- 1Department of Pediatric Surgery, Shanghai Key Laboratory of Birth Defect, and Key Laboratory of Neonatal Disease, Ministry of Health, Children’s Hospital of Fudan University, Shanghai, China
- 2Department of Data & Analytics, WuXi Diagnostics Innovation Research Institute, Shanghai, China
Purpose: Serum matrix metalloproteinase-7 (MMP-7) is significant in differentiating biliary atresia (BA). This study aims to develop a new peripheral blood quantitative collection device to detect MMP-7 levels via dried blood spot (DBS).
Methods: This is a diagnostic accuracy test. Serum and DBS MMP-7 concentrations were measured using an ELISA kit. Intraoperative cholangiography and subsequent histological examinations were used to confirm BA diagnoses.
Results: A total of 241 infants with obstructive jaundice were enrolled, among whom 168 were BA. Linear regression showed DBS MMP-7 correlated well with serum MMP-7 (R = 0.93, P < 0.001). The best cut-off value of serum MMP-7 for BA was 25.9 ng/ml, achieving the area under the ROC curve (AUC) of 0.962 (95% CI: 0.941, 0.983), and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 86.9%, 94.5%, 97.3% and 75.8%, respectively. The best cut-off value of DBS MMP-7 for BA was 12.5 ng/ml, achieving the AUC of 0.922 (95% CI: 0.888, 0.956), and the sensitivity, specificity, PPV, and NPV were 86.9%, 89.0%, 94.8%, and 74.7%, respectively. The dried blood spots were intervened under different storage conditions, including 1–5 days at room temperature, 2 or 3 days at 30 °C and 2 or 3 days at 37 °C. The DBS MMP-7 concentration under different storage conditions had good correlation and consistency with that at −80 °C.
Conclusions: Serum and DBS MMP-7 correlate well, both of which have high accuracy in the diagnosis of BA, while the requirements for the storage of DBS are low.
Introduction
Biliary atresia (BA) is a severe liver disease characterized by fibro-inflammatory destruction of bile ducts, resulting in cholestasis and liver fibrosis in neonates and infants (1). Kasai portoenterostomy (KPE) is the consensual treatment strategy to restore bile flow (1–5). To date, it remains difficult to accurately differentiate BA from other cholestatic diseases including Alagille syndrome based on liver function tests or grayscale ultrasound scans, which are the most widely used tests in clinical practice (6–8).
Matrix metalloproteinase-7 (MMP-7) plays an important role in remodeling the extracellular matrix (ECM), which is closely related to liver fibrosis progression (9–11). Recent studies have confirmed the clinical value of serum MMP-7 levels to discriminate BA, which can achieve the area under receiver operating characteristics (ROC) curve of more than 0.9, and both a sensitivity and specificity of between 90% and 95% (12–15).
However, the diagnostic value of MMP-7 expression has been reported on the basis of testing serum samples, which are challenging for sample collection, storage and transportation, and lead to unnecessary blood waste, as only 10–20 µl of serum is required for enzyme-linked immunosorbent assays (ELISAs). An innovative, less invasive method should be developed to further facilitate using MMP-7 levels and prospect its use in neonatal screening.
Thus, we conducted this study to develop an innovative approach to simplify sample collection and transportation that utilizes the dried blood spot (DBS) device and validate its diagnostic accuracy in BA.
Methods
Study design
This was a single-center diagnostic test. We developed a novel approach to simplify sample collection that involved collecting heel tip blood and detecting MMP-7 levels using dried blood spot (DBS).
This study was reviewed and approved by the Ethics Committee of Children's Hospital of Fudan University (No.: 2020–296). This work was performed in compliance with the Declaration of Helsinki and other relevant regulations. Informed consent was obtained from the guardian of each participant before enrollment.
Subjects
This study was conducted at Children's Hospital of Fudan University, an urban tertiary care academic children's hospital in Shanghai, China.
Infants who were admitted for cholestasis were consecutively enrolled. Cholestatic subjects were defined as having a serum direct bilirubin level >17 μmol/L and >20% of total bilirubin.
