
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 18 January 2024
Sec. Pediatric Critical Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1340360
We describe the case of an infant who presented with simple rhinovirus/enterovirus bronchiolitis whose condition worsened with rapid progression to multiple organ dysfunction syndrome (MODS). The patient was presumed to have either primary or secondary hemophagocytic lymphohistiocytosis (HLH), and treatment was initiated using dexamethasone, anakinra, and intravenous immunoglobulin to modulate the immune system. Due to the organ dysfunction, the use of etoposide was avoided and instead, emapalumab, an interferon gamma antagonist, was administered at a dose of 6 mg/kg. The patient's organ failure improved, and the levels of inflammatory markers decreased. The flow cytometry analysis revealed that cytotoxic cells lacked perforin expression, and subsequent genetic analysis confirmed homozygous pathogenic mutations in the perforin gene. This case highlights the potential avoidance of etoposide in cases of primary HLH, the possible benefit of an elevated initial dose of emapalumab, and the contribution offered by a multi-specialty team approach to complex diagnosis.
The patient is a 6-week old female infant, previously healthy, without any pertinent family history of the condition, who was admitted to Penn State Health Children's Hospital due to rhinovirus/enterovirus bronchiolitis and presented with a fever of 38.4°C. On hospital day (HD) #3, the patient was upgraded to the pediatric intensive care unit (PICU) for shock. Upon arriving at the PICU, the patient was intubated, and a right internal jugular central venous catheter was inserted. The laboratory tests demonstrated a condition of mixed acidosis (pH 7.15, PCO2 53 mmHg, base deficit −20). The complete metabolic panel revealed the presence of hyponatremia (Na 122 mEq/L), and the complete blood count (CBC) demonstrated thrombocytopenia (platelets 13 × 103 µl) (Figure 1A), a white blood cell (WBC) count of 26.53 × 103 µl, and mild anemia with a hemoglobin level of 9.6 g/dl. The patient also demonstrated an elevated international normalized ratio (INR) and total bilirubin (Figure 1A) and hypofibrinogenemia (<60 mg/dl). The inflammatory biomarkers demonstrated an elevated ferritin level (16,995 ng/ml) and C-reactive protein (CRP, 4.25 mg/dl). While the liver and spleen were palpable on the physical exam, the liver ultrasound reported only “top normal liver and spleen size(s).” The patient demonstrated a pediatric sequential organ failure (pSOFA) score of 11 (3—respiratory, 4—coagulation, 1—cardiovascular, 1—renal, 2—hepatic) (Figure 1B) (1).
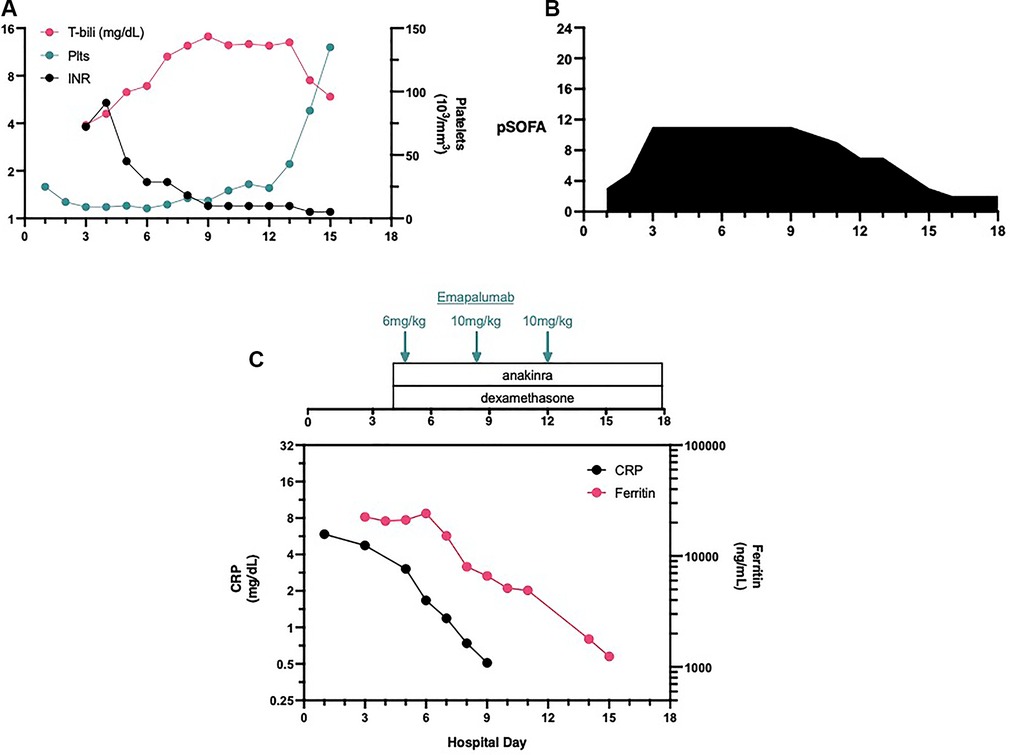
Figure 1. Laboratory values, organ dysfunction and interventions. The patient demonostrated thromobocytopenia on admission (A). When organ dysfunctioned worsened on HD#3 (B) additional laboratory values demonstrated coagulopathy and hyperbilirubinemia. Immune suppressive therapies were initiated late on HD#3 and the first dose of emapalumab was administered on HD#4 (C).
