- 1Institute for Central Laboratory, Weihai Central Hospital, Weihai, China
- 2Department of Laboratory Medicine, Weihai Central Hospital, Weihai, China
Objectives: The purpose of the network meta-analysis was to make a more comprehensive comparison of different interleukins in the detection of neonatal sepsis and to pose clues in the field of clinical practice.
Methods: Electronic databases of PubMed, Web of Science and Embase were systematically searched. Eligible studies included diagnostic tests utilizing interleukins to detect neonatal sepsis. We calculated pooled sensitivity, specificity, positive Likelihood Ratio (PLR) and negative Likelihood Ratio (NLR), diagnostic odds ratio (DOR), and superiority index.
Results: Fifteen studies including 1,369 neonates diagnosed of sepsis were included in this meta-analysis. For the detection of early-onset sepsis in neonates, the pooled sensitivity was 0.91 (95% CI: 0.81, 0.97; I2 = 0%, p = 0.946) and the pooled specificity was 0.98 (95% CI: 0.87, 0.97; I2 = 46.3%, p = 0.172) for IL-8. For the detection of late-onset sepsis in neonates. the sensitivity was 0.96 (95% CI: 0.85, 1.00; I2 = NA, p = NA) and the pooled specificity was 1.00 (95% CI: 0.92, 1.00; I2 = NA, p = NA) for IL-27. Results of ANOVA model revealed that the superiority index of IL-6, IL-8, IL-10, and IL-27 were 1.20 (0.14, 5.00), 5.14 (0.33, 7.00), 0.75 (0.14, 5.00), and 1.31 (0.14, 5.00) in the detection of early-onset neonatal sepsis. Superiority index of IL-8, IL-10, and IL-27 were 1.84 (0.20, 5.00), 1.04 (0.20, 5.00), and 2.21 (0.20, 5.00) in the detection of late-onset neonatal sepsis.
Conclusions: Findings of this network meta-analysis suggest that interleukins including IL-6, IL-8, IL-10, and IL-27 may have favorable performance in the detection of neonatal sepsis. IL-8 was more accurate in the detection of early-onset sepsis in neonates. IL-27 was more accurate in the detection of late-onset neonatal sepsis.
Introduction
Sepsis is a life-threatening organ dysfunction associated with a dysregulated body's response to infection, it remains one of the most common causes of deaths in critically ill individuals (1, 2). Sepsis has been recognized as a global health priority in The World Health Assembly and WHO (3, 4) and thereafter became a public issue. The incidence of severe sepsis reached more than 300/100,000 in the United States (5). In addition, it is estimated that approximately 2.8 million deaths are attributable to sepsis in high-resource countries every year. Sepsis is related to most deaths due to chest infections in neonates and infants in Africa and Asia, the morbidity of neonatal sepsis in premature infants and very low birth weight infants is significantly higher, while the mortality rate is inversely proportional to the gestational age, the mortality rate of premature infants or young infants is higher than that of full-term infants (6, 7). Subsequently, early and accurate diagnosis of sepsis plays an important role in successful treatment and improving survival rate of septic patients. However, the signs and symptoms of neonatal sepsis are similar to those with non-infectious inflammation, which brings more difficulties to clinical diagnosis when the source of infection cannot be determined (8, 9).
Blood culture is still the irreplaceable gold standard for sepsis diagnosis, which can identify pathogens and perform antibiotic sensitivity tests to guide the treatment of bacterial infections, although it is a time-consuming program, with a high false negative rate, especially after the use of antibiotics (10). In addition, several biomarkers were used to achieve the early detection of sepsis. Conventional test approaches, including C-reactive protein and procalcitonin, have been proved less inaccurate in detecting sepsis in previous meta-analyses (11, 12). Interestingly, host immune responses including cytokines and chemokines during neonatal sepsis may help to detect and evaluate the severity of sepsis (13). Activation of pathogen recognition receptor (PRR) leads to the production of inflammatory mediators, such as interleukin-11β (IL-1β), IL-6, IL-8, IL-12, IL-18, interferon-γ (INF-γ) and tumor necrosis factor-α (TNF-α) (14). Expression of transforming growth factors-β (TGF-β), IL-4, IL-10, IL-11, IL-13 and other anti-inflammatory cytokines are programed to manipulate and balance inflammation (15). The diagnostic validity of IL-6, IL-8, IL-10 and IL-27 for neonatal sepsis were investigated in several cross-sectional studies and meta-analyses (16–24). Nevertheless, the sample sizes of these studies were relatively limited and results are heterogeneous. We performed this network meta-analysis to compare the performance of different interleukins in the detection of sepsis and to provide potential evidence for future research and clinical practice.