Sample collection and storage
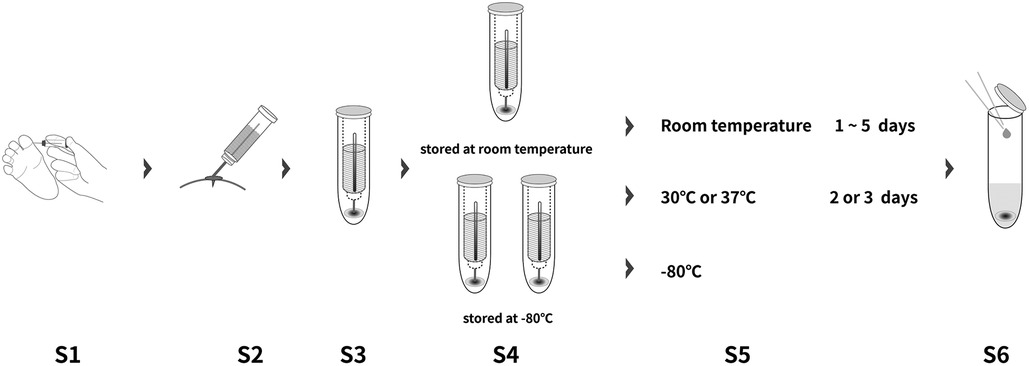
Serum and DBS samples were obtained simultaneously after enrollment. DBS samples were collected using quantificational device of 10 µl which was patented by Wuxi Diagnostics. Three duplicates were collected for each patient. One of the DBS samples was transported and stored at room temperature for 1–5 days before measurement, while the rest of DBS samples as well as the serum sample were transported with dry ice and stored at −80 °C.
The DBS samples were intervened under different storage conditions, including 2 or 3 days at 30 °C and 2 or 3 days at 37 °C after removing from −80 °C.
Measuring MMP-7 concentrations by ELISA
150 μl of Calibrator Diluent (RD6-28) of the ELISA kit (R&D Systems) was added to the DBS tower. After soaking with shaking (Thermo-shaker BE-9008, Thermo Fisher Scientific, Waltham, MA, USA) for 1 h, 50 μl of the solvent extraction was loaded to detect the MMP-7 concentration on DBS. Serum and DBS MMP-7 concentration were measured by ELISA (R&D system, DMP700), following the manufacturer's protocol. Each sample was provided with three technical replicates, and the mean was recorded. All measurements were performed by WuXi Diagnostics (Shanghai, China), who were blinded to other test results and final diagnosis (Figure 1).
Reference standard
Intraoperative cholangiography and subsequent histological examinations of liver biopsies were used to confirm BA diagnoses. Non-BA patients were confirmed by intraoperative cholangiography showing a patent biliary tree, and/or percutaneous transhepatic biopsy excluding BA, and/or genetic tests showing certain genetic mutations, and/or alleviation of symptoms without surgical intervention during follow-up for at least 3 months.
Statistical analysis
Demographic and clinical characteristics of patients were summarized using conventional descriptive statistics, n (%) for categorical variables and median and quartiles (Q1, Q3) for continuous variables. Due to failing Shapiro-Wilk W test for normal distribution, between-group comparisons were performed using the Chi-squared and Mann–Whitney U-tests for all continuous variables. The consistency of DBS MMP-7 under different storage conditions was compared by Bland-Altman method. Linear regression was applied to assess the correlation of serum MMP-7 and DBS MMP-7 levels. The Delong test was performed to compare the accuracy between the two methods. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) and 95% confidence intervals (CIs) were reported as a measure of accuracy. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were also used to show diagnostic accuracy. We used the maximum value of Youden's index as a criterion for selecting the optimal cut-off point. When the maximum value of Youden's index was achieved for multiple specificity values, the optimal cutoffs were defined based on achieving the maximum sensitivity.
A statistically significant difference was defined as a P-value <0.05. All data analyses were performed using R software 3.6.3 (R Foundation, Vienna, Austria). All authors had access to the study data and reviewed and approved the final manuscript.
