
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 08 January 2024
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1278978
 Jixin Yang1
Jixin Yang1 Xiaoqing Chen2
Xiaoqing Chen2 Wenjing Wang1
Wenjing Wang1 Yanwei Su3
Yanwei Su3 Keqin Liu3
Keqin Liu3 Adila Abudusalamu1
Adila Abudusalamu1 Dandan Li1
Dandan Li1 Ying He1
Ying He1 Pusu Wang1
Pusu Wang1 Xiaofeng Xiong1
Xiaofeng Xiong1 Jiexiong Feng1*
Jiexiong Feng1*
Objective: Biliary innervation is considered important in regulating the function of bile ducts, whereas the role of innervation in the hepatobiliary system of patients with biliary atresia (BA) remains unknown. This current study aims to investigate the role of innervation in biliary remnants and analyze the relationship between the innervation and prognosis of BA after surgery.
Methods: Eighty-seven patients with type III BA who underwent the Kasai procedure were consecutively enrolled from January 2017 to September 2020. Innervation and ductules in remnants were examined by pathologists. Liver function, onset of cholangitis, jaundice clearance, and survival with the native liver were recorded. Patients were followed up for 24 months. The relationship between innervation and prognosis was analyzed.
Results: In total, 67 patients had bile drainage postoperatively, and 21 biliary remnants contained neuronal plexuses where there was no neuron but nerve fiber bundles. Acetylcholinesterase staining was positive in all plexuses. In patients with bile drainage, those with plexuses had improved postoperative liver function, significantly better jaundice clearance 3 or 6 months postoperatively (50.0% vs. 19.1%, or 90.0% vs. 63.8%, respectively), fewer episodes of early cholangitis (10.0% vs. 34.0%), and better survival (80.0% vs. 61.7%) compared to those without. In addition, a larger area of plexuses was associated with a larger area of ductules (R2 = 0.786, p = 0.000), less frequent (p = 0.000) and later cholangitis onset (p = 0.012), and better jaundice clearance (p = 0.063).
Conclusions: Increased cholinergic innervation in biliary remnants may help reduce the onset of cholangitis and lead to better and earlier jaundice clearance. Thus, it improves the postoperative prognosis of patients with BA.
It is known that sympathetic and parasympathetic nerves exist around the intrahepatic and extrahepatic bile ducts (1). The parasympathetic nerve derives from the vagus nerve. Its anterior plexus surrounds the hepatic artery, and its posterior plexus surrounds the portal vein and bile duct. Research studies showed that the vagus nerve branches connect the liver and bile duct plexus and are distributed to each segment of the liver and around the bile ducts (2). This nerve distribution plays a vital role in regulating the functions of bile ducts, including promoting cholangiocyte proliferation and reducing apoptosis following bile duct injury (3). However, until now, there have been very few reports on the role of innervation in the hepatobiliary system in patients with biliary atresia (BA).
BA is the most common cause of obstructive jaundice in newborns (2). Patients may develop hepatic fibrosis 1 month after birth, and if untreated, the patients may die around 1 year of age (4). After the diagnosis of BA, the primary surgical treatment mainly involves Kasai portoenterostomy (KP). Although many factors affect the survival after KP, the favorable postoperative bile drainage and the onset of cholangitis are recognized as the most important determinants (5). In recent years, our studies have focused on the distribution of ductules in biliary remnants of patients with BA, and we observed that the number/area of ductules yielded by technical precision is closely related to effective bile drainage, jaundice clearance, and the first onset of cholangitis in patients after KP (6). When observing the ductules in biliary remnants, we noticed nerve plexuses in the biliary remnants of some BA patients in the meantime (Figure 1A). So far, there has not been any research focusing on the innervation in biliary remnants, but an early clinical investigation by Iwami et al. reported the abnormal proliferation of nerve fibers in the livers of patients with BA (7). They concluded that the innervation could be associated with immaturity or malformation in the biliary system. However, following this conclusion, they did not analyze the clinical significance or further discuss the possible mechanism of innervation. To understand the features and clinical significance of innervation in biliary remnants, we conducted this clinical study to reveal the characteristics of innervation and its impact at different postoperative stages after KP.