Methods
The meta-analysis was conducted under the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (25). This review was not registered in the Cochrane database. All included studies had declared ethical approvals in the original articles, no ethical approval was necessary for this study. Patients or the public were not involved in any aspect of this study.
Literature search and study selection
We performed a comprehensive search of PubMed, Web of Science, and Embase from inception to 31 January 2023. Only English language was considered. The following key terms were used for the database research: “Interleukin”, “IL”, “neonatal”, “neonate”, “sepsis”, “pyemias”, “pyohemias”, “septicemia”, “septic shock”, “septicemias”, and “poisoning, blood”. The references of related reviews were also screened for any possibly eligible tests. Inclusion criteria were as follows: (1) Different interleukins were used to detect early-onset or late-onset neonatal sepsis; (2) Absolute number of neonates with true positive (TP), false positive (FP), false negative (FN), and true negative (TN) were reported to form a 2 × 2 table or these outcomes could be calculated based on other reported indices; (3) Blood culture was used for the confirmation of neonatal sepsis. Exclusion criteria included: (1) Data to be analyzed cannot be extracted or calculated; (2) Case reports, review, letters, news, conference abstracts, animal studies, and animal experiments; (3) There was duplication or overlap in the research participants. If studies were done by the identical research group, those with the largest sample size or the most detailed data were included. Two independent researchers undertook the literature search and study screening. Disagreements were resolved through discussion.
Data extraction and quality assessments
Two investigators independently performed the title and abstract screening of citations on the basis of the inclusion criteria mentioned above. Then a full-text evaluation of the studies was conducted for the final inclusion. Moreover, the following information of each study included was extracted or calculated: first author's name, year of publication, country of participants, number of participants, cut-off level, type of interleukin, absolute numbers of participants evaluated as TP, FP, TN, FN under interleukin test. The updated quality assessment of diagnostic accuracy studies (QUADAS-2) tool was used as an assessment of methodological quality, study validity, and risk of bias within the study (26). This tool includes a list of probable sources of bias for diagnostic accuracy studies in the context of patient selection, index test, reference standards, flow and timing (26).
Statistical analysis
We used Meta-Disc software (Version 1.4) and the R (Version 4.1.2) for statistical analyses. A p value < 0.05 was considered to be statistically significant. Pooled sensitivity, specificity, positive Likelihood Ratio (PLR) and negative Likelihood Ratio (NLR), diagnostic odds ratio (DOR) and their 95% confidence intervals (CIs) were calculated using the random effects models. The Cochran Q test and the I2 statistics were introduced to qualitatively and quantitatively assess the heterogeneity between included studies. Insignificant, low, moderate, and high heterogeneity were identified if I2 values were 0%–25%, 25%–50%, 50%–75%, and 75%–100%, respectively (27). Furthermore, a Bayesian network meta-analysis (NMA) was performed to compare the diagnostic performance of different interleukins. Posterior estimates of absolute sensitivity, absolute specificity, diagnostic odds ratio, relative sensitivity, relative specificity and their corresponding 95% credible intervals were calculated using the two-way analysis of variance (ANOVA) model (28). Each analysis was based on noninformative priors for effect sizes and precision. Convergence and lack of autocorrelation were confirmed after 2 chains and a 1,000 simulation burn-in phase. Afterwards, direct probability statements were derived from an additional 10,000 simulation phase (29). The superiority of a diagnostic test could be quantified using a superiority (S) index, which ranges from 0 to ∞ with S tending to ∞ and S tending to 0 as the tests is superior and inferior increases, respectively, and S tending to 1 the more the tests are equal (30). Funnel plots were proposed to assess potential bias of publication. The Deeks' method was used to statistically check the asymmetry of the funnel plot and detect publication bias. Besides, we conducted sensitivity analysis to evaluate the impacts of single study on the overall outcomes.
