- 1Department of Allergy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2Department of Haematology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Department of Vascular Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 4Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 5Department of Pediatrics (Neonatology), Yancheng Third People’s Hospital, Yancheng, China
Background: Post-catheter removal sepsis (PCRS) is a notable complication of indwelling central venous catheters (CVCs) in neonates, which is postulated to be secondary to the disruption of biofilms formed along catheter tips up on CVCs removal. It remains controversial whether this could be prevented by antibiotic use upon CVCs removal. We aimed to evaluate the protective effect of antibiotic administration at the time of CVCs removal.
Methods: We searched through PubMed, EMBASE, Cochrane databases and reference lists of review articles for studies comparing the use of antibiotics versus no use within 12 h of CVCs removal. Risk of bias was assessed using the modified Newcastle-Ottawa Scale and Cochrane risk-of-bias tool accordingly. Results of quantitative analyses were presented as mean differences (MD) or odds ratio (OR). Subgroup and univariate meta-regression analyses were performed to identify heterogeneity.
Results: The review included 470 CVCs in the antibiotic group and 658 in the control group. Antibiotic use within 12 h of CVCs removal did not significantly reduce the incidence of PCRS (OR = 0.35, 95% CI: 0.08–1.53), but was associated with a lower incidence of post-catheter removal blood stream infection (OR = 0.31, 95% CI: 0.11–0.86). Dosage of vancomycin and world region were major sources of heterogeneity.
Conclusion: Antibiotic administration upon CVCs removal does not significantly reduce the incidence of PCRS but offers less post-catheter removal blood stream infection. Whether this will be converted to better clinical outcomes lacks evidential support. Further randomized controlled studies with longer follow-up are needed.
Summary: Results of our meta-analysis suggest that antibiotic use at planned central line removal removal does not significantly reduce the incidence of PCRS but offers less blood stream infection, which might contribute to future management of central lines in neonates.
Systematic Review Registration: https://www.crd.york.ac.uk/, PROSPERO (CRD42022359677).
Introduction
Central venous catheters (CVCs) are commonly used in the neonatal intensive care unit (NICU), contributing to better survival outcomes in critically ill newborn infants. Post-catheter removal sepsis (PCRS) is an important complication of indwelling CVCs with an incidence reported varying from 1.9% to 11.6% (1, 2). PCRS is predominantly caused by late-onset central line-associated blood stream infection (CLABSI) which is defined as a primary blood stream infection developing within 48 h after CVCs removal in the absence of other known infection sites (3). It is hypothesized that a biofilm forms along the inserted catheter, which is disrupted and washed into blood stream at the removal of CVCs, leading to bacteremia (2, 4). CLABSI is correlated with increased morbidity and mortality, additional antibiotic use and prolonged hospitalization (5, 6). Fortunately, implementation of central-line bundles and prophylactic systemic antibiotics use in the 72 h preceding PICC removal may help to reduce the incidence of late-onset CLABSIs (7, 8). However, the relatively long exposure to antibiotics during infancy is challenged by the selection of antibiotic-resistant organisms and gut microbiome dysbiosis, and thus prophylactic antibiotics use is not recommended (8, 9). Hence, concurrent antibiotics given at the time of CVCs removal might be an alternative strategy. Inconsistent results have been reported in several interventional or observational studies, which underscores the need to perform a systematic review and meta-analysis to quantitatively evaluate whether antibiotics administration at the time of CVCs removal prevents late-onset sepsis in neonates.
Materials and methods
We performed the systematic review based on a protocol with the registration number CRD42022359677) and complied with the Preferred Reporting terms for Systematic Review and Meta-Analysis (PRISMA) statement (10). Reporting items were detailed in the PRISMA checklist (Supplementary Table S1).
The purpose of this review was to evaluate whether antibiotics administration within 12 h of planned CVCs removal can reduce the incidence of post-catheter removal sepsis in neonates.
Literature search
We searched through PubMed, EMBASE and Cochrane databases. The search strategy in PubMed was: [central AND (catheter OR line)] AND (removal OR remove OR removing) AND (infection OR sepsis OR bacteremia) AND (infant OR neonate OR neonatus OR neonatal OR newborn) AND (antibiotic OR prevention OR prevent OR preventing OR prophylaxis OR prophylactic). The search strategy was adapted for EMBASE and Cochrane databases. We also searched references of review articles for relevant studies. The last search update was August 2023.
Selection of studies
Studies were selected according to the PICOS (patients/participants, intervention, comparison, outcome, study type) approach. Inclusion criteria were:
Patients/participants: neonates aged ≤28 days admitted in NICU, undergoing planned removal of CVCs.
