
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 05 January 2024
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1286400
Introduction: Finding non-invasive methods to predict the degree of liver fibrosis is very important in managing children with biliary atresia. Therefore, we explored the predictive value of APRI, FIB-4, and serological markers for liver fibrosis in children with biliary atresia.
Methods: This study retrospectively reviewed data from children diagnosed with BA between March and December 2022. Liver tissue pathology specimens were obtained during surgery. The serum markers were measured within 2 days before the Kasai procedure or liver transplantation. The aspartate aminotransferase-to-platelet ratio index (APRI) and the four-factor-based fibrosis index (FIB-4) were calculated. The outcome was the diagnosis of progressive liver fibrosis.
Results: This study reviewed the data from 41 children with biliary atresia. APRI had 52% sensitivity and 83% specificity for progressive liver fibrosis, while FIB-4 had 83% sensitivity and 67% specificity. Their areas under the curve were not significantly different from those of conventional markers.
Conclusion: Although they were not better than conventional markers, APRI and FIB-4 can be used as follow-up markers for progressive liver fibrosis in patients with biliary atresia, but their predictive value was moderate. Additional studies are necessary to determine whether they could be combined with other markers to improve their predictive value.
Biliary atresia (BA) is a rare liver disease in infants, leading to bile flow obstruction to the intestines and resulting in bile accumulation, liver cell injury, and liver fibrosis (1). Evaluating the extent of liver fibrosis in children with biliary BA is crucial for treatment and prognosis. Conventional histopathological assessment may be unsuitable for children with BA as it requires liver tissue biopsy (2). Therefore, finding non-invasive methods to predict the degree of liver fibrosis is very important in managing children with BA. Among the possible biomarkers, the aspartate aminotransferase (AST)-to-platelet (PLT) ratio index (APRI), previously studied in children with hepatitis B, hepatitis C, and BA, can serve as a non-invasive marker of fibrosis and cirrhosis (3). The four-factor-based fibrosis index (FIB-4) has been used to predict the degree of liver fibrosis in children with liver cystic fibrosis (4, 5). Therefore, we explored the predictive value of APRI, FIB-4, and serological markers for liver fibrosis in children with BA.
This study retrospectively reviewed data from 41 children diagnosed with BA between March and December 2022. Liver tissue pathology specimens were obtained during surgery. This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of our hospital, and all participants provided written informed consent.
The serum markers measured within 1 week before the Kasai procedure or liver transplantation were collected: alanine aminotransferase (ALT), AST, γ-glutaryl transferase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), and PLT.
The pathological examinations were performed by a single pathologist with 20 years of experience, who assessed the liver fibrosis levels and inflammation grade based on the New Inuyama classification (6) using liver tissue pathology slides collected in this study. Fibrosis was staged as F0 (no fibrosis), F1 (fibrous portal expansion), F2 (bridging fibrosis, either portal-portal or portal-central linkage), F3 (bridging fibrosis with lobular distortion or disorganization), and F4 (cirrhosis). Inflammation was graded as A0 (no necro-inflammatory reaction), A1 (mild necro-inflammatory reaction), A2 (moderate necro-inflammatory reaction), and A3 (severe necro-inflammatory reaction). The patients were classified into non-progressive liver fibrosis (<F3) and progressive liver fibrosis (≥F3).
The APRI was calculated as APRI = (AST/upper limit of normal AST) × 100/PLT (7). FIB-4 was calculated as FIB-4 = (years of age × AST)/(PLT × √ALT) (8).
Statistical analysis was conducted using GraphPad Prism version 9.0.0 for Windows (GraphPad Software, San Diego, CA, USA) and MedCalc version 20.010 (MedCalc Software Ltd., Ostend, Belgium). The continuous variables were presented as means ± standard deviations or medians (interquartile ranges) and analyzed using the independent sample t-test or Mann–Whitney U-test. Categorical data were analyzed using the chi-squared test. Spearman correlation analysis was performed to examine the correlations between pairs of variables. Received operating characteristics (ROC) analysis was used to explore the predictive value of APRI, FIB-4, and serological markers for liver fibrosis levels. The Delong test was used to compare the area under the curve (AUC) of the biomarkers. Two-sided P-values <0.05 were considered statistically significant.