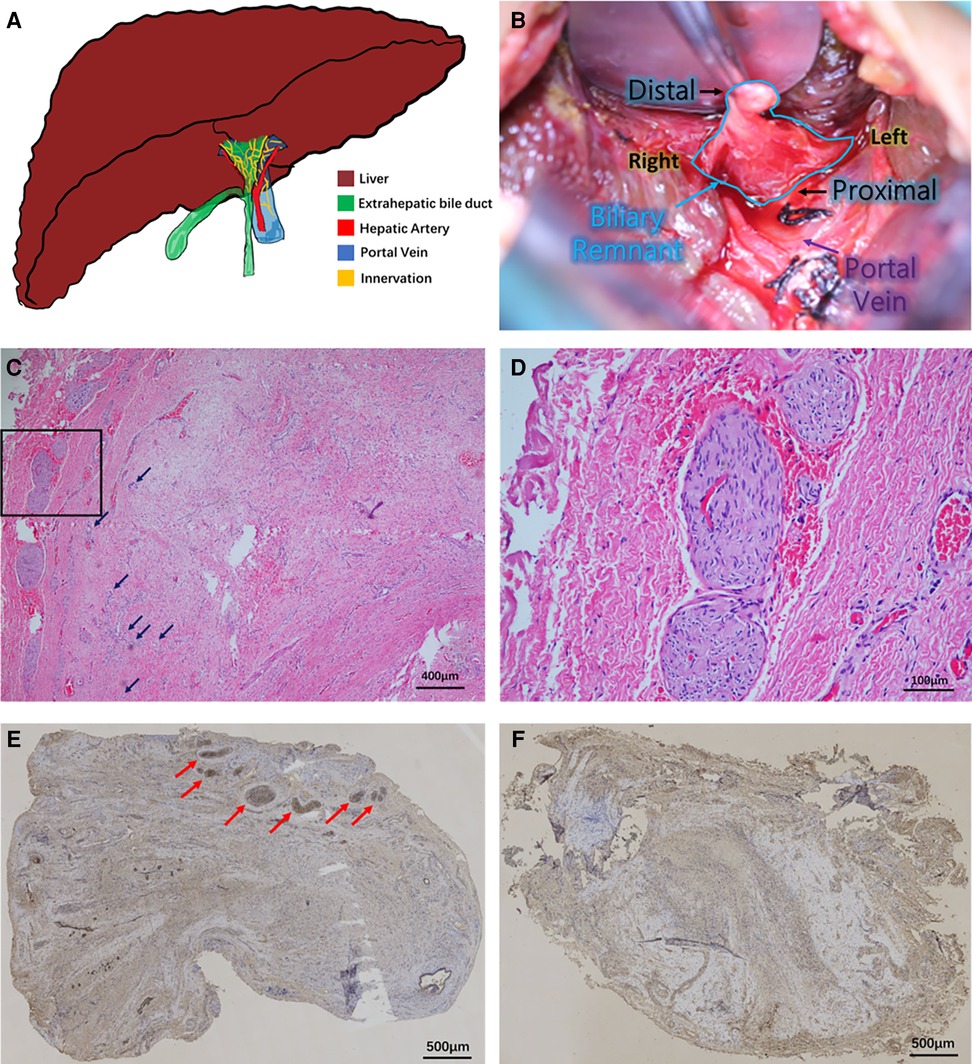
Figure 1. Innervation in biliary remnants. (A) Illustration of the hepatobiliary system in patients with biliary atresia. The atresia of the extrahepatic biliary system (green) is visible outside the fibrotic liver (brown). The right hepatic artery (red) and portal vein (blue) are behind the biliary remnants. Innervation (yellow) is present in the bile ducts and vascular system. (B) Intraoperative view of a biliary remnant in the Kasai procedure. The triangular remnant is stripped to the depth above the portal vein bifurcation and enlarged to the outer edges of the portal vein. (C) Hematoxylin–eosin staining of biliary remnants and neuronal plexuses. Growth of giant plexuses was observed in the marginal area of remnants of some cases. Some ductules were noted in the remnant (black arrow). The black frame area was further magnified in (D). Magnification: ×100. (D) Details of plexuses. Larger magnification of plexuses showed no neurons in the bundles but only thick nerve fiber bundles. The bundles were “wavy” or “vortex”-shaped. Magnification: ×400. (E) Acetylcholinesterase staining of plexuses. The plexuses were all positive, shown by immunohistochemical staining (red arrow). Magnification: ×40. (F) Biliary remnant without plexuses. No plexus was observed in some other cases. Magnification: ×40.