Intervention: antibiotics use within 12 h of planned CVCs removal.
Comparison: no antibiotics use within 12 h of planned CVCs removal.
Primary outcomes: PCRS which is defined as the appearance of clinical signs and symptoms of infection or the initiation of anti-infection therapy, with or without confirmatory blood markers or cultures within 72 h after catheter removal (11);
Secondary outcomes: (1) late-onset blood stream infection which is defined as clinical or laboratory signs of infection plus a positive blood culture or specific non-culture based microbiologic testing methods which is not related to the infection at another site (12); (2) CLABSI which is defined as clinical or laboratory signs of infection plus a positive blood culture developing within 48 h of CVCs removal in the absence of other known infection sites (3); (3) neonatal mortality.
Studies: retrospective or prospective human studies.
Exclusion criteria included: (1) noncomparative studies; (2) prophylactic antibiotics use for the duration of the CVCs; (3) therapeutic antibiotics use for known or suspected catheter-related bloodstream infections; (4) insufficient data for quantitative analyses; (5) grey literature lacking details or peer review. We set no restriction on language, publication type or date. Study selection was conducted by two researchers (RYJ and ZYTH) independently, with disagreements resolved through discussion with a senior investigator (LLG).
Data extraction
We extracted the following data: (1) study information: publication (article title, authors, year, journal title), study design (patient inclusion and exclusion criteria, grouping, sample size of each) and bias control; (2) baseline characteristics: gestational age, sex, birth weight, races and country or region; (3) CVCs management: type, duration of insertion and indications for removal. (4) antibiotic use: type, dosage, frequency, start and end time. (5) outcomes: incidence of PCRS, late-onset blood stream infection and CLABSIs after catheter removal, neonatal mortality. Data extraction was conducted by two researchers (RYJ and ZYTH) independently, with disagreements resolved through discussion with a senior investigator (LLG).
Risk of bias assessment
Risk of bias for randomized clinical trial (RCT) studies was assessed using the Cochrane risk-of-bias tool (13) based on seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias (Supplementary Figure S1). For observational studies, risk of bias was assessed using a modified Newcastle-Ottawa Scale (NOS) (14) with the intention of best evaluating our phenomenon of interest (Supplementary Table S2). Assessment was performed based on three domains: selection, comparability and exposure, with a maximum score of 10. A total score of 5 or less, 6–7 and 8 or more was considered low, moderate and high quality, respectively. Risk of bias assessment was conducted by two researchers (RYJ and ZYTH) independently, with disagreements resolved through discussion with a senior investigator (LLG).
Statistical analysis
Basic characteristics of enrolled studies were firstly tabulated. Variables reported by three or more studies were evaluated through quantitative analyses. For continuous data, the mean differences (MD) with 95% confidence intervals (CI) were calculated as the effect measurements. Data reported as the median with interquartile range were converted into the mean with standard deviation through a recommended formula (15). For binary data, the odds ratio (OR) and 95% CI were calculated as the effect measurements. Heterogeneity across studies were evaluated by Cochrane chi-square (χ2) and quantified with the I2 statistics (16). I2 values of 25, 50% and 75% represented low, moderate and high heterogeneity, respectively (17). For valuables with I2 values ≤25%, the fixed-effect model will be used, otherwise, we used the random-effect model for data synthesis. We performed the following subgroup analyses to explore sources of heterogeneity: gestational age, birth weight, duration of catheter insertion, world region, type of study design and type, dosage and frequency of antibiotics use. Univariate meta-regression analyses were further performed to identify heterogeneity sources across studies. Multivariate meta-regression analyses were not performed due to limited number of studies. Publication bias was not evaluated as no more than ten studies were enrolled (18). All analyses were performed using Review Manager 5.3.3 (Nordic Cochrane Centre, Copenhagen, Denmark) and P < 0.05 was considered statistically significant.