The study included 23 patients with BA and progressive liver fibrosis and 18 with BA and non-progressive liver fibrosis. The between-group comparison is presented in Table 1. There were no significant differences between the two groups regarding age (at admission and surgery), sex, ALT, AST, GGT, TBIL, and DBIL (all P > 0.055). Compared with the non-progressive liver fibrosis group, the progressive liver fibrosis group showed lower PLT (255.0 ± 28.6 vs. 399.7 ± 59.8 × 109/L, P = 0.039), higher APRI score (median, 2.3 vs. 11.32, P = 0.033), and higher FIB-4 score (median, 0.0805 vs. 0.0071, P = 0.019). Figure 1 shows the APRI and FIB-4 scores according to the fibrosis stage (Figures 1A,B) and inflammation grade (Figures 1C,D).
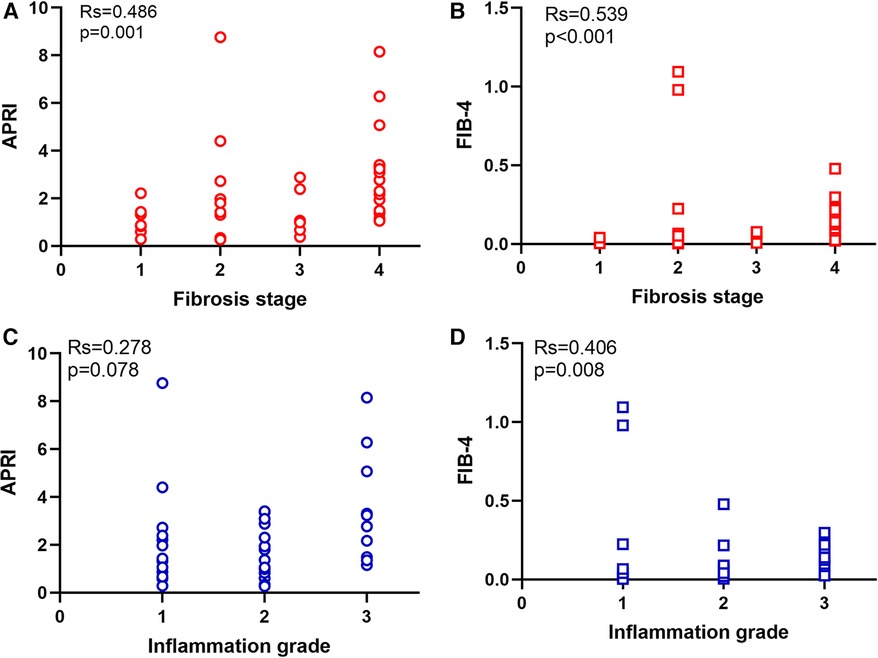
Figure 1. Distribution of APRI and FIB-4 in different fibrosis stage and inflammation grade. (A) APRI in different fibrosis stage. (B) FIB-4 in different fibrosis stage. (C) APRI in different inflammation grade. (D) FIB-4 in different inflammation grade.
Spearman correlation analysis showed that the APRI was significantly correlated to the fibrosis stage (r = 0.486, P = 0.001), ALT (r = 0.376, P = 0.015), AST (r = 0.512, P = 0.001), and PLT (r = 0.735, P < 0.001). FIB-4 was significantly correlated to the fibrosis stage (r = 0.539, P < 0.001), inflammation grade (r = 0.406, P = 0.008), GGT (−0.387, P = 0.012), and PLT (r = −0.828, P < 0.001). The APRI and FIB-4 were correlated (r = 0.818, P < 0.001) (Table 2).