From January 1, 2017, to September 30, 2020, 87 patients with BA who underwent KP at our department were consecutively enrolled. This study was approved by the Institutional Review Board, and informed consent forms were signed. All patients were diagnosed with type Ⅲ BA during the operation. The same group of surgeons performed the operation throughout the research, employing consistent operative techniques and postoperative management. We adopted the modified extensive KP (8). The anatomic depth of the fibrous remnant was above the portal vein bifurcation to the outer edges of the portal vein (Figure 1B).
The resected fibrous remnants were oriented by the methods established in our previous research (6). Sections at the proximal end from the whole remnants were used to analyze neuronal plexuses and ductules. Sections with a thickness of 6 μm were prepared from paraffin-embedded biliary remnants at the cutting edge. The sections were further subjected to hematoxylin–eosin (HE) staining and acetylcholinesterase (AChE) immunochemical staining. Heat-induced antigen retrieval was performed using 10 mM sodium citrate (pH 6.0) buffer to expose target proteins. The sections were subjected to microwave treatment for 10 min. Following antigen retrieval, tissues were blocked in 3% bovine serum albumin–phosphate-buffered saline for 30 min at room temperature. Tissues were then probed at a dilution of 1:20 with the monoclonal AChE antibody (Ab2803, Abcam, Cambridge, MA, USA) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBS, and endogenous peroxidase activity was quenched with a peroxidase suppressor. The specific signals were detected using a commercial SP-0024 Histostain-Plus Kit (Bioss, Beijing, China). Colorimetric detection was performed using diaminobenzidine. Tissues were counterstained with hematoxylin and prepared for mounting. Two independent pathologists examined the results of HE staining and AChE staining of tissue specimens, focusing on the shape and extent of innervation in biliary remnants, AChE positivity, and the distribution and area of biliary ductules. The number and area of AChE-positive plexuses were measured and recorded.
In total, 67 patients exhibited stable bile drainage within 1 month after KP (Group A), while the other 20 patients showed no bile drainage during the follow-up periods (Group B). “Stable bile drainage” is defined as bile drainage that turns stool brown or dark green without the appearance of acholic stool. Patients with bile drainage were postoperatively treated with ursodeoxycholic acid capsules at 10 mg/kg bodyweight (three times a day) immediately after observation of stool containing bile. The other medications were given as previously described (6).
All patients were followed up for 24 months by phone. Various parameters including jaundice clearance at 3 or 6 months postoperatively, survival with the native liver at 1 and 2 years postoperatively, onset of cholangitis within 1 or 3 months postoperatively, total episodes of cholangitis onset, and preoperative/postoperative (at 1 month postoperatively) liver function markers (such as total bilirubin or TBIL, direct bilirubin or DBIL, γ-glutamyl transpeptidase or γ-GT, total bile acid or TBA, albumin or ALB, alanine aminotransferase or ALT, and aspartate aminotransferase or AST) were recorded and compared. The criterium for jaundice clearance was defined as total bilirubin <20 μmol/L.
SPSS19.0 software was used for statistical analysis. The counting data were expressed as relative composition ratios (%) or rates (%). Fisher's exact test was used to compare the jaundice clearance rate, incidence of cholangitis, and survival rate with the native liver. The results of liver function markers by blood tests were expressed as means ± standard deviations. All continuous data were compared using a two-way analysis of variance followed by the Mann–Whitney U-test. Spearman’s statistic was used to illustrate correlation. The 2-year survival with the native liver was assessed by the log rank test. The difference was considered statistically significant if the p-value was less than 0.05.
Patients were not involved in the design, conduct, reporting, or dissemination plans of our research.
Diffused fibrous connective tissue and varying numbers of proliferative biliary ductules in biliary remnants were seen in all cases. Small vessel dilation/hyperemia and diffused infiltration of inflammatory cells were also observed. In some cases, the growth of giant neuronal plexuses was observed (Figure 1C), and some proliferated ductules were also noted. No neuron existed in the bundles, but only thick nerve fiber bundles gathered in plexuses. The bundles were “wavy” or “vortex”-shaped (Figure 1D). Their morphology was similar to the exogenous neuronal plexuses in aganglionic colonic segments of patients with Hirschsprung's disease in our previous study (9). AChE in these plexuses was all positive, as shown by immunohistochemical staining (Figure 1E). However, in some other cases, no neuronal plexus was observed in biliary remnants (Figure 1F). In total, plexuses were seen in 21 biliary remnants, while they were absent in 66. In Group B, only one out of 20 had any plexus, whereas 20 out of 67 patients in Group A had plexuses (p = 0.034). Then, Group A was further divided into two subgroups, A1 and A2, according to the presence or absence of plexuses in biliary remnants.