Results
Baseline characteristics
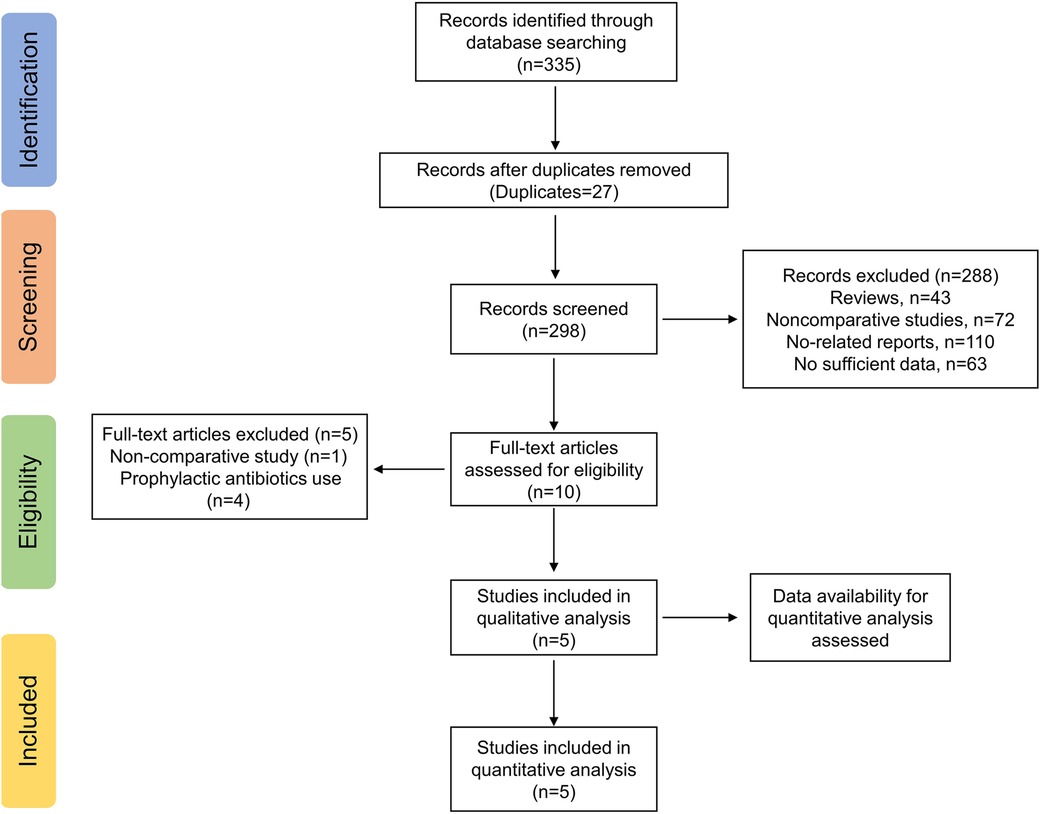
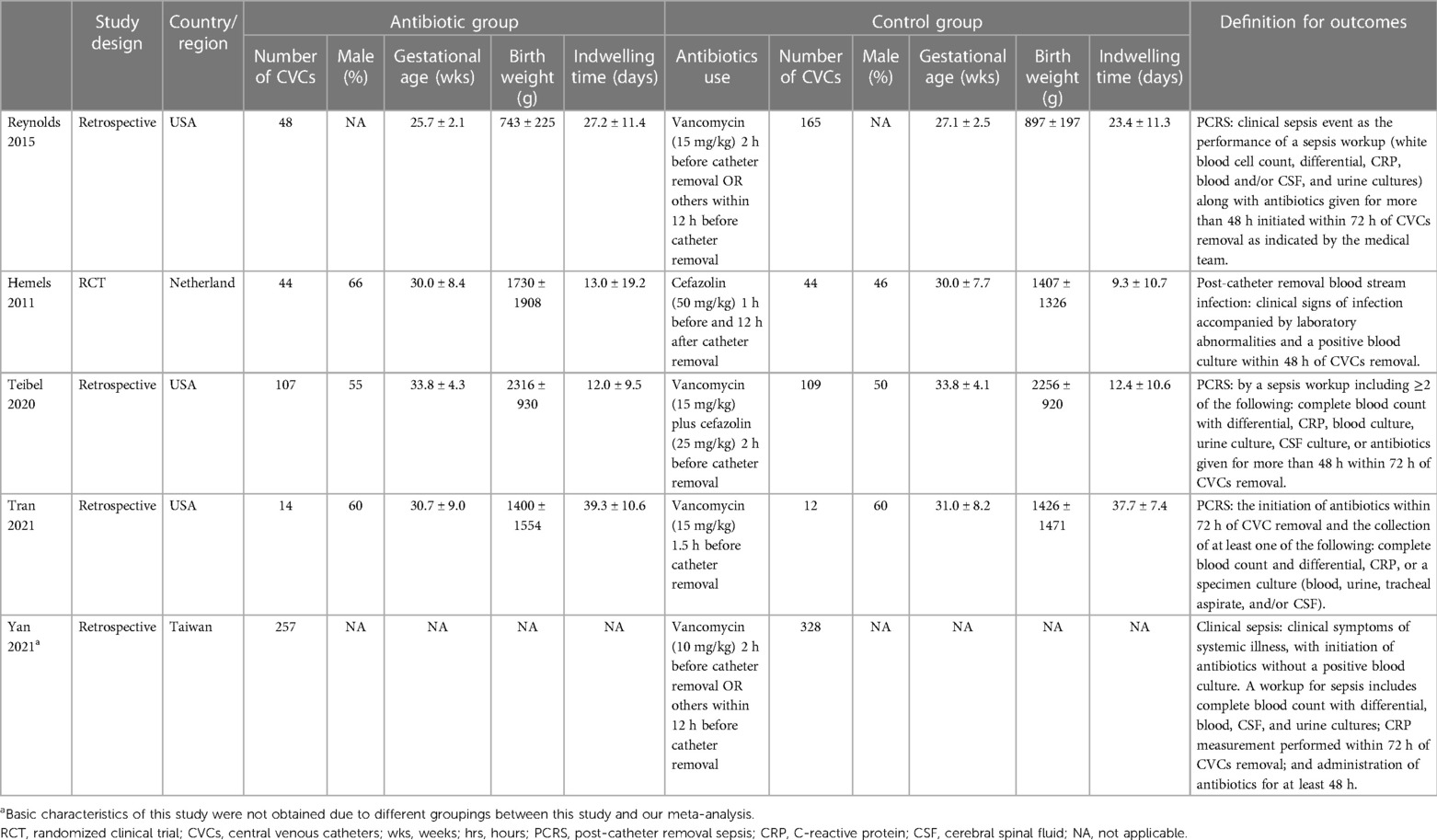
The electronic search yielded a total of 335 potentially relevant studies (Figure 1). All records were imported into the Endnote with 37 duplicates removed. After reading the titles and abstracts, 288 irrelevant studies were further eliminated. Among the remaining 10 studies, four studies regarding routine prophylactic or therapeutic antibiotics use (2, 19–21) and one non-comparative study (22) were excluded. Therefore, a total of five studies, including one RCT (23) and four retrospective studies (1, 24–26) were ultimately enrolled in the quantitative analyses (Table 1).
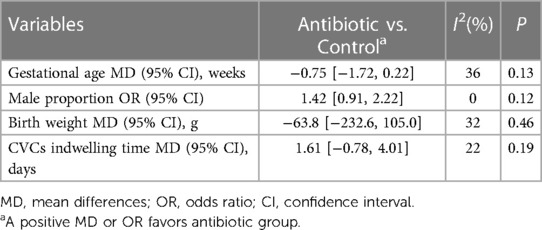
All enrolled studies were conducted in the health setting of NICU. Altogether, 470 central lines in the antibiotic group and 658 central lines in the control group based on 1,054 neonates were included. The commonly used antibiotic regimen was a single dose of vancomycin (10 or 15 mg/kg) given at 2 h prior to CVCs removal. Other regimens included one dose of vancomycin plus cefazolin or two doses of cefazolin. There were no statistically significant differences between two groups in basic demographic characteristics including gestational age (Mean difference (MD) = −0.75 weeks, 95% confidence intervals (CI): −1.72 to 0.22, P = 0.13), male proportion (OR = 1.42, 95% CI: 0.91–2.22, P = 0.12) and birth weight (MD = −63.8 g, 95% CI: −232.6 to 105.0, P = 0.46). Also, the length of CVCs indwelling is comparable between two groups (MD = 1.61 days, 95% CI: −0.78 to 4.01, P = 0.19) (Table 2).
Incidence of PCRS
In total, 15 of 470 (3.2%) and 50 of 658 (7.6%) CVCs removal episodes had PCRS in the antibiotic group and control group, respectively. The random-effects meta-analysis demonstrated that antibiotics given within 12 h of CVCs removal non-significantly reduced the incidence of PCRS (OR = 0.35, 95% CI: 0.08–1.53, P = 0.16, I2 = 63%) (Figure 2A).