Results
Study population
From December 2020 to October 2021, 241 infants with cholestasis undertook both serum and DBS MMP-7 measurement. 168 infants were diagnosed as biliary atresia through surgical exploration and subsequent histological examination (BA group). 73 infants were excluded of BA by intraoperative cholangiography showing a patent biliary tree, percutaneous transhepatic biopsy excluding BA, genetic tests showing certain genetic mutations, or alleviation of symptoms without surgical intervention during follow-up for at least three months (Non-BA group).
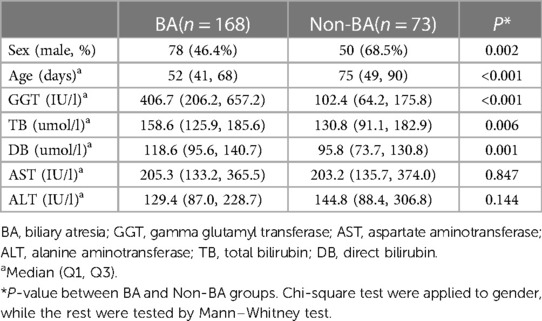
Demographic and baseline characteristics of the patients were summarized in Table 1. A majority of non-BA patients were male (68.5%), while the sex distribution was nearly equal in the BA group (46.4% male) (P = 0.002). The median age of the patients was 52 (IQR: 41, 68) days in the BA group and 75 (IQR: 49, 90) days in the Non-BA group (P < 0.001). The detailed description of other characteristics including TB, DB, GGT, ALT, AST were also listed in Table 1. As a result, GGT, TB and DB were identified to have significant differences between the BA and Non-BA groups (P < 0.05), whereas the two groups did not show any differences in ALT or AST (P > 0.05).
Correlation and consistency of serum and DBS MMP-7
Linear regression showed DBS MMP-7 correlated well with serum MMP-7 (R = 0.93, P < 0.001), and the regression formula was DBS MMP-7 = 0.47* Serum MMP-7 + 2.55 (Figure 2A). The Bland-Altman method showed that DBS MMP-7 was consistent with serum MMP-7 (95% values within 95% LoA) (Figure 2B).
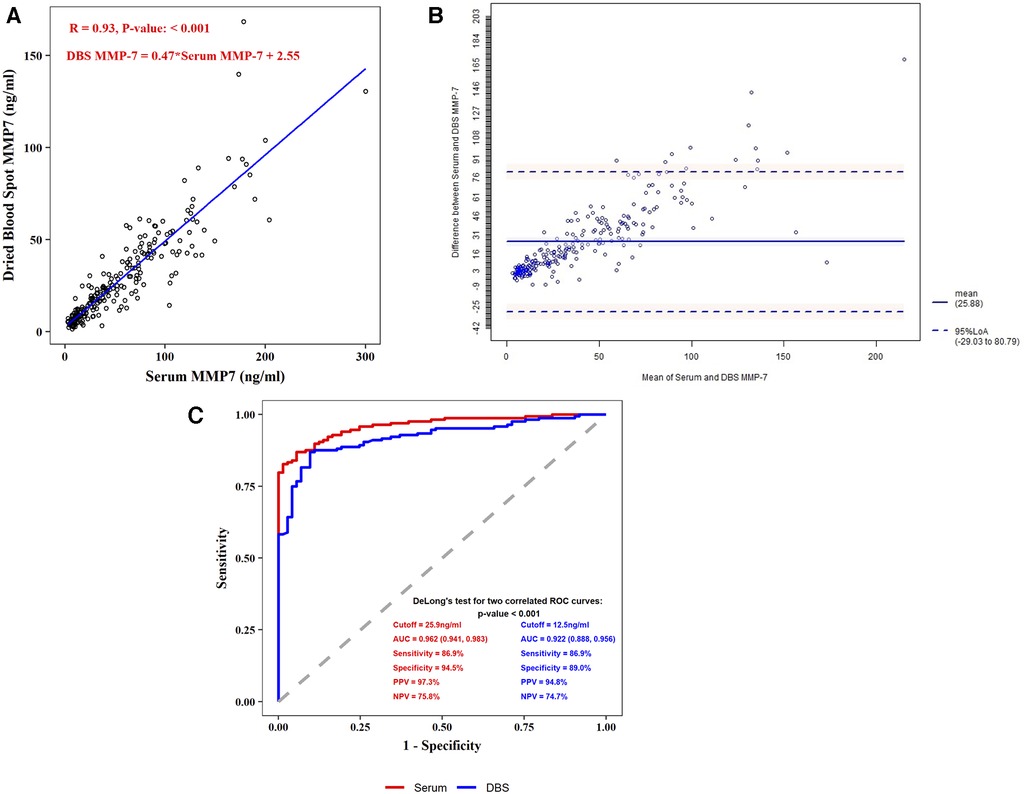