In total, 77.0% (67/87) of patients had bile drainage within 1 month postoperatively. As shown in Table 1, there were 20 patients with postoperative bile drainage and plexuses in biliary remnants in Group A1, 47 patients with postoperative bile drainage but no plexuses seen in biliary remnants in Group A2, and 20 patients with neither bile drainage nor plexuses in Group B. There was no significant difference in gender distribution, mean age at operation, or preoperative liver function in the three groups (all p’s > 0.05). At 1 month postoperatively, in Group A1 and A2, except for the insignificant change in ALB levels, TBA (p = 0.022 and p = 0.153, respectively), TBIL (p = 0.000 and p = 0.000, respectively), DBIL (p = 0.000 and p = 0.000, respectively), ALT (p = 0.010 and p = 0.179, respectively), and AST (p = 0.000 and p = 0.000, respectively) levels appeared decreased to varying degrees compared with those preoperatively. However, the liver function in Group B worsened, especially the levels of TBIL (p = 0.022), DBIL (p = 0.052), and TBA (p = 0.006), which increased to varying degrees compared to preoperative levels.
We next assessed jaundice clearance rates at 3 and 6 months postoperatively (Table 2). In Group A1, patients had the highest clearance rates at both time points (50% and 90%) compared to those in Groups A2 (19.1%, p = 0.010; 63.8%, p = 0.030) and B (10.0%, p = 0006; 0, p = 0.000). Episodes of early cholangitis onset within 1 month postoperatively appeared to be significantly fewer in Group A1 than in Group A2 (10.0% vs. 34.0%, p = 0.042). Within 3 months postoperatively, significantly fewer patients in Group A1 had onset of cholangitis than in Group A2 (55% vs. 78.7%, p = 0.048). During the follow-up period, 30% of patients in Group A1 had frequent (≥3 episodes) onset of cholangitis, which was significantly lower than in Group A2 (57.4%, p = 0.040). There was no significant difference between Group A1 and A2 in the overall survival with the native liver at 1 year postoperatively (80.0% vs. 61.7%, p = 0.144), whereas patients in these groups had significantly better survival with the native liver than those in Group B (30%, p = 0.001 and p = 0.017, respectively). However, a follow-up at 2 years postoperatively showed that patients’ survival rate with the native liver in Group A1 was significantly higher than that in Group A2 (p = 0.038) (Figure 2).
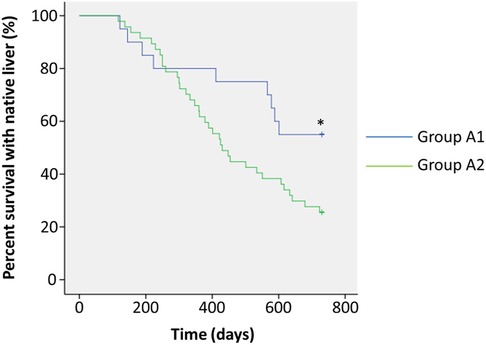
Figure 2. Analysis of survival with the native liver. Patients in Group A1 (n = 20) showed postoperative bile drainage and plexuses in biliary remnants (blue line). Patients in Group A2 (n = 47) showed postoperative bile drainage, but no plexuses were seen in biliary remnants (green line). *p < 0.05.
We measured the areas of biliary ductules according to our previously established methods (6). We have previously examined the variation of innervation in remnants of 10 patients (from January 1, 2017, to September 30, 2017) by using continuous section; the shape and area of plexuses were insignificant within one remnant from the proximal to the distal end (data not shown). For the rest of the patients, we did not further compare the variation at different ends but only analyzed neuronal plexuses and ductules at the proximal end from the whole remnants. The area of ductules in Group A1 was significantly larger than that in Group A2 [(18.80 ± 10.53) × 104 μm2 vs. (12.57 ± 9.37) × 104 μm2, p = 0.011], whereas patients in Group B had the smallest areas [(18.80 ± 10.53) × 104 μm2 vs. (2.92 ± 4.46) × 104 μm2, p = 0.000; (12.57 ± 9.37) × 104 μm2 vs. (2.92 ± 4.46) × 104 μm2, p = 0.000] (Figure 3A). The number of plexuses ranged from 2 to 11 (average 3.85 ± 2.08), and the average area was (57.71 ± 36.83) × 104 μm2. After regression analysis, the number of plexuses was found to be positively correlated with area (R2 = 0.871, p = 0.000) (Figure 3B). In Group A1, a positive correlation was found between ductule areas and plexus areas (R2 = 0.786, p = 0.000) (Figure 3C).