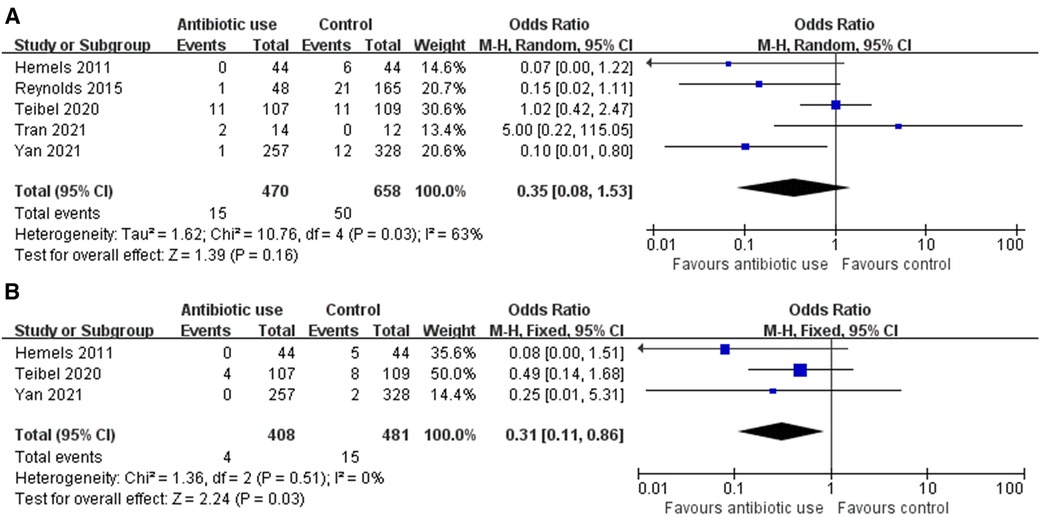
Figure 2. (A) Forrest plot of incidence of post-catheter removal sepsis (PCRS) for antibiotic use versus control. (B) Forrest plot of incidence of post-catheter removal blood stream infection for antibiotic use versus control.
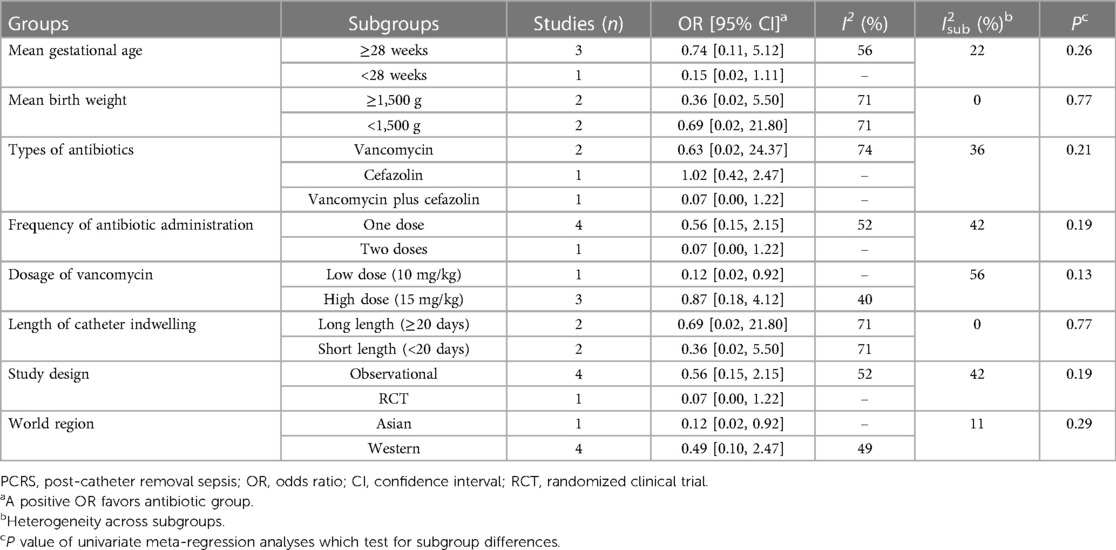
Results of subgroup analyses and univariate meta-regression were detailed in Table 3. No significant subgroup difference was tested. Regarding antibiotic regimens, the vancomycin subgroup (OR = 0.63, 95% CI: 0.02–24.37) and the cefazolin subgroup (OR = 0.07, 95% CI: 0.00–1.22) showed non-significant protective effect, while the combination subgroup suggested a near equal incidence of PCRS (OR = 1.02, 95% CI: 0.42–2.47) with and without antibiotics use. In addition, the single-dose subgroup (OR = 0.56, 95% CI: 0.15–2.15) and two-doses (OR = 0.07, 95% CI: 0.00–1.22) subgroups both demonstrated non-significant protective effect. Similar results were reached in subgroup analyses according to gestational age, birth weight, types of study design and length of CVCs indwelling. In the Asian region and low-dose (10 mg/kg) vancomycin subgroups, a single-dose of vancomycin (10 mg/kg) given at 2 h prior to CVCs removal significantly reduced the incidence of PCRS (OR = 0.12, 95% CI: 0.02–0.92), but such significant protective effect was not observed in the Western region subgroup (OR = 0.49, 95% CI: 0.10–2.47), and the high-dose (15 mg/kg) vancomycin subgroup (OR = 0.87, 95% CI: 0.18–4.12).
Incidence of post-catheter removal blood stream infection
In total, 4 of 408 (0.1%) and 15 of 481 (3.1%) CVCs removal episodes resulted in post-catheter removal blood stream infection, as was proven by blood culture, in the antibiotic group and control group, respectively. The fixed-effects meta-analysis demonstrated that antibiotics given within 12 h of CVCs removal significantly reduced the incidence of post-catheter removal blood stream infection (OR = 0.31, 95% CI: 0.11–0.86, P = 0.03, I2 = 0%) (Figure 2B). Subgroup analysis was not performed due to limited number of relevant studies.
