Margotto PR, Nascimento Costa K, Carvalho Garbi Novaes MR. Hypertension induced by pregnancy and neonatal outcome: results from a retrospective cohort study in preterm under 34 weeks. PLoS One. (2021) 16:e0255783. doi: 10.1371/journal.pone.0255783
34. Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children’s eating behaviours. Nutrients. (2018) 10:706. doi: 10.3390/nu10060706
35. Wang T, Chen L, Ni B, Sheng X, Huang P, Zhang S, et al. Maternal pre-pregnancy/early-pregnancy smoking and risk of congenital heart diseases in offspring: a prospective cohort study in central China. J Glob Health. (2022) 12:11009. doi: 10.7189/jogh.12.11009
36. Smith RB, Fecht D, Gulliver J, Beevers SD, Dajnak D, Blangiardo M, et al. Impact of London’s road traffic air and noise pollution on birth weight: retrospective population based cohort study. Br Med J. (2017) 359:j5299. doi: 10.1136/bmj.j5299
37. Ristovska G, Laszlo H, Hansell A. Reproductive outcomes associated with noise exposure—a systematic review of the literature. IJERPH. (2014) 11:7931–52. doi: 10.3390/ijerph110807931
38. Sartika AN, Khoirunnisa M, Meiyetriani E, Ermayani E, Pramesthi IL, Nur Ananda AJ. Prenatal and postnatal determinants of stunting at age 0–11 months: a cross-sectional study in Indonesia. PLoS One. (2021) 16:e0254662. doi: 10.1371/journal.pone.0254662
39. Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. (2019) 574:117–21. doi: 10.1038/s41586-019-1560-1
40. Iddrisu I, Monteagudo-Mera A, Poveda C, Pyle S, Shahzad M, Andrews S, et al. Malnutrition and gut microbiota in children. Nutrients. (2021) 13:2727. doi: 10.3390/nu13082727
41. Costello CL, Gellatly M, Daniel J, Justo RN, Weir K. Growth restriction in infants and young children with congenital heart disease. Congenit Heart Dis. (2015) 10:447–56. doi: 10.1111/chd.12231
42. Arodiwe I, Chinawa J, Ujunwa F, Adiele D, Ukoha M, Obidike E. Nutritional status of congenital heart disease (CHD) patients: burden and determinant of malnutrition at university of Nigeria teaching hospital Ituku—Ozalla, Enugu. Pak J Med Sci. (2015) 31:1140–5. doi: 10.12669/pjms.315.6837
43. Poryo M, Paes LA, Pickardt T, Bauer UMM, Meyer S, Wagenpfeil S, et al. Somatic development in children with congenital heart defects. J Pediatr. (2018) 192:136–43.e4. doi: 10.1016/j.jpeds.2017.09.059
44. de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. (2020) 396:786–98. doi: 10.1016/S0140-6736(20)31550-6
45. Zwittink RD, Renes IB, van Lingen RA, van Zoeren-Grobben D, Konstanti P, Norbruis OF, et al. Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants. Eur J Clin Microbiol Infect Dis. (2018) 37:475–83. doi: 10.1007/s10096-018-3193-y
46. Davis JA, Spatz DL. Human milk and infants with congenital heart disease: a summary of current literature supporting the provision of human milk and breastfeeding. Adv Neonatal Care. (2019) 19:212–8. doi: 10.1097/ANC.0000000000000582
47. Irarrázaval B, Barja S, Bustos E, Doirsaint R, Senethmm G, Guzmán MP, et al. Influence of feeding practices on malnutrition in Haitian infants and young children. Nutrients. (2018) 10:382. doi: 10.3390/nu10030382
Keywords: congenital heart disease, undernutrition, associated factors, dietary factor, gestational complication
Citation: Ruan X, Ou J, Chen Y, Diao J, Huang P, Song X, Wei J, Sun M, Shi H, Li L, Tang J, Liu H and Qin J (2024) Associated factors of undernutrition in children with congenital heart disease: a cross-sectional study. Front. Pediatr. 12:1167460. doi: 10.3389/fped.2024.1167460
Received: 10 April 2023; Accepted: 17 January 2024;
Published: 29 January 2024.
Edited by:
Sajid Bashir Soofi, Aga Khan University, PakistanReviewed by:
Brekhna Aurangzeb, University of Bergen, NorwayGabriela Corina Zaharie, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
© 2024 Ruan, Ou, Chen, Diao, Huang, Song, Wei, Sun, Shi, Li, Tang, Liu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiabi Qin cWluamlhYmkxMjNAMTYzLmNvbQ==
 Xiaorui Ruan1
Xiaorui Ruan1 Xinli Song
Xinli Song Jianhui Wei
Jianhui Wei Jiabi Qin
Jiabi Qin