Figure 2. Correlation and comparison between serum MMP-7 and DBS MMP-7. (A) Spearman correlation coefficient test of serum and DBS MMP-7 (B) bland-altman consistency test of serum and DBS MMP-7 (C) receiver operating characteristic (ROC) plots for serum and DBS MMP-7.
Diagnostic performance of serum and DBS MMP-7
In the BA group, the median concentration of serum MMP-7 was 64.25 ng/ml (IQR: 36.65, 94.13), and the median concentration of DBS MMP-7 stored at −80 °C was 30.38 ng/ml (IQR: 18.24, 47.57). In the Non-BA group, the median concentration of serum MMP-7 was 9.97 ng/ml (IQR: 7.34, 13.20), and the median concentration of DBS MMP-7 was 6.68 ng/ml (IQR: 4.86, 9.97). Further analysis showed both serum and DBS MMP-7 correlated with age in BA group, while the age has no significant correlation with MMP-7 levels in non-BA group (Supplementary Figures S1,S2). The best cut-off value of serum MMP-7 in the diagnosis of BA was 25.9 ng/ml, achieving the area under the ROC curve (AUC) of 0.962 (95% CI: 0.941, 0.983), and the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 86.9%, 94.5%, 97.3% and 75.8%, respectively. The best cut-off value of DBS MMP-7 in the diagnosis of BA at −80 °C was 12.5 ng/ml, achieving the AUC of 0.922 (95% CI: 0.888, 0.956), and the sensitivity, specificity, PPV, and NPV were 86.9%, 89.0%, 94.8%, and 74.7%, respectively (Figure 2C).
Thermal stability of DBS MMP-7
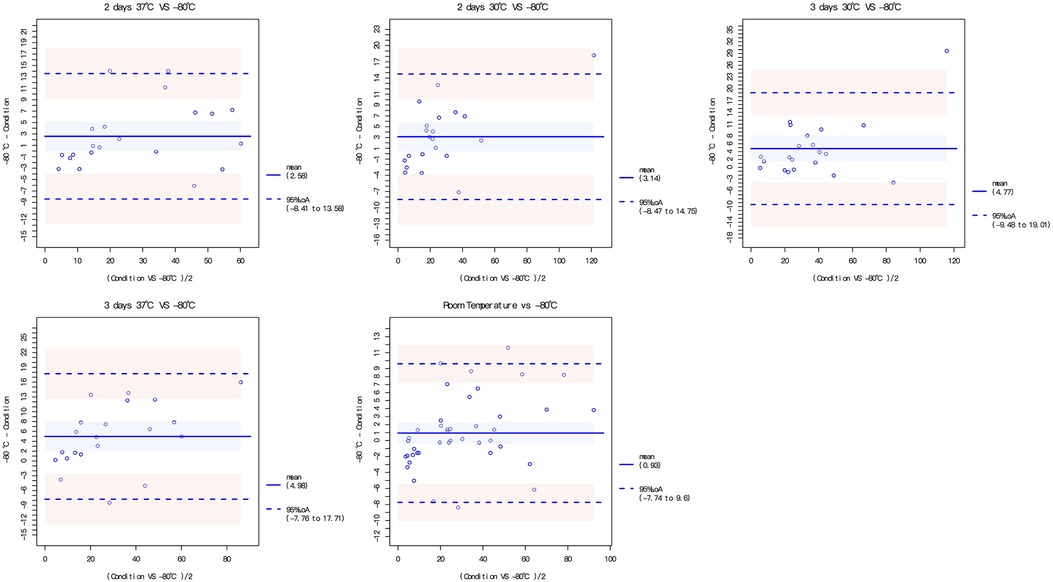
The dried blood spots were intervened under different storage conditions, including 1–5 days at room temperature, 2 or 3 days at 30 °C and 2 or 3 days at 37 °C. The DBS MMP-7 concentration under different storage conditions all had good correlation and consistency with that at −80 °C (Figure 3, Supplementary Table S1; Figure S3).
