- Department of Pediatrics, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Objective: The purpose of this study is to examine the prognostic significance of the amalgamated indicators, reticulocyte percentage (RET%), lactate dehydrogenase (LDH), and γ-Glutamyltransferase (γ-GT), in neonatal ABO hemolytic disease.
Methods: A total of 137 hospitalized children with pathological jaundice were included. Based on their medical conditions, they were categorized into two groups, hemolytic (67 cases) and non-hemolytic (70 cases). Pearson linear correlation and binary logistic multivariate analysis were used to analyze LDH, γ-GT, RET% and hemolysis. Furthermore, the predictive value of the combined predictors of RET%, LDH, and γ-GT on ABO neonatal hemolytic disease was evaluated using the ROC curve analysis.
Results: The laboratory indexes of the two groups were subject to analysis using binary logistic regression to identify suspicious influencing factors. The study revealed that RET%, LDH, and γ-GT were independent risk factors for hemolysis. Pearson linear correlation analysis indicated a positive correlation between LDH and γ-GT with RET% (r = 0.529, P < 0.01; r = 0.526, P = <0.01, respectively). Furthermore, the predictive value of each combined predictor was obtained using the ROC curve, and it was observed that combined predictor L (RET% + LDH + γ-GT)>L1 (RET% + LDH)>L2 (RET% + γ-GT).
Conclusion: Combined predictor L (RET% + LDH + γ-GT)demonstrate its optimal diagnostic efficacy, offering a novel approach towards diagnosing early-onset ABO hemolytic disease of the newborn.
Introduction
Hemolytic disease, which is caused by ABO blood type incompatibility, is a frequently occurring ailment in newborns, particularly those who are type A or B and are born to mothers with blood type O (1). Despite 10% of pregnancies with ABO blood group incompatibility resulting in neonatal hemolytic disease, ABO hemolytic disease accounts for a significant 85.3% of neonatal hemolytic disease, with an incidence rate ranging from 2.23% to 4.55% (2–4). Untimely diagnosis and delayed treatment may result in various symptoms, including jaundice, anemia, and bilirubin encephalopathy. In severe cases, it may lead to hepatosplenomegaly and even more severe heart failure, posing a severe threat to the newborn's life (5). Furthermore, reports have indicated that this ailment may harm the newborn's central system, which can significantly impact their intellectual development (6). Therefore, this ailment necessitates utmost attention.
Currently, the hemolysis three tests (direct antiglobulin test, free antibody test, and antibody release test) are the commonly employed diagnostic method (7, 8). However, this testing method is lacking in many non-pediatric specialized hospitals and grassroots hospitals. Moreover, there are few reports on the routine tests that are correlated with hemolysis. Typically, hemolysis is manifested as mild anemia with normal hemoglobin levels and without liver and spleen enlargement. However, it does lead to a certain degree of hyperbilirubinemia, which can be easily misinterpreted as physiological jaundice. Diagnosis of typical cases is uncomplicated, based on the incompatibility between the blood types of the mother and child, age, elevated bilirubin levels, and decreased hemoglobin levels. Nonetheless, it is worthwhile to explore the utilization of basic routine examinations to accurately diagnose hemolytic disease in newborns, particularly in children with mild anemia in clinical practice.
Several research studies have reported that the determination of reticulocyte (9–12) and lactate dehydrogenase percentages (13–15) is useful in the diagnosis of ABO hemolytic disease in newborns. The γ-Glutamate transferase is also present in the liver, and it has not been reported whether it is also related to the occurrence of neonatal hemolytic disease (16). The potential of combined predictive factors comprising these non-specific detection indicators for accurate diagnosis of ABO hemolytic disease in newborns warrants investigation by clinical practitioners. This study seeks to investigate the value of RET%, LDH, γ-GT, and ABO as combined predictive factors in accurately diagnosing ABO hemolytic disease in newborns, thereby providing a reference basis for clinical diagnosis.
Materials and methods
Population and design of the study
This retrospective case-control study was conducted in the department of pediatrics, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine during the period of June 2015–June 2021. The study was approved by the Ethics Committee of the Shanghai Ninth People's Hospital (SH9H-2021-T404-1, November, 2021).
137 hospitalized neonates with pathologic jaundice were above 37 weeks of gestation and 2.5 kg of birth weight and day ages less than 7 days were divided into hemolytic group (67 cases) and non-hemolytic group (70 cases). The three hemolytic tests are utilized as diagnostic tools for ABO hemolytic disease in newborns. The presence of any of the following three conditions indicates a positive result for the hemolysis test: 1. Positive results for the antibody release test, direct anti-human globulin test, and free antibody test. 2. Positive results for the antibody release test and direct anti-human globulin test, but negative result for the free antibody test. 3. Positive result for either the antibody release test or direct anti-human globulin test, but negative result for the free antibody test (Table 1). Neonates are excluded if they have congenital malformation, metabolic disease, metabolic disorders caused by dehydration and sever infection, hemolytic disease resulting from Rh incompatibility, glucose 6-phosphate dehydrogenase (G6PD) deficiency, low birth weight, anoxia, asphyxia, septicemia, cephalhematoma, or polycythemia vera.