The comparison of the AUCs between APRI and ALT, AST, GGT, TBIL, DBIL, and PLT showed no statistically significant differences (P = 0.11, P = 0.53, P = 0.40, P = 0.21, P = 0.28, and P = 0.32, respectively). Similarly, the comparison of AUC between FIB-4 and ALT, AST, GGT, TBIL, DBIL, and PLT also revealed no statistically significant difference (P = 0.07, P = 0.53, P = 0.44, P = 0.21, P = 0.30, and P = 0.65, respectively). The comparison of AUC between FIB-4 and APRI was not statistically significant (P = 0.74). APRI had 52% sensitivity and 83% specificity for progressive liver fibrosis, while FIB-4 had 83% sensitivity and 67% specificity (Figure 2 and Table 3).
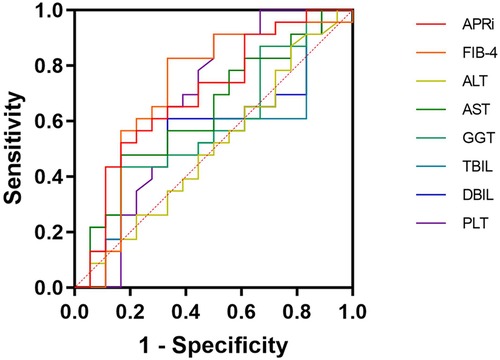
Figure 2. Receiver operating characteristics curve. APRI, aspartate aminotransferase to platelet ratio index; FIB-4, four-factor-based fibrosis index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutaryl transferase; TBIL, total bilirubin; DBIL, direct bilirubin; PLT, platelets.
Patients with BA usually undergo the Kasai procedure to buy time pending liver transplantation. Patients with non-progressive liver fibrosis can undergo transplantation at an older age, but patients with progressive liver fibrosis must be carefully monitored to determine the optimal timing of transplantation. Even though conventional routine blood tests and liver function indicators (e.g., AST, ALT, GGT, TBIL, and DBIL) are useful in the diagnosis and monitoring of BA, these biochemical markers have limited diagnostic efficacy in identifying progressive liver fibrosis (F ≥ 3) and are unable to provide effective clinical assistance for accurate judgment in patients with BA (9).
Histopathological examination is the only definitive diagnostic tool for liver fibrosis, but the invasiveness of liver biopsy limits its use in neonates, and it cannot be repeated periodically in the context of long-term condition monitoring (2). Liver elastography assessed by ultrasound can provide a fair assessment of liver fibrosis (10, 11), including in children (12), but it is operator-dependent and has limited reliability and reproducibility (13). Still, specific ultrasound modalities have advantages and disadvantages. Vibration-controlled transient elastography can provide a quick bedside assessment but does not provide real-time ultrasound guidance and performs poorly in congestion, obesity, and inflammation (2). Two-dimensional shear-wave elastography can be implemented in regular scanners, shows the liver parenchyma, and can measure several levels at the same time, but it is affected by steatosis, inflammation, age, and BMI, and different manufacturers use different cutoff points (2). Point shear-wave elastography has the same advantages and disadvantages as two-dimensional shear-wave elastography, and it can evaluate a single region of interest (2). Shear-wave elastography combined with serum markers could be used to assess fibrosis after the Kasai procedure (14). Acoustic radiation force pulse imaging (ARFI) appears promising to evaluate liver fibrosis (15), but little data are available for its use in BA. Magnetic resonance elastography and diffusion-weighted imaging are promising modalities but are limited by scanner availability, high costs, long scanning times, and the necessity for holding breath, which is impossible in newborns and difficult in infants (2, 16, 17). As reviewed by Nallagangula et al. (18), Lew-Tusk et al. (19), and Ozdogan et al. (2), blood biomarkers for liver fibrosis include indirect markers (albumin, bilirubin, AST, ALT, GGT, ALP, and prothrombin time), direct markers (collagens, glycoproteins and polysaccharides, collagenases, hyaluronic acid, type IV collagen, procollagen III aminopeptide, laminin, YKL-40, MCP-1, sFas, CK18, and autotaxin), and combinational markers (APRI, AST/ALT, Bonacini index, ELF index, FIB-4, Fibro index, fibrometer test, FibroSpect II, Foma test, Hepascore, Fibrotest, and Lok index). Their AUCs are highly variable, even for a given biomarker (2, 18). Still, MMP-7 appears promising (20–27) and could be included in test packages or composite scores for BA, including in newborn screening (28). In addition, some of these tests are not routinely performed in the clinical setting or require special devices. Hence, the main advantage of the APRI and FIB-4 is that they can be calculated using routine blood test results without additional tests. It is an important point to consider in infants with an already highly morbid condition like BA. Of note, the present study did not evaluate the APRI and FIB-4 for diagnosing BA but for progressive liver fibrosis. Therefore, they could be of value for determining the timing of interventions like liver transplantation. Nevertheless, a major issue is that the available studies (including the present one) examined only a few modalities or biomarkers at a time; future studies should examine multiple imaging modalities, serum markers, and composite scores within the same patient samples and perform comparisons among them.