The Penn State University Department of Pediatrics recently assembled the Dysregulated Immune Response Team (DIRT), a group of pediatric subspecialists interested in hyperinflammatory disease processes. The team, comprised of Pediatric subspecialists from the Divisions of Pediatric Rheumatology, Pediatric Hematology/Oncology, Pediatric Infectious Disease, and Pediatric Critical Care offered differential diagnoses including viral (e.g., herpes simplex virus) or bacterial sepsis, primary and secondary HLH, and rickettsial infections. As of HD#3, the patient fulfilled four to five (depending on the interpretation of the abdominal ultrasound) of the HLH-2004 criteria (fever, cytopenias, hypofibrinogenemia, hyperferritinemia, and hepatosplenomegaly) (2). Given the similarity of the case to two previous documented cases of secondary HLH at our institution (3, 4), the diagnosis of HLH was presumed, and empiric treatment with dexamethasone and the IL-1 receptor antagonist, anakinra, was initiated. Meanwhile, an expedited order was placed for the interferon gamma antagonist, emapalumab (24 h transit time). Additional labs to confirm HLH were sent to the Diagnostic Immunology Lab at the Cincinnati Children's Hospital Medical Center (CCHMC).
We administered a moderate/high starting dose of emapalumab (6 mg/kg) very early in the morning on HD#5. Even prior to the administration of the second dose of emapalumab, the ferritin levels started to decline, indicating a decrease in macrophage activation (Figure 1C). However, organ failure persisted so the DIRT team recommended administering a higher dosage of emapalumab (10 mg/kg, maximal dose) for refractory disease. On HD#8, the patient’s organ functions showed improvement. The patient was extubated on HD#11 and transferred out of the PICU on HD#13.
The reference laboratory results were obtained from CCHMC around the time of extubation. On HD#9, the soluble CD25 (IL-2RA) level of 26,020 IU/ml was reported, and on HD#10, CXCL9 and IL-18 were reported at 108,813 pg/ml and 12,941 pg/ml, respectively. A bone marrow biopsy was performed on HD#11 once the coagulopathy had improved, revealing the presence of hemophagocytosis without the presence of malignancy. Finally, the flow cytometry results were received from CCHMC on HD#13 and demonstrated the absence of perforin expression by cytotoxic cells and NK cytotoxicity was absent, thus confirming a form of familial HLH. On HD#21, the genetic analysis demonstrated the presence of homozygous pathogenic early termination variants in PRF1, c.1122G>A (p.Trp374*). This finding confirmed our suspicion of primary HLH, specifically familial HLH type 2 (FHL2) (5).
This case highlights several interesting points pertaining to the diagnosis and treatment of HLH. MODS from sepsis/HLH/MAS is an end-stage disease process that can have many different infectious and rheumatologic etiologies (6, 7). Given the clinical overlap of these etiologies, we at Penn State Children's Hospital created the DIRT to help decipher the complex picture using a multi-specialty approach. At presentation on HD#3, we were fairly convinced that the patient, similar to a previously published case report (3, 4), had a disseminated viral infection based on symptomology and laboratory values. However, her elevated ferritin level with worsening organ failure with hepatic involvement led us to favor the diagnosis of HLH and initiate immune modulation. In cases of MODS due to HLH, emapalumab may be an attractive alternative to etoposide, which has a wide range of toxicities including the risk of treatment-related myelodysplastic syndrome or acute myeloid leukemia. Further, optimal etoposide dosing can be challenging in an infant with renal and hepatic dysfunction. Emapalumab has a minimal side effect profile compared with etoposide due to its specificity to interferon gamma. Given the similarity of the presentation of this patient to another case in which the CXCL9 level was measured at 39,000 pg/ml (4), we chose to administer a moderate/high starting dose of emapalumab (6 mg/kg). Furthermore, emapalumab pharmacokinetic clearance is enhanced with higher levels of IFN-gamma (8). Because of the clinical efficacy of emapalumab, paired with its minimal side effect profile (9), this is likely an acceptable approach.