Results
Study selection and characteristics
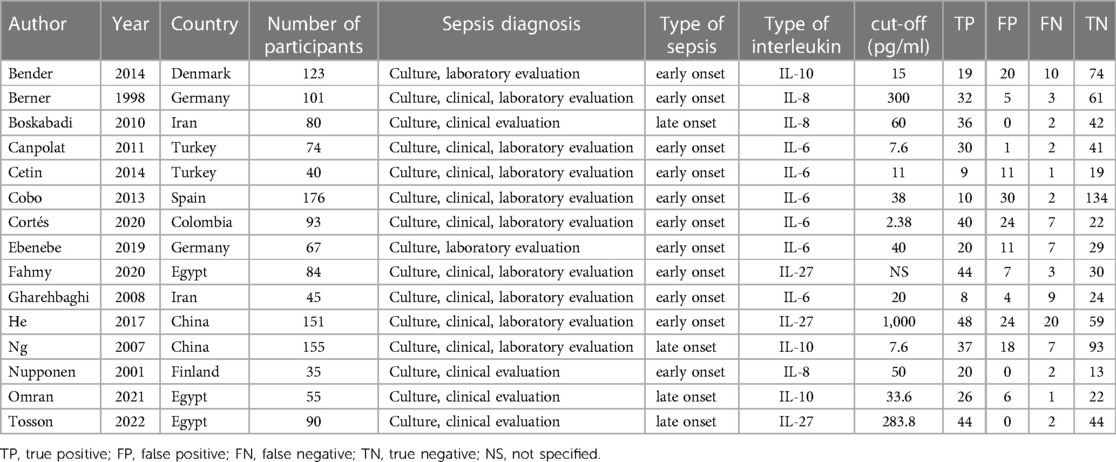
A total of 3,644 articles were identified from the databases search, in which 897 duplicates were removed and 2,747 studies were excluded through an initial screening. Based on the full text assessment for eligibility of the remaining 72 citations, 15 studies with 1,369 neonates were identified for inclusion in this meta-analysis (22, 31–44). No additional studies were found through bibliography screening of the relevant reviews. Eleven enrolled studies were performed among patients with early-onset sepsis, four were in late-onset sepsis (Table 1). Years of publication of included studies ranged from 1998 to 2022. The included studies were done in 9 countries including China, Colombia, Denmark, Egypt, Finland, Germany, Iran, Spain, and Turkey (Table 1). Figure 1 shows the detailed flow of the literature search and study selection. Risk of bias for each included study were rated as low (Figure 2).
Detection performance for early-onset sepsis
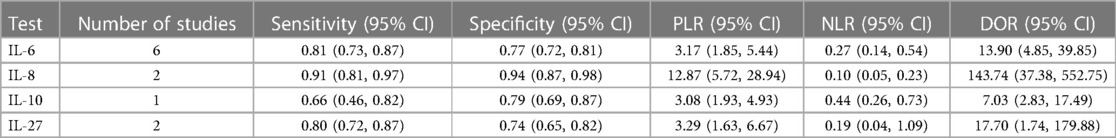
For the detection of early-onset sepsis in neonates. Six studies reported detection outcomes of IL-6 in the detection of sepsis, the pooled sensitivity was 0.81 (95% CI: 0.73, 0.87; I2 = 68.8%, p = 0.007) and the pooled specificity was 0.77 (95% CI: 0.72, 0.81; I2 = 87.5%, p < 0.001), respectively. PLR, NLR, and DOR were 3.17 (95% CI: 1.85, 5.44), 0.27 (95% CI: 0.14, 0.54), and 13.90 (95% CI: 4.85, 39.85) (Table 2A). Two studies reported outcomes of IL-8 in the detection of sepsis, the pooled sensitivity was 0.91 (95% CI: 0.81, 0.97; I2 = 0%, p = 0.946) and the pooled specificity was 0.98 (95% CI: 0.87, 0.97; I2 = 46.3%, p = 0.172), respectively. PLR, NLR, and DOR were 12.87 (95% CI: 5.72, 28.94), 0.10 (95% CI: 0.05, 0.23), and 143.74 (95% CI: 37.38, 552.75) (Table 2A). One study reported outcomes of IL-10 in the detection of sepsis, the sensitivity was 0.66 (95% CI: 0.46, 0.82; I2 = NA, p = NA) and the specificity was 0.79 (95% CI: 0.69, 0.87; I2 = NA, p = NA), respectively. PLR, NLR, and DOR were 3.08 (95% CI: 1.93, 4.93), 0.44 (95% CI: 0.26, 0.73), and 7.03 (95% CI: 2.83, 17.49) (Table 2A). Two studies reported outcomes of IL-27 in the detection of sepsis, the pooled sensitivity was 0.80 (95% CI: 0.72, 0.87; I2 = 94.0%, p = 0.001) and the pooled specificity was 0.74 (95% CI: 0.65, 0.82; I2 = 28.0%, p = 0.239), respectively. PLR, NLR, and DOR were 3.29 (95% CI: 1.63, 6.67), 0.19 (95% CI: 0.04, 1.09), and 17.70 (95% CI: 1.74, 179.88) (Table 2A).