Discussion
Accurately differentiating BA from other causes of non-BA cholestasis remains a challenge due to overlapping symptoms preoperatively. According to previous studies, serum MMP-7 holds great promise for discriminating BA (12–14).
This study aimed to develop an innovative approach of measuring MMP-7 using dried blood spot to collect micro-volume blood from heel tips, laying the foundation for its significant role in the diagnostic and screening algorithm for BA. We found that: (1) DBS MMP-7 levels correlated very well with serum MMP-7 levels; (2) DBS MMP-7 levels had a good accuracy in discriminating BA; (3) DBS MMP-7 had good thermal stability under different storage conditions.
This study further confirmed the promising diagnostic accuracy of MMP-7 as a biomarker for BA. To avoid investigator bias, all MMP-7 levels were measured by Wuxi Diagnostics, who were blinded to the clinical data. MMPs are involved in ECM remodeling, which is closely related to various physical and pathological processes, including liver fibrosis (16, 17). Biliary atresia is different from other cholestatic diseases in rapid fibrotic process in early stage. This might be why MMP-7 levels are significantly elevated in BA group.
In recent years, serum MMP-7 has been gradually integrated into diagnostic algorithm of BA during routine clinical practice in our center. However, fresh serum samples should be applied for serum MMP-7 measurements in case of protein degradation, leading to high requirements for sample collection and storage. Besides, only 10–20 µl of serum is required for ELISAs, resulting in unnecessary blood waste.
Thus, we developed a noninvasive method to collect heel tip blood, which can easily be used to detect MMP-7 concentrations via DBS. The DBS device in this study is different from standard DBS. It comprises a quantificational collection vessel, a sample collection tube and a sample collection filter paper. The quantificational collection vessel is a 10-μl capillary tube, which is necessary for DBS MMP-7 measurement. The sample collection tube helped to separate the DBS from outside environment to facilitate its transportation. The filter paper is made of glass fiber and attached with calcium chloride, which can shorten the drying time after blood sample collection. It can effectively absorb whole blood including red blood cells. However, the standard DBS is usually made of cotton fiber and is not quantificational for blood collection. A more recent study using standard DBS has confirmed the screening performance of MMP-7 as early as 3 days old (18). The novel quantificational DBS device in this study may provide a more accurate measurement.
DBS and serum MMP-7 couldn't be equivalent because there is a matrix effect. That is, the serum accounts for about half of the whole blood, and that's why the slope of relationship between DBS and serum MMP-7 is around 0.5. DBS MMP-7 correlated well with serum MMP-7, with R of 0.93. DBS MMP-7 has good accuracy for diagnosing BA, though serum MMP-7 showed better accuracy. However, DBS is less invasive given the need for only 10 μl of blood. Thermal stability showed that it is convenient to be transported at room temperature. Thus, it could be possible to add BA screening to the myriad tests performed on neonatal heel blood screening and for parents to collect the samples themselves at home and mail them to an institution for measurement.