It has well been established that the HLH-2004 criteria lack specificity (6). Specificity may be increased by raising the ferritin threshold from 500 to 10,000 ng/ml (10). However, the fact that this patient, with proven Type 2 familial HLH, did not fulfill the HLH-2004 diagnostic criteria until HD#3 (Table 1) also speaks to the lack of sensitivity, or better, the lack of real-time clinical usefulness, of the HLH-2004 criteria. This is due to the specialized immunologic testing needed to fulfill many of the criteria. Lin et al. (7) recently published proteomic-based approach to differentiate HLH from sepsis using the CXCL9/IL-6 ratio. They reported that a CXCL9/IL-6 ratio of approximately 4:1 differentiated HLH from sepsis. Interestingly, the patient’s in-house clinical IL-6 level on HD#4 was measured at 444 pg/ml. Simultaneously, a CXCL9 level was submitted to CCHMC and was ultimately measured at 108,813 pg/ml. Therefore, our patient had a CXCL9/IL-6 ratio of 245:1. In addition, IL-18 has been used to differentiate HLH from sJIA/MAS (11). The IL-18 level of our patient at12,941 pg/ml is significantly elevated, but it remains 10-fold lower than that of patients diagnosed with true sJIA/MAS (unpublished data). These data indicate that there is both a need and an opportunity to improve the HLH-2004 diagnostic criteria.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Penn State University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
TH: Writing – original draft, Writing – review & editing. DM: Writing – review & editing. JB: Writing – review & editing. JE: Writing – review & editing. EH: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
EH serves as a member of SOBI pharmaceuticals’ speaker bureau whose goal is to educate healthcare providers regarding hemophagocytic lymphohistiocytosis (HLH) and the drug, emapalumab.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. (2017) 171(10):e172352. doi: 10.1001/jamapediatrics.2017.2352
2. Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007) 48(2):124–31. doi: 10.1002/pbc.21039
3. Halstead ES, Rajasekaran S, Fitzgerald JC, Weiss SL. Hyperferritinemic sepsis: an opportunity for earlier diagnosis and intervention? Front Pediatr. (2016) 4:77. doi: 10.3389/fped.2016.00077
4. McKeone DJ, DeMartini TKM, Kavanagh RP, Halstead ES. Case report: rapid recognition and immune modulation of secondary HLH due to disseminated HSV infection. Front Pediatr. (2021) 9:681055. doi: 10.3389/fped.2021.681055
5. Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. (2007) 166(2):95–109. doi: 10.1007/s00431-006-0258-1
6. Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. (2009) 10(3):387–92. doi: 10.1097/PCC.0b013e3181a1ae08
7. Lin H, Scull BP, Goldberg BR, Abhyankar HA, Eckstein OE, Zinn DJ, et al. IFN-gamma signature in the plasma proteome distinguishes pediatric hemophagocytic lymphohistiocytosis from sepsis and SIRS. Blood Adv. (2021) 5(17):3457–67. doi: 10.1182/bloodadvances.2021004287
8. Jacqmin P, Laveille C, Snoeck E, Jordan MB, Locatelli F, Ballabio M, et al. Emapalumab in primary haemophagocytic lymphohistiocytosis and the pathogenic role of interferon gamma: a pharmacometric model-based approach. Br J Clin Pharmacol. (2022) 88(5):2128–39. doi: 10.1111/bcp.15133
9. Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. (2020) 382(19):1811–22. doi: 10.1056/NEJMoa1911326
10. Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2008) 50(6):1227–35. doi: 10.1002/pbc.21423
Keywords: MODS, sepsis, emapalumab, HLH, ferritin
Citation: Hahn TJ, McKeone DJ, Beal JW, Ericson JE and Halstead ES (2024) Case Report: Successful avoidance of etoposide for primary hemophagocytic lymphohistiocytosis-induced multiple organ dysfunction syndrome using emapalumab. Front. Pediatr. 11:1340360. doi: 10.3389/fped.2023.1340360
Received: 17 November 2023; Accepted: 18 December 2023;
Published: 18 January 2024.
Edited by:
Kenneth E. Remy Case Western Reserve University, United StatesReviewed by:
Katherine Bline, Nationwide Children's Hospital, United States© 2024 Hahn, McKeone, Beal, Ericson and Halstead. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. Scott Halstead ZWhhbHN0ZWFkQHBlbm5zdGF0ZWhlYWx0aC5wc3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.