Figure 3. Analyses of ductules, plexuses, and postoperative prognosis of patients with biliary atresia. (A) Area of ductules in Groups A1, A2, and B. Group A1 had the largest area of ductules followed by Groups A2 and B. Group A1: postoperative bile drainage (+), nervous plexuses (+); Group A2: postoperative bile drainage (+), nervous plexuses (-); Group B: postoperative bile drainage (-), nervous plexuses (-). **p < 0.01 or *p < 0.05 were considered statistically significant compared to preoperative levels. (B) The number of plexuses was positively correlated with the area of plexuses. (C) The area of ductules was positively correlated with the area of plexuses. (D) Episodes of cholangitis and area of plexuses. Patients with ≥3 episodes had smaller areas than those with <3 episodes. **p < 0.01. (E) Early cholangitis and area of plexuses. Patients with early cholangitis had smaller areas of plexuses compared to those with cholangitis after 1 month postoperatively. *p < 0.05. (F) Area of plexuses and onset of cholangitis before or after 3 months postoperatively. Taking the 3-month postoperative period as the demarcation point, there was no significant difference in the area of the plexuses between the two subsets of patients. (G) Area of plexuses and jaundice clearance before or after 3 months postoperatively. Taking the 3-month postoperative period as the demarcation point, there was no significant difference in the area of the plexuses between the two subsets of patients. (H) Area of plexuses and jaundice clearance before or after 6 months postoperatively. Taking the 6-month postoperative period as the demarcation point, there was no significant difference in the area of the plexuses between the two subsets of patients.
Patients in Group A1 with cholangitis episodes ≥3 or <3 were further subgrouped. We found that children with more episodes (median = 5.5) of cholangitis had significantly smaller areas of nerve plexuses than those with fewer episodes (median = 1.0) [(20.92 ± 6.43) × 104 μm2 vs. (73.47 ± 32.77) × 104 μm2, p = 0.000] (Figure 3D). Only two patients had early cholangitis in Group A1 within 1 month after surgery; the mean plexus area of the two cases was significantly smaller compared to the other 18 patients without early cholangitis onsets [(14.50 ± 1.27) × 104 μm2 vs. (63.62 ± 36.44) × 104 μm2, p = 0.0117] (Figure 3E). However, children with cholangitis onsets within 3 months after surgery had similar plexus areas to those without [(56.17 ± 32.36) × 104 μm2 vs. (59.58 ± 43.64) × 104 μm2, p = 0.941] (Figure 3F). At 3 months postoperatively, there was no significant difference in the areas of the nerve plexus between children who had jaundice clearance and those who did not [(57.54 ± 22.03) × 104 μm2 vs. (57.87 ± 48.77) × 104 μm2, p = 0.393] (Figure 3G). At 6 months postoperatively, although the plexus areas in children with jaundice clearance (n = 18) seemed larger than those in children without jaundice clearance (n = 2) [(62.07 ± 36.23) × 104 μm2 vs. (18.40 ± 4.24) × 104 μm2] (Figure 3H), the difference was not statistically significant (p = 0.063).
The porta hepatis in normal children is the major outlet of intrahepatic innervation, forming an anterior plexus around the hepatic artery and a posterior plexus around the portal vein and bile ducts (3). Instead of normal bile ducts in the porta hepatis, patients with BA only have biliary remnants, where the innervation appears, as shown in our current study. The morphological details revealed no neuron but only nerve fiber bundles in “thickened” plexuses. Because we cannot acquire normal bile ducts as control specimens, especially those of the same age, we do not know the characteristics of normal plexuses in 1–3-month-old children. The description of “thickened plexuses” is derived from our previous experience when examining exogenous neuronal plexuses in the aganglionic colons in patients with Hirschsprung's disease (9) and other previous studies (10). The plexus shape, cell shape, and positive AChE staining were three major similarities of plexuses between the two different diseases. Nevertheless, the plexuses in biliary remnants are gathered at marginal areas, while the plexuses in aganglionic colons are distributed around the colonic lumens. At least, based on the current evidence, we speculate that innervation inside the remnants is presented in some BA cases, yet we have not elucidated why not all remnants contain plexuses.