In the present study, APRI and FIB-4 showed a similar diagnostic value for progressive liver fibrosis (F ≥ 3; pathological diagnosis) compared with the traditional indicators, with FIB-4 outperforming APRI. Still, their AUCs were <0.75, indicating a suboptimal diagnostic value. These results are supported by a meta-analysis that revealed pooled sensitivity and specificity of 61% and 80% for significant liver fibrosis after surgery for BA (29). A study that used liver ultrasound for fibrosis diagnosis revealed AUCs of 0.897 for APRI and 0.856 for FIB-4 (14). However, it is worth noting that the predictive value of FIB-4 could be affected by age, as observed in adults (30). Since the patients in this study were relatively young, it could lead to lower FIB-4 values. In the previous studies, APRI and FIB-4 were evaluated postoperatively, while the present study evaluated these indexes before surgery. It will be necessary to examine such models in large-scale multicenter studies. Nevertheless, in the meantime, the APRI and FIB-4 can still be considered auxiliary monitoring biomarkers of liver fibrosis in children with BA, together with other indicators to reflect the situation of liver fibrosis in BA.
This study had limitations. It was a single-center study, and we enrolled all eligible patients during the study period. However, biliary atresia is a rare disease with an incidence of about 1 in 10,000, and it is not easy to collect cases. In addition, some patients had either passed away or returned to their residential areas during this time, making their inclusion unfeasible. Consequently, this situation introduced a bias as these patients could no longer be included in the consecutive enrollment process. These resulted in a small sample size. Even though enrollment was prospective, the available data were limited to the routine tests performed in infants with BA for ethical considerations against additional testing in infants with severe conditions and poor functional reserves. The study did not enroll control infants.
Since children with BA undergo the Kasai procedure and are observed until liver transplantation, and although they were not better than conventional markers, APRI and FIB-4 can be used as follow-up markers for progressive liver fibrosis in patients with biliary atresia, but their predictive value was moderate. Additional studies are necessary to determine whether they could be combined with other markers to improve their predictive value.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethic Committee of Shenzhen Children's Hospital (#202106602). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
HL: Conceptualization, Methodology, Writing – original draft. YY: Formal analysis, Writing – original draft. BW: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Sanming Project of Medicine in Shenzhen (#SZSM201812055) and the Guangdong High-level Hospital Construction Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. (2009) 374(9702):1704–13. doi: 10.1016/S0140-6736(09)60946-6
2. Ozdogan E, Arikan C. Liver fibrosis in children: a comprehensive review of mechanisms, diagnosis, and therapy. Clin Exp Pediatr. (2023) 66(3):110–24. doi: 10.3345/cep.2022.00367