This study has some remaining limitations. First, the BA and non-BA groups in our study were not age-matched because many of the non-BA patients had been first referred to local hospitals, thereby delaying the first visit to our hospital. However, age showed no significant correlation with both DBS and serum MMP-7 levels in the non-BA group; thus, the chance of induced bias was small. Second, subgroup analysis of neonates was not performed since the sample size was small. Further study is warranted to validate the diagnostic accuracy of serum and DBS MMP-7 before its application in neonates screening. Third, operator bias may exist because this was a single center study and the DBS samples were all collected by the same investigator (JJ). It may take some time to have a command of DBS collection since quantification is necessary for DBS MMP-7 measurement. Thus, the standardized protocol of the use of DBS collection device should be made before its clinical application and multicenter studies are needed to further validate its consistency and accuracy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Children's Hospital of Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JJ: Data curation, Formal Analysis, Investigation, Writing – original draft. SL: Formal Analysis, Writing – original draft. MD: Data curation, Writing – original draft. JD: Data curation, Formal Analysis, Writing – review & editing. GC: Data curation, Project administration, Writing – review & editing. YY: Data curation, Project administration, Writing – review & editing. RD: Conceptualization, Project administration, Supervision, Writing – review & editing. ZF: Conceptualization, Project administration, Supervision, Writing – review & editing. SZ: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study received financial support from Clinical Research Plan of SHDC (No. SHDC2020CR2009A), Shanghai Municipal Key Clinical Specialty (No. shslczdzk05703), National Natural Science Foundation of China (Nos. 81770519, 81771633, 81873545, 81974059 and 82001595), The Science Foundation of Shanghai (Nos. 18411969100 and 19ZR1406600), Children’s National Medical Center (Nos. EK1125180104, EKYY20180204, EK112520180211 and EK112520180310) and Shanghai Pujiang Program (21PJ1423100).
Acknowledgments
We would like to thank professor Weili Yan from Department of Clinical Epidemiology, Children's Hospital of Fudan University for the help in study design and statistical analyses. We are thankful to professor Yongfu Yu from Department of Biostatistics, School of Public Health, Fudan University for the help in statistical review and revision. We are grateful to professor Xinbao Xie, Jianshe Wang from Department of Hepatology, Jing Wang from Department of Neonatology and all the doctors and nurses involved for their contributions in the process of the study.
Conflict of interest
SL, JD, and ZF work for WuXi Diagnostics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1293329/full#supplementary-material
References
1. Alagille D. Extrahepatic biliary atresia. Hepatology. (1984) 4:7s–10s. doi: 10.1002/hep.1840040704
2. Feldman AG, Mack CL. Biliary atresia: clinical lessons learned. J Pediatr Gastroenterol Nutr. (2015) 61:167–75. doi: 10.1097/MPG.0000000000000755
3. Davenport M. Biliary atresia: clinical aspects. Semin Pediatr Surg. (2012) 21:175–84. doi: 10.1053/j.sempedsurg.2012.05.010
4. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. (2009) 374:1704–13. doi: 10.1016/S0140-6736(09)60946-6
5. Ando H, Inomata Y, Iwanaka T, Kuroda T, Nio M, Matsui A, et al. Clinical practice guidelines for biliary atresia in Japan: a secondary publication of the abbreviated version translated into English. J Hepatobiliary Pancreat Sci. (2021) 28:55–61. doi: 10.1002/jhbp.816