Detection performance for late-onset sepsis
For the detection of late-onset sepsis in neonates. Two studies reported outcomes of IL-8 in the detection of sepsis, the pooled sensitivity was 1.00 (95% CI: 0.94, 1.00; I2 = NA, p = NA) and the pooled specificity was 0.05 (95% CI: 0.01, 0.18; I2 = NA%, p = NA), respectively. PLR, NLR, and DOR were 1.06 (95% CI: 0.97, 1.15), 0.13 (95% CI: 0.01, 2.59), and 8.29 (95% CI: 0.39, 177.47) (Table 2B). Two studies reported outcomes of IL-10 in the detection of sepsis, the pooled sensitivity was 0.89 (95% CI: 0.79, 0.95; I2 = 65.3%, p = 0.090) and the pooled specificity was 0.83 (95% CI: 0.75, 0.89; I2 = 0%, p = 0.523), respectively. PLR, NLR, and DOR were 4.98 (95% CI: 3.42, 7.26), 0.13 (95% CI: 0.04, 0.45), and 34.24 (95% CI: 13.23, 88.64) (Table 2B). One study reported outcomes of IL-27 in the detection of sepsis, the sensitivity was 0.96 (95% CI: 0.85, 1.00; I2 = NA, p = NA) and the pooled specificity was 1.00 (95% CI: 0.92, 1.00; I2 = NA, p = NA), respectively. PLR, NLR, and DOR were 85.21 (95% CI: 5.41, 1,342.6), 0.05 (95% CI: 0.02, 0.18), and 1,584.2 (95% CI: 73.93, 33,945.3) (Table 2B).
ANOVA model for NMA
Results of ANOVA model revealed that the superiority index of IL-6, IL-8, IL-10, and IL-27 were 1.20 (0.14, 5.00), 5.14 (0.33, 7.00), 0.75 (0.14, 5.00), and 1.31 (0.14, 5.00) in the detection of early-onset neonatal sepsis. Superiority index of IL-8, IL-10, and IL-27 were 1.84 (0.20, 5.00), 1.04 (0.20, 5.00), and 2.21 (0.20, 5.00) in the detection of late-onset neonatal sepsis. Results on the posterior estimates and their respective 95% credible intervals are shown in Tables 3A,B.
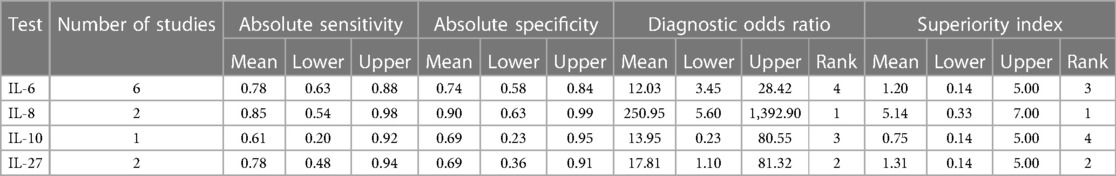
Table 3A. Posterior estimates of detection performance of different interleukins in early-onset neonatal sepsis.
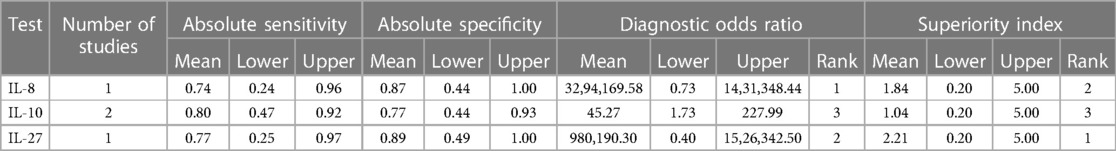
Table 3B. Posterior estimates of detection performance of different interleukins in late-onset neonatal sepsis.
Publication bias
Deeks' funnel plot asymmetry test was not performed because the number of included studies in each meta-analysis was <10.