Cholinergic innervation was reported to derive from the vagus, with the major biological function of secreting acetylcholine and other neurotransmitters (11). As an important transmitter of the vagus nerve, acetylcholine can directly inhibit the activation of macrophages, thereby effectively reducing the release of a variety of pro-inflammatory factors, and it can act rapidly and persistently on specific tissues. Thus, it has a significant inhibitory effect on local and systemic inflammation (12). This recently discovered anti-inflammatory pathway associated with acetylcholine is known as the cholinergic anti-inflammatory pathway (13). Although the mechanism of cholinergic innervation in biliary remnants of children with BA remains unknown, in some experimental models of chronic hepatobiliary injury, such as the rat cirrhosis model induced by carbon tetrachloride, the cholinergic innervation in the damaged area was reported to be increased (14). Ungvary and Donath observed secondary changes in the distribution of intrahepatic nerves in cavies after ligation of the common bile duct, confirming a significant increase in nerve fibers in the porta hepatis (15). These early studies have shown the increase of innervation at various degrees in different types of liver injury, and they speculated that these changes could be associated with an increase in innate anti-inflammatory activities. It seems that in these above models, innervation is a consequence of inflammation or hepatic injury.
Viral infection is considered one of the possible theories for the pathogenesis of BA in neonates (16). Our serial studies previously showed the importance of viral antigens (17), innate immunity (18), and acquired immunity (19) in the development of experimental BA. Despite having not been fully elucidated, undeniable inflammation existed throughout the pathophysiological processes of BA and postoperative cholangitis, which is the most frequently encountered medical complication after KP. As known, postoperative cholangitis remains a significant challenge. It is closely related to a relatively delayed jaundice clearance (20) and may lead to increased early and late mortalities after KP for BA (21). We have also tried to explore the factors that may influence the onset frequency and severity of cholangitis and its therapeutic effect (6, 22, 23), but to date, doctors are still unable to link the onset of cholangitis and the morphological changes in biliary remnants. Through our current investigation, we claimed that the existence of cholinergic innervation at the transection where we cut the remnants may decrease the early onset of cholangitis and frequencies of recurrent cholangitis after KP.
Although it is yet unclear whether innervation is the consequence of inflammation, which serves as a feedback mechanism of bile duct injury, or the reason for ductule proliferation, which determines the number or area of ductules, a previous study has shown an extensive association between nerves and biliary ductules (24), and some other studies have shown that the activated cholinergic system may regulate ductal bile secretion (25). On the other hand, hepatic vagotomy is believed to inhibit cholangiocyte proliferation after bile duct ligation (26). These studies showed some positive roles of cholinergic innervation in the biliary system, but they did not show any direct relation between innervation and the growth of ductules. Researchers also believe that the loss of parenchymal nerve fibers is a consequence of worsening disease course in hepatic fibrotic diseases (3), and patients with BA lack intrahepatic bile duct innervation (7). Their research studies implicated that biliary innervation may form normally and then degrade as BA progresses. However, we found some connections between innervation and the number of ductules, which was crucial in postoperative bile flow drainage. As shown in Figures 1C,E, neuronal plexuses at the transection were clustered in the margin area of remnants, and the ductules distributed at lateral sides of remnants (6). Although there have been no studies showing mechanisms of how neuronal plexuses promote the hyperplasia of ductules or prevent ductules from damage, we found that the distribution of cholinergic plexuses depends on the number of biliary ductules (positive correlation referring to Figures 3B,C), indicating that the development or say destruction of cholinergic plexuses is subordinate to the capillary bile ducts. Although it is not a direct predictive factor that may give better bile drainage to patients with BA after KP, we need to pay attention to the potential research values of biliary innervation. Therefore, favorable bile flows may flush ductules persistently; thus, it may prevent the occurrence of reflux cholangitis.