Risk of bias assessment
Risk of bias of retrospective studies were assessed by a modified NOS (Supplementary Table S2). The total score of the four studies (1, 24–26) was 7, 8, 9, 7, respectively, indicating a moderate to low risk of bias. The risk of bias of the RCT (23) was assessed by using the Cochrane risk-of-bias tool, which was detailed in the Supplementary Table S1. This study is an open RCT with no detailed randomization and allocation procedures reported. Also, the actual enrolled number of patients was fewer than the planned value. Therefore, we considered this RCT to be at high risk of bias.
Publication bias
Publication bias was not evaluated because of a lack of test power when ten or fewer studies are enrolled (18).
Discussion
Conflicting evidence surrounds the use of antibiotic at the time of CVCs removal to prevent late-onset sepsis. In this meta-analysis, we quantitatively evaluated the preventive effect of antibiotic administration within 12 h of planned CVCs removal on late-onset sepsis based on five studies with a total of 1,128 central lines. Results demonstrated that antibiotic use upon CVCs removal did not significantly alter PCRS rates (OR = 0.35, 95% CI: 0.08–1.53), but was correlated with a lower incidence of post-catheter removal blood stream infection (OR = 0.31, 95% CI: 0.11–0.86).
Our results suggested a non-significant protective effect of antibiotic use at the time of CVCs removal in reducing rates of PCRS, with a pooled rate of 3.2%. Similar results were also found in most subgroup analyses. However, the evidence is still inadequate to examine this clinical issue as prospective, high-quality studies regarding this issue are largely insufficient. More attention has been devoted to prophylactic antibiotic use where antibiotics were given during the whole period of CVCs insertion or within 72 h prior to CVCs removal. A large retrospective study demonstrated a protective effect (OR = 0.26, P < 0.001) of prophylactic antibiotics in preventing culture-negative sepsis. In the intervention group, PCRS was found in 17 of the 322 (5.3%) central lines that were free from infection before removal (27). Inconsistently in an earlier RCT, infants were randomly assigned to receive amoxicillin prophylaxis or no antibiotic prior to CVCs removal (21). PCRS was found in 3 of 75 (4.0%) lines and 8 of 73 (11.0%) lines in two groups (P = 0.107), indicating non-significant benefit brought by routine antibiotic prophylaxis. A Cochrane meta-analysis enrolling three RCTs further affirmed the effect of prophylactic antibiotics in reducing rates of PCRS (RR = 0.40, 95% CI: 0.20–0.78), with a pooled PCRS rate of 8.8% (8). Though the absolute incidence of PCRS was comparable between antibiotic prophylaxis and antibiotics on CVCs removal, the superiority of one or another could not be determined due to a lack of comparative studies. However, there is no doubt that a single or two doses of antibiotics on CVCs removal could help to avoid antimicrobial resistance and microbiome dysbiosis brought by long-term antibiotic prophylaxis in neonates (9, 28).
Although the antibiotic use upon CVCs removal did not exhibit a significant protective effect against PCRS, it was correlated a lower risk of post-catheter removal blood stream infection (culture-positive sepsis), as suggested by our analysis. The specific mechanisms underlying this is unclear. It might be explained that the infusion of antibiotic disrupts the catheter biofilm formed along the catheter tip, decreasing the load of bacteria showered into blood stream upon catheter removal and therefore preventing culture-positive sepsis (27). However, a culture-negative sepsis could still be caused by the inflammatory response to unculturable bacteremia, especially by gram-negative bacteria which is largely non-susceptible to vancomycin. Though correlated with less blood stream infection, whether antibiotic use on CVCs removal will contribute to better clinical outcomes was not identified in our systematic review due to insufficient data reported. A recent large-scale meta-analysis indicated that despite similar mortality rate of sepsis shared by culture-positive and culture negative sepsis, patients with culture-positive sepsis had significantly longer hospitalization and mechanical ventilation duration (29). A retrospective study based on the pediatric setting reported a significantly lower mortality rate and organ-dysfunction in the culture-negative group (30). We thus speculate that antibiotic use on CVCs removal may bring clinical benefits by reducing the rate of culture-positive sepsis, which should be further examined with more relevant data reported.