Sensitivity analysis
The sensitivity analysis was performed to evaluate the impacts of individual study on the overall results. No outlier was identified in all sensitivity analyses.
Discussion
Neonatal sepsis remains one of the leading sources of morbidity and mortality in the neonatal intensive care unit (NICU) (45). Owing to the variable and non-specific signs and symptoms, the diagnosis and treatment of neonatal sepsis is still a challenging task (46). Active management rather than prospective management can be adopted to reduce the possibility of accidental use of antibiotics, treatment costs and over treatment (47).
Previous studies have demonstrated that cytokines such as IL-6, IL-8, IL-10, and IL-27 were biomarkers of neonatal sepsis and their diagnostic properties have been investigated. However, the outcomes of these investigations are heterogeneous. The primary aim of this study was to explore the diagnostic performance of different subtypes of interleukins by pooling the evidence in published articles.
The level of IL-6 in healthy people is very low, generally not more than 7 pg/ml, while the level of IL-6 in serum of septic patients increases rapidly in the early stage of infection, and can reach the peak within 2 h (48). Likewise, IL-8 regulates the migration and activation of leukocytes, and its level is rapidly assessed within 1–3 h after infection, with a half-life of less than 4 h (19). Furthermore, IL-10 is expressed by many innate and adaptive immune response cells, thus it plays an important role in the early diagnosis of sepsis in neonates (49). In recent years, IL-27 has been used as a biological marker for sepsis diagnosis (20, 22, 40, 44). Results of conventional meta-analysis showed that IL-8 manifested the highest pooled sensitivity and specificity in the detection of early-onset sepsis in neonates, which indicates the superior diagnostic properties of IL-8 for neonates as compared to IL-6, IL-8, IL-10. In the detection of late-onset neonatal sepsis, IL-8 showed the highest sensitivity, and IL-27 demonstrated the highest specificity. Moreover, these aforementioned results were consistent with those of the network meta-analysis using the ANOVA model. IL-8 ranked the best in the detection of early-onset sepsis which may be associated with its short half-life (19). The detection performance of IL-27, IL-8, and IL-10 in late-onset sepsis ranked from superior to inferior based on the superiority index. Studies have shown that the effect of IL-27 may be related to the pathogen that infects the bacteria, the host's immune status, and the duration of infection, a better understanding of pathogen specific IL-27 responses during sepsis may have clinical benefits (50). The underlying mechanism needs to be furtherly investigated. However, results of this study may provide evidence for clinical application with regard to the choice of different subtypes of interleukins for the diagnosis of neonatal sepsis. Moreover, it was proved in He's et al. study that the combined use of IL-27 and PCT (AUC = 0.792) revealed greater performance than PCT or IL-27 alone in the detection of neonatal sepsis (40). Zeitoun's et al. study showed that the combination was IL-10 and nCD64 together provided sensitivity of 95% and specificity of 83% in the detection of neonatal sepsis (51). The potential roles of interleukins in combination with other inflammatory markers are promising and need to be further investigated.
This is the first network meta-analysis of detection test accuracy on neonatal sepsis. Compared to conventional meta-analysis, network meta-analysis holds the process of combining and summarizing direct and indirect evidence from independent studies to assess the diagnostic accuracy of different tests for the same disease. Network meta-analysis provides a unified reasoning framework and uses data more effectively. Superiority index was introduced to quantify the superiority of a specific interleukin in the diagnosis of neonatal sepsis. In this study, conventional meta-analysis was also performed, we firstly endeavored to complete a detailed and comprehensive literature research of electronic databases as to retrieve as much related studies as we can. Two independent reviewers screened the titles, abstracts and full-text of the articles and undertook the process of data extraction. In addition, heterogeneity in studies include were assessed. The detection properties of interleukins in the detection of early-onset sepsis and late-onset sepsis were performed. Sensitivity analysis indicated that the pooled outcomes were robust after omitting study one after another in this meta-analysis. We planned to perform subgroup analysis and meta-regression to explore the potential source of heterogeneity in studies enrolled, nevertheless, we were not able to complete these analyses due to the limited number of studies eligible for the inclusion criteria. A meta-analysis based on individual patient data is warranted to further address the issue on the optimal cut-offs of different interleukins in the detection of early-onset or late-onset sepsis. Moreover, although considered the gold standard for detecting sepsis, it is reported that the positive rate of blood culture in infant cases is as low as 3.3% (52), which may cause potential bias in this study.