Finally, we aimed to analyze the influence of the number/area of neuronal plexuses on the onset of cholangitis in patients with cholinergic innervation. A smaller area of plexuses was correlated to more episodes of cholangitis and a greater possibility of early cholangitis onset (within 1 month). Theoretically, increased cholinergic innervation should mean a potential increase in acetylcholine activity, which plays a potential anti-inflammatory role (13). However, within 3 months, patients with different areas of plexuses showed no statistical difference in the episodes of cholangitis, nor did the time of jaundice clearance between them show any differences. Owing to that, we cannot observe the change in the hepatojejunal anastomosis during the following months after surgery, and we are unable to analyze the change patterns of neuronal plexuses in remnants at the transection in patients after surgery. Nevertheless, we speculate that there may be certain aspects of morphological or functional changes in innervation after surgery, similar to the report by Iwami et al. showing that biliary innervation may form normally and then degrade as BA progresses (7). Thus, after months of bile drainage, innervation at the transection may have become similar in patients who previously had different levels of innervation when undergoing KP. Therefore, the difference in anti-inflammatory effect, which exists in the early days after KP, may no longer exist in these patients several months postoperatively.
Cholinergic innervation at the transection of biliary remnants may help reduce the onset of cholangitis and lead to better and earlier jaundice clearance. Innervation may lead to better bile drainage by affecting the areas of biliary ductules or by increasing acetylcholine activity. Children with larger plexus areas are less likely to have an early episode of cholangitis. Based on our current findings, we believe that it is worth exploring the roles of acetylcholine and its associated anti-inflammatory pathway during the development of BA and in postoperative cholangitis in the future.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Review Board of Tongji Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
JY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. XC: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. WW: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. YS: Data curation, Methodology, Software, Writing – review & editing. KL: Data curation, Formal Analysis, Investigation, Project administration, Writing – review & editing. AA: Data curation, Investigation, Methodology, Writing – review & editing. DL: Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. YH: Formal Analysis, Investigation, Methodology, Project administration, Writing – review & editing. PW: Data curation, Formal Analysis, Investigation, Writing – review & editing. XX: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. JF: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This work was funded by the Natural Science Foundation of Hubei Province (2022CFB134) and National Natural Science Foundation of China (81401240, JY; 81873541, JF).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken). (2008) 291(6):653–60. doi: 10.1002/ar.20664
2. Teratani T, Mikami Y, Nakamoto N, Suzuki T, Harada Y, Okabayashi K, et al. The liver-brain-gut neural arc maintains the Treg cell niche in the gut. Nature. (2020) 585(7826):591–6. doi: 10.1038/s41586-020-2425-3
3. Miller BM, Oderberg IM, Goessling W. Hepatic nervous system in development, regeneration, and disease. Hepatology. (2021) 74(6):3513–22. doi: 10.1002/hep.32055
4. Kelay A, Davenport M. Long-term outlook in biliary atresia. Semin Pediatr Surg. (2017) 26(5):295–300. doi: 10.1053/j.sempedsurg.2017.09.003
5. Kelley-Quon LI, Shue E, Burke RV, Smith Caitlin, Kling K, Mahdi E, et al. The need for early Kasai portoenterostomy: a western pediatric surgery research consortium study. Pediatr Surg Int. (2022) 38(2):193–9. doi: 10.1007/s00383-021-05047-1
6. Yang J, Wei N, Su Y, Wei M, Yi B, Feng J. A morphology-based analysis of biliary ductules after Kasai procedure and a review of the literature. J Surg Res. (2020) 251:180–6. doi: 10.1016/j.jss.2019.11.013
7. Iwami D, Ohi R, Nio M, Shimaoka S, Sano N, Nagura H. Abnormal distribution of nerve fibers in the liver of biliary atresia. Tohoku J Exp Med. (1997) 181(1):57–65. doi: 10.1620/tjem.181.57