To the best of our knowledge, this meta-analysis provides the most updated assessments of current evidence regarding the use of antibiotics at the time of CVCs removal in reducing late-onset sepsis. Despite this, several limitations exist. Due to the small number of published studies, we include both RCT and retrospective observational studies in our analyses, which might limit the quality of generated evidence. Even though the subgroup analysis based on types of study design did not detect significant interstudy heterogeneity, our results should still be interpreted with caution as the robustness and convincingness of subgroup analysis could be weakened by small number of included studies. The neonatal sepsis lacks a consensus definition, and its definition varies among enrolled studies. The updated regional practice manual and recommendations define CLABSI as a laboratory confirmed bloodstream infection where an eligible organism is identified, and an eligible central line (in place for over 48 h) is present, and further categorized the CLABSI into various types (12, 31). Once a global consensus definition is established, our outcome definition and study selection should be modified accordingly, and a re-analysis should be performed. Several critical clinical outcomes such as mortality rate, subsequent antibiotics, and other treatments for PCRS, length of stay as well as long-term outcomes are unable to evaluate due to insufficient data reported. Also, there is a moderate to high interstudy heterogeneity for the primary outcome, even though sources of heterogeneity were partly identified by subgroup analyses. Therefore, with continuous publication of articles, the update of the meta-analysis is still warranted to improve the above deficiencies.
Conclusions
In conclusion, results of our review suggests that antibiotic administration in neonates within 12 h of planned CVCs removal does not significantly reduce the incidence of PCRS but offers less post-catheter removal blood stream infection. However, whether this will be converted to clinical benefits lacks evidential support. These findings should be interpreted with caution due to limitations stated above. The update of meta-analysis is warranted with more randomized designed studies having a longer follow-up performed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
This study complied with the Declaration of Helsinki and the ethics approval was waived by the Ethics Committee of Peking Union Medical College Hospital and Yancheng Third People’s Hospital.
Author contributions
RJ: Formal Analysis, Investigation, Methodology, Software, Writing – original draft. ZH: Formal Analysis, Investigation, Methodology, Writing – review & editing. JZ: Software, Writing – review & editing. SF: Supervision, Writing – review & editing. LG: Conceptualization, Supervision, Validation, Writing – review & editing, Resources.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1324242/full#supplementary-material
References
1. Tran VL, Greenberg J, Nuibe A. Evaluating the incidence of sepsis post-central catheter removal when using prophylactic vancomycin in the neonatal intensive care unit. J Pediatr Pharmacol Ther. (2021) 26(7):728–33. doi: 10.5863/1551-6776-26.7.728
2. van den Hoogen A, Brouwer MJ, Gerards LJ, Fleer A, Krediet TG. Removal of percutaneously inserted central venous catheters in neonates is associated with the occurrence of sepsis. Acta Paediatr. (2008) 97(9):1250–2. doi: 10.1111/j.1651-2227.2008.00864.x
3. O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. (2011) 52(9):e162–93. doi: 10.1093/cid/cir257
4. Mermel LA. What is the predominant source of intravascular catheter infections? Clin Infect Dis. (2011) 52(2):211–2. doi: 10.1093/cid/ciq108
5. Rosenthal VD. Central line-associated bloodstream infections in limited-resource countries: a review of the literature. Clin Infect Dis. (2009) 49(12):1899–907. doi: 10.1086/648439
6. Payne V, Hall M, Prieto J, Johnson M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2018) 103(5):F422–9. doi: 10.1136/archdischild-2017-313362
7. Ista E, van der Hoven B, Kornelisse RF, van der Starre C, Vos MC, Boersma E, et al. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis. (2016) 16(6):724–34. doi: 10.1016/S1473-3099(15)00409-0
8. Jardine LA, Inglis GD, Davies MW. Prophylactic systemic antibiotics to reduce morbidity and mortality in neonates with central venous catheters. Cochrane Database Syst Rev. (2008) 2008(1):Cd006179. doi: 10.1002/14651858.CD006179.pub2
9. McDonnell L, Gilkes A, Ashworth M, Rowland V, Harries TH, Armstrong D, et al. Association between antibiotics and gut microbiome dysbiosis in children: systematic review and meta-analysis. Gut Microbes. (2021) 13(1):1–18. doi: 10.1080/19490976.2020.1870402
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
11. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2008) 36(5):309–32. doi: 10.1016/j.ajic.2008.03.002
12. National Healthcare Safety Network. Bloodstream Infection Event (Central Line-associated Bloodstream Infection and Non–central Line-associated Bloodstream Infection. Centers for Disease Control and Prevention Website. (2023). Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf (Accessed November 12, 2023).
13. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK): John Wiley & Sons (2019).