Conclusion
Interleukins including IL-6, IL-8, IL-10, and IL-27 demonstrated favorable performance in the detection of neonatal sepsis. IL-8 showed the most optimal properties in the diagnosis of early-onset sepsis in neonates as compared to IL-6, IL-8, IL-10. IL-27 revealed the most superior validity in the detection of late-onset sepsis. Evidence on the detection performance of combined biomarkers including interleukins is warranted to pose indications for clinical practitioners.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
WX: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft. YW: Data curation, Formal Analysis, Methodology, Writing – review & editing. JL: Data curation, Formal Analysis, Methodology, Writing – review & editing. JP: Writing – review & editing. CY: Supervision, Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. (2018) 392(10141):75–87. doi: 10.1016/s0140-6736(18)30696-2
2. Dugar S, Choudhary C, Duggal A. Sepsis and septic shock: guideline-based management. Cleve Clin J Med. (2020) 87(1):53–64. doi: 10.3949/ccjm.87a.18143
3. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority - a WHO resolution. N Engl J Med. (2017) 377(5):414–7. doi: 10.1056/NEJMp1707170
4. Kumar V. Sepsis roadmap: what we know, what we learned, and where we are going. Clin Immunol. (2020) 210:108264. doi: 10.1016/j.clim.2019.108264
5. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. (2001) 29(7):1303–10. doi: 10.1097/00003246-200107000-00002
6. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. (2010) 126(3):443–56. doi: 10.1542/peds.2009-2959
7. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. (2015) 385(9966):430–40. doi: 10.1016/s0140-6736(14)61698-6
8. Liu C, Fang C, He Q, Xie L. The value of interleukin-6 (IL-6) within 6 h after birth in the prompt diagnosis of early-onset neonatal sepsis. Transl Pediatr. (2020) 9(5):629–35. doi: 10.21037/tp-20-239
9. Zhang W, Wang W, Hou W, Jiang C, Hu J, Sun L, et al. The diagnostic utility of IL-10, IL-17, and PCT in patients with sepsis infection. Front Public Health. (2022) 10:923457. doi: 10.3389/fpubh.2022.923457
10. Cheng MP, Stenstrom R, Paquette K, Stabler SN, Akhter M, Davidson AC, et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: a diagnostic study. Ann Intern Med. (2019) 171(8):547–54. doi: 10.7326/m19-1696
11. Pontrelli G, De Crescenzo F, Buzzetti R, Jenkner A, Balduzzi S, Calò Carducci F, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: a meta-analysis. BMC Infect Dis. (2017) 17(1):302. doi: 10.1186/s12879-017-2396-7
12. Shabuj KH, Hossain J, Moni SC, Dey SK. C-reactive protein (CRP) as a single biomarker for diagnosis of neonatal sepsis: a comprehensive meta-analysis. Mymensingh Med J. (2017) 26(2):364–71.28588174
13. Celik IH, Hanna M, Canpolat FE, Mohan P. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. (2022) 91(2):337–50. doi: 10.1038/s41390-021-01696-z
14. Cornell TT, Wynn J, Shanley TP, Wheeler DS, Wong HR. Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics. (2010) 125(6):1248–58. doi: 10.1542/peds.2009-3274
15. Sikora JP, Chlebna-Sokół D, Krzyzańska-Oberbek A. Proinflammatory cytokines (IL-6, IL-8), cytokine inhibitors (IL-6sR, sTNFRII) and anti-inflammatory cytokines (IL-10, IL-13) in the pathogenesis of sepsis in newborns and infants. Arch Immunol Ther Exp. (2001) 49(5):399–404.