8. Hashimoto T, Otobe Y, Shimizu Y, Suzuki T, Nakamura T, Hayashi S, et al. A modification of hepatic portoenterostomy (Kasai operation) for biliary atresia. J Am Coll Surg. (1997) 185(6):548–53. doi: 10.1016/S1072-7515(97)00104-X
9. Li N, Xiang L, Wu X, Yang J, Wei J, Feng J. A rapid lactate dehydrogenase histochemical method for the intraoperative assessment of Hirschsprung’s disease. Int J Colorectal Dis. (2012) 27(9):1175–80. doi: 10.1007/s00384-012-1443-5
10. Akiyoshi H, Gonda T, Terada T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver. (1998) 18(5):352–9. doi: 10.1111/j.1600-0676.1998.tb00817.x
11. Kandilis AN, Koskinas J, Vlachos I, Skaltsas S, Karandrea D, Karakitsos P, et al. Liver regeneration: immunohistochemical study of intrinsic hepatic innervation after partial hepatectomy in rats. BMC Gastroenterol. (2014) 14:202. doi: 10.1186/s12876-014-0202-1
12. Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. (2014) 182:65–9. doi: 10.1016/j.autneu.2013.12.007
13. Alen NV. The cholinergic anti-inflammatory pathway in humans: state-of-the-art review and future directions. Neurosci Biobehav Rev. (2022) 136:104622. doi: 10.1016/j.neubiorev.2022.104622
14. Akiyoshi H, Terada T. Mast cell, myofibroblast and nerve terminal complexes in carbon tetrachloride-induced cirrhotic rat livers. J Hepatol. (1998) 29(1):112–9. doi: 10.1016/S0168-8278(98)80185-2
15. Ungvary G, Donath T. Changes of the peripheral autonomic nervous system in altered internal environment. Z Mikrosk Anat Forsch. (1980) 94(6):985–98.7281881
16. Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. (2007) 27(3):233–42. doi: 10.1055/s-2007-985068
17. Feng J, Yang J, Zheng S, Qiu Y, Chai C. Silencing of the rotavirus NSP4 protein decreases the incidence of biliary atresia in murine model. PLoS One. (2011) 6(8):e23655. doi: 10.1371/journal.pone.0023655
18. Qiu Y, Yang J, Wang W, Zhao W, Peng F, Xiang Y, et al. HMGB1-promoted And TLR2/4-dependent NK cell maturation and activation take part in rotavirus-induced murine biliary atresia. PLoS Pathog. (2014) 10(3):e1004011. doi: 10.1371/journal.ppat.1004011
19. Zheng S, Zhang H, Zhang X, Peng F, Chen X, Yang J, et al. CD8+ T lymphocyte response against extrahepatic biliary epithelium is activated by epitopes within NSP4 in experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol. (2014) 307(2):G233–240. doi: 10.1152/ajpgi.00099.2014
20. Liu J, Dong R, Chen G, Dong K, Zheng S. Risk factors and prognostic effects of cholangitis after Kasai procedure in biliary atresia patients: a retrospective clinical study. J Pediatr Surg. (2019) 54(12):2559–64. doi: 10.1016/j.jpedsurg.2019.08.026
21. Gad EH, Kamel Y, Salem TA, Ali MA, Sallam AN. Short- and long-term outcomes after Kasai operation for type III biliary atresia: twenty years of experience in a single tertiary Egyptian center—a retrospective cohort study. Ann Med Surg (Lond). (2021) 62:302–14. doi: 10.1016/j.amsu.2021.01.052
22. Li D, Chen X, Fu K, Yang J, Feng J. Preoperative nutritional status and its impact on cholangitis after Kasai portoenterostomy in biliary atresia patients. Pediatr Surg Int. (2017) 33(8):901–6. doi: 10.1007/s00383-017-4118-z
23. Li D, Wang P, He Ying, Jiao C, Zhuansun D, Wei N, et al. Intravenous immunoglobulin for the treatment of intractable cholangitis after Kasai portoenterostomy in biliary atresia patients. Pediatr Surg Int. (2018) 34(4):399–404. doi: 10.1007/s00383-018-4240-6
24. Zanchi A, Reidy J, Feldman HJ, Qualter J, Gouw AS, Osbeck J, et al. Innervation of the proximal human biliary tree. Virchows Arch. (2020) 477(3):385–92. doi: 10.1007/s00428-020-02761-4
25. LeSage G, Glaser S, Alpini G. Regulatory mechanisms of ductal bile secretion. Dig Liver Dis. (2000) 32(7):563–6. doi: 10.1016/S1590-8658(00)80836-1
Keywords: cholangitis, biliary ductile, jaundice, acetylcholinesterase, parasympathetic nerve
Citation: Yang J, Chen X, Wang W, Su Y, Liu K, Abudusalamu A, Li D, He Y, Wang P, Xiong X and Feng J (2024) Role of cholinergic innervation in biliary remnants of patients with biliary atresia. Front. Pediatr. 11:1278978. doi: 10.3389/fped.2023.1278978
Received: 17 August 2023; Accepted: 13 December 2023;
Published: 8 January 2024.
Edited by:
Francesco Savino, University Hospital of the City of Health and Science of Turin, ItalyReviewed by:
Jun Fujishiro, The University of Tokyo, Japan© 2024 Yang, Chen, Wang, Su, Liu, Abudusalamu, Li, He, Wang, Xiong and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiexiong Feng MjAwMnRqMDUxNUBodXN0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.