6. Dong R, Jiang J, Zhang S, Shen Z, Chen G, Huang Y, et al. Development and validation of novel diagnostic models for biliary atresia in a large cohort of Chinese patients. EBioMedicine. (2018) 34:223–30. doi: 10.1016/j.ebiom.2018.07.025
7. Yoon HM, Suh CH, Kim JR, Lee JS, Jung AY, Cho YA. Diagnostic performance of sonographic features in patients with biliary atresia: a systematic review and meta-analysis. J Ultrasound Med. (2017) 36:2027–38. doi: 10.1002/jum.14234
8. Sandberg JK, Sun Y, Ju Z, Liu S, Jiang J, Koci M, et al. Ultrasound shear wave elastography: does it add value to gray-scale ultrasound imaging in differentiating biliary atresia from other causes of neonatal jaundice? Pediatr Radiol. (2021) 51(9):1654–66. doi: 10.1007/s00247-021-05024-9
9. Nomden M, Beljaars L, Verkade HJ, Hulscher JBF, Olinga P. Current concepts of biliary atresia and matrix metalloproteinase-7: a review of literature. Front Med (Lausanne. (2020) 7(617261). doi: 10.3389/fmed.2020.617261
10. Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, et al. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol. (2005) 18:941–50. doi: 10.1038/modpathol.3800374
11. Hsieh CS, Chuang JH, Huang CC, Chou MH, Wu CL, Lee SY, et al. Evaluation of matrix metalloproteinases and their endogenous tissue inhibitors in biliary atresia-associated liver fibrosis. J Pediatr Surg. (2005) 40:1568–73. doi: 10.1016/j.jpedsurg.2005.06.028
12. Wu JF, Jeng YM, Chen HL, Ni YH, Hsu HY, Chang MH. Quantification of Serum matrix metallopeptide 7 levels may assist in the diagnosis and predict the outcome for patients with biliary atresia. J Pediatr. (2019) 208:30–37.e31. doi: 10.1016/j.jpeds.2018.12.006
13. Jiang J, Wang J, Shen Z, Lu X, Chen G, Huang Y, et al. Serum MMP-7 in the diagnosis of biliary atresia. Pediatrics. (2019) 144(5):e20190902. doi: 10.1542/peds.2019-0902
14. Yang L, Zhou Y, Xu PP, Mourya R, Lei HY, Cao GQ, et al. Diagnostic accuracy of Serum matrix metalloproteinase-7 for biliary atresia. Hepatology. (2018) 68:2069–77. doi: 10.1002/hep.30234
15. Lertudomphonwanit C, Mourya R, Fei L, Zhang Y, Gutta S, Yang L, et al. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med. (2017) 9(417). doi: 10.1126/scitranslmed.aan8462
16. Chan ZC, Oentaryo MJ, Lee CW. MMP-mediated modulation of ECM environment during axonal growth and NMJ development. Neurosci Lett. (2020) 724:134822. doi: 10.1016/j.neulet.2020.134822
17. Berg G, Barchuk M, Miksztowicz V. Behavior of metalloproteinases in adipose tissue, liver and arterial wall: an update of extracellular matrix remodeling. Cells. (2019) 8(2):158. doi: 10.3390/cells8020158
Keywords: biliary atresia, diagnosis, dried blood spot, MMP-7, screening
Citation: Jiang J, Liu S, Du M, Deng J, Chen G, Yang Y, Dong R, Fang Z and Zheng S (2023) Measurement of MMP-7 in micro-volume peripheral blood: development of dried blood spot approach. Front. Pediatr. 11:1293329. doi: 10.3389/fped.2023.1293329
Received: 27 September 2023; Accepted: 31 October 2023;
Published: 15 November 2023.
Edited by:
Kejun Zhou, Human Metabolomics Institute Inc., ChinaReviewed by:
Jianghua Zhan, Tianjin Medical University, ChinaZhang Ruizhong, Guangzhou Medical University, China
© 2023 Jiang, Liu, Du, Deng, Chen, Yang, Dong, Fang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Dong cmRvbmdAZnVkYW4uZWR1LmNu Zhuo Fang bWlubmllX2Zhbmd6aHVvQHFxLmNvbQ== Shan Zheng c3poZW5nQHNobXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Abbreviations ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under curve; BA, biliary atresia; CI, confidence interval; DB, direct bilirubin; DBS, dried blood spot; GGT, gamma glutamyl transferase; IQR, interquartile range; MMP-7, matrix metalloproteinase-7; NPV, negative predictive value; PPV, positive predictive value; TB, total bilirubin; TBA, total bile acid.
 Jingying Jiang
Jingying Jiang Shuyang Liu2,†
Shuyang Liu2,† Rui Dong
Rui Dong Zhuo Fang
Zhuo Fang Shan Zheng
Shan Zheng