16. Santana Reyes C, García-Muñoz F, Reyes D, González G, Dominguez C, Domenech E. Role of cytokines (interleukin-1β, 6, 8, tumour necrosis factor-α, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr Int J Paediatr. (2003) 92(2):221–7. doi: 10.1111/j.1651-2227.2003.tb00530.x
17. Hatzidaki E, Gourgiotis D, Manoura A, Korakaki E, Bossios A, Galanakis E, et al. Interleukin-6 in preterm premature rupture of membranes as an indicator of neonatal outcome. Acta Obstet Gynecol Scand. (2005) 84(7):632–8. doi: 10.1111/j.0001-6349.2005.00747.x
18. Sherwin C, Broadbent R, Young S, Worth J, McCaffrey F, Medlicott NJ, et al. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am J Perinatol. (2008) 25(10):629–36. doi: 10.1055/s-0028-1090585
19. Zhou M, Cheng S, Yu J, Lu Q. Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. PLoS One. (2015) 10(5):e0127170. doi: 10.1371/journal.pone.0127170
20. Abo El Magd NM, Abdel Salam SA, Aly YA, Fahim NA. The role of serum interleukin-27 as a diagnostic biomarker for diagnosis of neonatal sepsis. Egypt J Immunol. (2018) 25(2):87–95.30600951
21. Qiu X, Zhang L, Tong Y, Qu Y, Wang H, Mu D. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of the membranes: a meta-analysis. Medicine. (2018) 97(47):e13146. doi: 10.1097/md.0000000000013146
22. Fahmy EM, Kamel NM, Abdelsadik A, Botrous OE, Sheemy MS, Soliman SR, et al. Assessment of interleukin-27 and chemokine RANTES as biomarkers for early onset neonatal sepsis. Egypt J Immunol. (2020) 27(1):9–18.33180383
23. Wang Q, Peng G, Gan L, Deng Z, Zeng L, Deng J. The value of interleukin-10 in the early diagnosis of neonatal sepsis: a meta-analysis. Pediatr Crit Care Med. (2021a) 22(9):e492–501. doi: 10.1097/pcc.0000000000002706
24. Wang Y, Zhao J, Yao Y, Zhao D, Liu S. Interleukin-27 as a diagnostic biomarker for patients with sepsis: a meta-analysis. Biomed Res Int. (2021b) 2021:5516940. doi: 10.1155/2021/5516940
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
26. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
27. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
28. Nyaga VN, Aerts M, Arbyn M. ANOVA model for network meta-analysis of diagnostic test accuracy data. Stat Methods Med Res. (2018) 27(6):1766–84. doi: 10.1177/0962280216669182
29. Tu YK, Needleman I, Chambrone L, Lu HK, Faggion CM Jr. A Bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. J Clin Periodontol. (2012) 39(3):303–14. doi: 10.1111/j.1600-051x.2011.01844.x
30. Deutsch R, Mindt MR, Xu RH, Cherner M, Grant I, Group TH. Quantifying relative superiority among many binary-valued diagnostic tests in the presence of a gold standard. J Data Sci. (2009) 7(2):161–77. doi: 10.6339/JDS.2009.07(2).450
31. Berner R, Niemeyer CM, Leititis JU, Funke A, Schwab C, Rau U, et al. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. (1998) 44(4):469–77. doi: 10.1203/00006450-199810000-00002
32. Nupponen I, Andersson S, Järvenpää AL, Kautiainen H, Repo H. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. (2001) 108(1):E12. doi: 10.1542/peds.108.1.e12
33. Ng PC, Li K, Chui KM, Leung TF, Wong RP, Chu WC, et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. (2007) 61(1):93–8. doi: 10.1203/01.pdr.0000250207.95723.96
34. Bender L, Thaarup J, Varming K, Krarup H, Ellermann-Eriksen S, Ebbesen F. Early and late markers for the detection of early-onset neonatal sepsis. Dan Med Bull. (2008) 55(4):219–23.19232162
35. Gharehbaghi MM, Peirovifar A, Gharehbaghi PM. Comparison of umbilical cord interleukin-6 in preterm infants with premature rupture of membranes and intact membranes. Saudi Med J. (2008) 29(2):224–8.18246231
36. Boskabadi H, Maamouri G, Afshari JT, Ghayour-Mobarhan M, Shakeri MT. Serum interleukin 8 level as a diagnostic marker in late neonatal sepsis. Iran J Pediatr. (2010) 20(1):41–7.23056680
37. Canpolat FE, Yiğit S, Korkmaz A, Yurdakök M, Tekinalp G. Procalcitonin versus CRP as an early indicator of fetal infection in preterm premature rupture of membranes. Turk J Pediatr. (2011) 53(2):180–6.21853656