3. Grieve A, Makin E, Davenport M. Aspartate aminotransferase-to-platelet ratio index (APRi) in infants with biliary atresia: prognostic value at presentation. J Pediatr Surg. (2013) 48(4):789–95. doi: 10.1016/j.jpedsurg.2012.10.010
4. Leung DH, Khan M, Minard CG, Guffey D, Ramm LE, Clouston AD, et al. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology. (2015) 62(5):1576–83. doi: 10.1002/hep.28016
5. Sellers ZM. Barrier to using APRI and GPR as identifiers of cystic fibrosis liver disease. J Cyst Fibros. (2021) 20(3):551. doi: 10.1016/j.jcf.2020.07.018
6. Ichida F, Tsuji T, Omata M, Ichida T, Inoue K, Kamimura T, et al. New inuyama classification; new criteria for histological assessment of chronic hepatitis. Intl Hepatol Comm. (1996) 6:112–9. doi: 10.1016/S0928-4346(96)00325-8
7. Petersen JR, Stevenson HL, Kasturi KS, Naniwadekar A, Parkes J, Cross R, et al. Evaluation of the aspartate aminotransferase/platelet ratio index and enhanced liver fibrosis tests to detect significant fibrosis due to chronic hepatitis C. J Clin Gastroenterol. (2014) 48(4):370–6. doi: 10.1097/MCG.0b013e3182a87e78
8. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. (2006) 43(6):1317–25. doi: 10.1002/hep.21178
9. Kong M, Xiang B. Identifying biomarkers to predict the prognosis of biliary atresia by weighted gene co-expression network analysis. Front Genet. (2021) 12:760182. doi: 10.3389/fgene.2021.760182
10. Dhyani M, Anvari A, Samir AE. Ultrasound elastography: liver. Abdom Imaging. (2015) 40(4):698–708. doi: 10.1007/s00261-015-0373-4
11. Zhang YN, Fowler KJ, Ozturk A, Potu CK, Louie AL, Montes V, et al. Liver fibrosis imaging: a clinical review of ultrasound and magnetic resonance elastography. J Magn Reson Imaging. (2020) 51(1):25–42. doi: 10.1002/jmri.26716
12. Mjelle AB, Mulabecirovic A, Havre RF, Rosendahl K, Juliusson PB, Olafsdottir E, et al. Normal liver stiffness values in children: a comparison of three different elastography methods. J Pediatr Gastroenterol Nutr. (2019) 68(5):706–12. doi: 10.1097/MPG.0000000000002320
13. Pirmoazen AM, Khurana A, El Kaffas A, Kamaya A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics. (2020) 10(9):4277–89. doi: 10.7150/thno.40249
14. Hwang J, Yoon HM, Kim KM, Oh SH, Namgoong JM, Kim DY, et al. Assessment of native liver fibrosis using ultrasound elastography and serological fibrosis indices in children with biliary atresia after the Kasai procedure. Acta Radiol. (2021) 62(8):1088–96. doi: 10.1177/0284185120948489
15. Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. (2012) 19(2):e212–9. doi: 10.1111/j.1365-2893.2011.01537.x
16. Kim J, Shin HJ, Yoon H, Han SJ, Koh H, Kim MJ, et al. Diffusion-weighted imaging for differentiation of biliary atresia and grading of hepatic fibrosis in infants with cholestasis. Korean J Radiol. (2021) 22(2):253–62. doi: 10.3348/kjr.2020.0055
17. Dong B, Lyu G, Chen Y, Lin G, Wang H, Qin R, et al. Comparison of two-dimensional shear wave elastography, magnetic resonance elastography, and three serum markers for diagnosing fibrosis in patients with chronic hepatitis B: a meta-analysis. Expert Rev Gastroenterol Hepatol. (2021) 15(9):1077–89. doi: 10.1080/17474124.2021.1880894
18. Nallagangula KS, Nagaraj SK, Venkataswamy L, Chandrappa M. Liver fibrosis: a compilation on the biomarkers status and their significance during disease progression. Future Sci OA. (2018) 4(1):FSO250. doi: 10.4155/fsoa-2017-0083