14. Wells GA, S B, Oc D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. (2015). Available at: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
16. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
18. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. (2000) 53(11):1119–29. doi: 10.1016/s0895-4356(00)00242-0
19. Lindquist S, Hentz E, Tessin I, Elfvin A. Very low birthweight infants face an increased risk of bloodstream infections following the removal of umbilical catheters. Acta Paediatr. (2016) 105(4):391–6. doi: 10.1111/apa.13240
20. Brooker RW, Keenan WJ. Catheter related bloodstream infection following PICC removal in preterm infants. J Perinatol. (2007) 27(3):171–4. doi: 10.1038/sj.jp.7211655
21. Harms K, Herting E, Kron M, Schiffmann H, Schulz-Ehlbeck H. Randomized, controlled trial of amoxicillin prophylaxis for prevention of catheter-related infections in newborn infants with central venous silicone elastomer catheters. J Pediatr. (1995) 127(4):615–9. doi: 10.1016/S0022-3476(95)70126-5
22. Hoffman MA, Snowden JN, Simonsen KA, Nenninger TM, Lyden ER, Anderson-Berry AL. Neonatal late-onset sepsis following peripherally inserted central catheter removal: association with antibiotic use and adverse line events. J Infus Nurs. (2015) 38(2):129–34. doi: 10.1097/NAN.0000000000000096
23. Hemels MA, van den Hoogen A, Verboon-Maciolek MA, Fleer A, Krediet TG. Prevention of neonatal late-onset sepsis associated with the removal of percutaneously inserted central venous catheters in preterm infants. Pediatr Crit Care Med. (2011) 12(4):445–8. doi: 10.1097/PCC.0b013e3182070f5d
24. Reynolds GE, Tierney SB, Klein JM. Antibiotics before removal of percutaneously inserted central venous catheters reduces clinical sepsis in premature infants. J Pediatr Pharmacol Ther. (2015) 20(3):203–9. doi: 10.5863/1551-6776-20.3.203
25. Teibel H, Hood K, Manasco K, Bhatia J. Antibiotic administration prior to central venous catheter removal in neonates. J Pharm Pract. (2021) 34(6):894–900. doi: 10.1177/0897190020932800
26. Yan PR, Chi H, Chiu NC, Huang CY, Huang DT, Chang L, et al. Reducing catheter related bloodstream infection risk of infant with a prophylactic antibiotic therapy before removing peripherally inserted central catheter: a retrospective study. J Microbiol Immunol Infect. (2021) 55(6):1318–25. doi: 10.1016/j.jmii.2021.09.016
27. Casner M, Hoesli SJ, Slaughter JC, Hill M, Weitkamp JH. Incidence of catheter-related bloodstream infections in neonates following removal of peripherally inserted central venous catheters. Pediatr Crit Care Med. (2014) 15(1):42–8. doi: 10.1097/PCC.0b013e31829f5feb
28. Krediet TG, Fleer A. Should we use vancomycin as prophylaxis to prevent neonatal nosocomial coagulase-negative staphylococcal septicemia? Pediatr Infect Dis J. (1998) 17(8):763–4. doi: 10.1097/00006454-199808000-00027
29. Li Y, Guo J, Yang H, Li H, Shen Y, Zhang D. Comparison of culture-negative and culture-positive sepsis or septic shock: a systematic review and meta-analysis. Crit Care. (2021) 25(1):167. doi: 10.1186/s13054-021-03592-8
30. Hazwani TR, Kazzaz YM, Alsugheir S, Aldelaijan S, Alsugheir F, Alali H, et al. Association between culture-negative versus culture-positive sepsis and outcomes of patients admitted to the pediatric intensive care unit. Cureus. (2020) 12(8):e9981. PMCID: PMC7447147; PMID: 32855896.32855896
Keywords: neonate, sepsis, critical care, central venous catheters, meta-analysis
Citation: Ji R, He Z, Zhou J, Fang S and Ge L (2024) Antibiotic use at planned central line removal in reducing neonatal post-catheter removal sepsis: a systematic review and meta-analysis. Front. Pediatr. 11:1324242. doi: 10.3389/fped.2023.1324242
Received: 19 October 2023; Accepted: 18 December 2023;
Published: 8 January 2024.
Edited by:
Rozeta Sokou, Nikaia General Hospital “Aghios Panteleimon”, GreeceReviewed by:
Eleftheria Hatzidaki, University of Crete, GreeceGokhan Ceylan, University of Health Sciences, Türkiye
© 2024 Ji, He, Zhou, Fang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Ge NDkyMjQ4MjU3QHFxLmNvbQ==
 Ruoyu Ji
Ruoyu Ji Zhangyuting He
Zhangyuting He Jiawei Zhou
Jiawei Zhou Shiyuan Fang
Shiyuan Fang Lili Ge5*
Lili Ge5*