38. Cobo T, Kacerovsky M, Andrys C, Drahosova M, Musilova I, Hornychova H, et al. Umbilical cord blood IL-6 as predictor of early-onset neonatal sepsis in women with preterm prelabour rupture of membranes. PLoS One. (2013) 8(7):e69341. doi: 10.1371/journal.pone.0069341
39. Cetin O, Dokurel Cetin I, Uludag S, Sen C, Verit FF, Guralp O. Serial ultrasonographic examination of the fetal thymus in the prediction of early neonatal sepsis in preterm premature rupture of membranes. Gynecol Obstet Invest. (2014) 78(3):201–7. doi: 10.1159/000364871
40. He Y, Du WX, Jiang HY, Ai Q, Feng J, Liu Z, et al. Multiplex cytokine profiling identifies interleukin-27 as a novel biomarker for neonatal early onset sepsis. Shock. (2017) 47(2):140–7. doi: 10.1097/shk.0000000000000753
41. Ebenebe CU, Hesse F, Blohm ME, Jung R, Kunzmann S, Singer D. Diagnostic accuracy of interleukin-6 for early-onset sepsis in preterm neonates. J Matern Fetal Neonatal Med. (2019) 34(2):253–8. doi: 10.1080/14767058.2019.1606194
42. Cortés JS, Losada PX, Fernández LX, Beltrán E, DeLaura I, Narváez CF, et al. Interleukin-6 as a biomarker of early-onset neonatal sepsis. Am J Perinatol. (2020) 38(S 01):e338–46. doi: 10.1055/s-0040-1710010
43. Omran A, Sobh H, Abdalla MO, El-Sharkawy S, Rezk AR, Khashana A. Salivary and serum interleukin-10, C-reactive protein, mean platelet volume, and CRP/MPV ratio in the diagnosis of late-onset neonatal sepsis in full-term neonates. J Immunol Res. (2021) 2021:4884537. doi: 10.1155/2021/4884537
44. Tosson AMS, Koptan DMT, Kamal M, Abd Elhady M. Assessment of serum interleukin-27 and mean platelet volume in late-onset neonatal sepsis. Am J Perinatol. (2022). doi: 10.1055/s-0042-1748165
45. Bakhuizen SE, de Haan TR, Teune MJ, van Wassenaer-Leemhuis AG, van der Heyden JL, van der Ham DP, et al. Meta-analysis shows that infants who have suffered neonatal sepsis face an increased risk of mortality and severe complications. Acta Paediatr. (2014) 103(12):1211–8. doi: 10.1111/apa.12764
46. Eichberger J, Resch E, Resch B. Diagnosis of neonatal sepsis: the role of inflammatory markers. Front Pediatr. (2022) 10:840288. doi: 10.3389/fped.2022.840288
47. van Herk W, Stocker M, van Rossum AM. Recognising early onset neonatal sepsis: an essential step in appropriate antimicrobial use. J Infect. (2016) 72(Suppl):S77–82. doi: 10.1016/j.jinf.2016.04.026
48. Wakabayashi A, Sawada K, Nakayama M, Toda A, Kimoto A, Mabuchi S, et al. Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation. Mol Hum Reprod. (2013) 19(11):718–26. doi: 10.1093/molehr/gat057
49. Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. (2012) 129(5):1006–15. doi: 10.1542/peds.2012-0541
50. Morita Y, Masters EA, Schwarz EM, Muthukrishnan G. Interleukin-27 and its diverse effects on bacterial infections. Front Immunol. (2021) 12:678515. doi: 10.3389/fimmu.2021.678515
51. Zeitoun AA, Gad SS, Attia FM, Abu Maziad AS, Bell EF. Evaluation of neutrophilic CD64, interleukin 10 and procalcitonin as diagnostic markers of early- and late-onset neonatal sepsis. Scand J Infect Dis. (2010) 42(4):299–305. doi: 10.3109/00365540903449832
Keywords: interleukins, neonates, sepsis, detection, network meta-analysis
Citation: Xing W, Wang Y, Liu J, Pei J and Yu C (2023) Role of interleukins in the detection of neonatal sepsis: a network meta-analysis. Front. Pediatr. 11:1267777. doi: 10.3389/fped.2023.1267777
Received: 27 July 2023; Accepted: 24 October 2023;
Published: 2 November 2023.
Edited by:
Michael Hermon, Medical University of Vienna, AustriaReviewed by:
Lukas Wisgrill, Medical University of Vienna, AustriaFlorian Kipfmueller, University Hospital Bonn, Germany
© 2023 Xing, Wang, Liu, Pei and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengyong Yu enh5dWNoZW5neW9uZ0BzaW5hLmNvbQ==
 Wei Xing
Wei Xing Ying Wang2
Ying Wang2 Chengyong Yu
Chengyong Yu