19. Lew-Tusk A, Peksa M, Stachowicz-Stencel T. Recent studies on non-invasive biomarkers useful in biliary atresia—a literature review. Acta Biochim Pol. (2023) 70(3):475–80. doi: 10.18388/abp.2020_6858
20. Karbasian F, Mashhadiagha A, Anbardar MH, Ataollahi M, Dehghani SM, Honar N, et al. Questioning diagnostic value of serum matrix metalloproteinase 7 for biliary atresia. J Clin Exp Hepatol. (2023) 13(2):265–72. doi: 10.1016/j.jceh.2022.10.001
21. Aldeiri B, Si T, Huang Z, Torner N, Ma Y, Davenport M, et al. Matrix metalloproteinase-7 and osteopontin serum levels as biomarkers for biliary atresia. J Pediatr Gastroenterol Nutr. (2023) 77(1):97–102. doi: 10.1097/MPG.0000000000003792
22. Wu B, Zhou Y, Tian X, Cai W, Xiao Y. Diagnostic values of plasma matrix metalloproteinase-7, interleukin-8, and gamma-glutamyl transferase in biliary atresia. Eur J Pediatr. (2022) 181(11):3945–53. doi: 10.1007/s00431-022-04612-7
23. Salvi PS, Fawaz R, Cowles RA. Comparing serum matrix metalloproteinase-7 in parenteral nutrition-associated liver disease and biliary atresia. J Pediatr. (2022) 249:97–100. doi: 10.1016/j.jpeds.2022.06.014
24. Sakaguchi H, Konishi KI, Yasuda R, Sasaki H, Yoshimaru K, Tainaka T, et al. Serum matrix metalloproteinase-7 in biliary atresia: a Japanese multicenter study. Hepatol Res. (2022) 52(5):479–87. doi: 10.1111/hepr.13753
25. Rohani P, Mirrahimi SB, Bashirirad H, Rahmani P, Kamran N, Alimadadi H, et al. Serum matrix metalloproteinase-7 levels in infants with cholestasis and biliary atresia. BMC Pediatr. (2022) 22(1):351. doi: 10.1186/s12887-022-03409-9
26. Chi S, Xu P, Yu P, Cao G, Wang H, Ye Y, et al. Dynamic analysis of serum mmp-7 and its relationship with disease progression in biliary atresia: a multicenter prospective study. Hepatol Int. (2022) 16(4):954–63. doi: 10.1007/s12072-022-10322-x
27. Nomden M, Beljaars L, Verkade HJ, Hulscher JBF, Olinga P. Current concepts of biliary atresia and matrix metalloproteinase-7: a review of literature. Front Med. (2020) 7:617261. doi: 10.3389/fmed.2020.617261
28. Lee CS, Ni YH, Chen HL, Wu JF, Hsu HY, Chien YH, et al. A pilot study of biliary atresia newborn screening using dried blood spot matrix metalloproteinase-7. J Pediatr Gastroenterol Nutr. (2023) 76(4):418–23. doi: 10.1097/MPG.0000000000003701
29. He L, Ip DKM, Tam G, Lui VCH, Tam PKH, Chung PHY. Biomarkers for the diagnosis and post-Kasai portoenterostomy prognosis of biliary atresia: a systematic review and meta-analysis. Sci Rep. (2021) 11(1):11692. doi: 10.1038/s41598-021-91072-y
Keywords: predictive value of tests, liver fibrosis, children, biliary atresia, aspartate aminotransferase, platelet
Citation: Lyu H, Ye Y and Wang B (2024) FIB-4 and APRI scores for progressive liver fibrosis diagnosis in children with biliary atresia. Front. Pediatr. 11:1286400. doi: 10.3389/fped.2023.1286400
Received: 31 August 2023; Accepted: 11 December 2023;
Published: 5 January 2024.
Edited by:
Thomai Karagiozoglou-Lampoudi, International Hellenic University, GreeceReviewed by:
Jianghua Zhan, Tianjin Medical University, China© 2024 Lyu, Ye and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wang c3p3YjE5NjdAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.