Data collection
Blood routine, reticulocyte percentage, blood type, hemolysis and liver function indexes were performed for 137 hospitalized neonates on admission. The semi-automatic immune turbidimeter, Turbo X-plus kit, provided by Orion company, the XT-2000i full-automatic blood cell analyzer manufactured by Sysmex company, and the cobasintegra 400plus full-automatic biochemical analyzer manufactured by Roche company in Germany were used.
Statistical analysis
All of the data were analyzed using an unpaired Student t test for continuous variables and the χ2 test or Fisher's exact test for categorical variables using SPSS version 25. Pearson linear correlation and binary logistic regression were used to analyze RET%, LDH, γ-GT on the correlation of ABO hemolytic disease of newborn. Taking hemolysis as the state variable, the predictive value of nonspecific laboratory indicators combined with predictors for hemolytic disease of newborn was analyzed by a receiver-operating characteristic (ROC) curve. P < 0.05 was considered statistically significant.
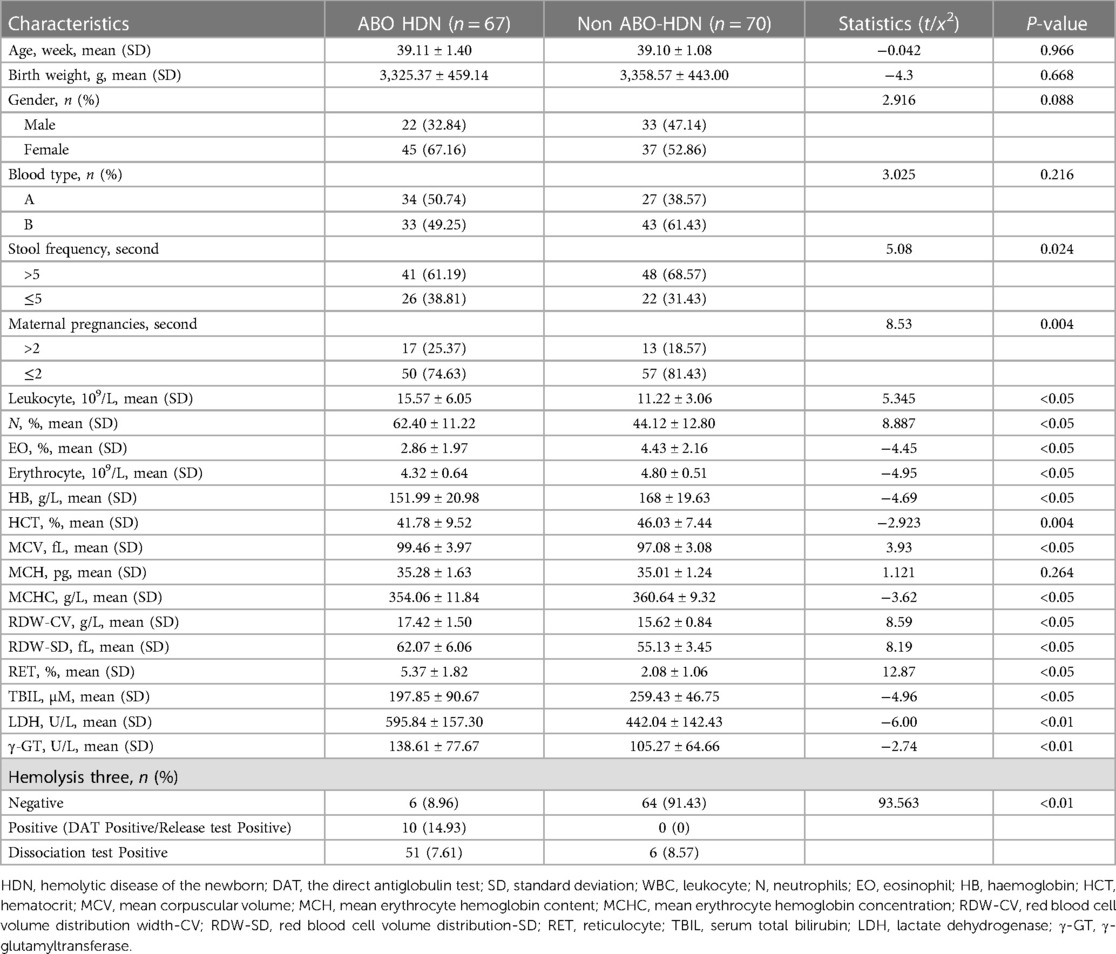
Results
There were 67 neonates in hemolytic group and 70 in non-hemolytic group. There was no significant difference in gestational age, sex (male/female) and birth weight between the two groups (P > 0.05). Notably, significant differences were found in pregnancy times and stool times between the two groups (P < 0.05). Additionally, the data demonstrated that the white blood cell (WBC), neutrocyte (N)%, eosinophilic granulocyte (EO)%, red blood cell (RBC), hemoglobin (Hb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood corpuscular volume distribution width (RDW-CV), red blood corpuscular volume distribution width (RDW-SD), RET, total bilirubin (TBIL), γ-GT, and LDH values were significantly different in the hemolytic group when compared to the non-hemolytic group (P < 0.05). There was a significant difference in the hemolytic three items between the two groups (P < 0.01), indicating that the maternal pregnancy, stool frequency, WBC, N%, EO%, RBC, Hb, HCT, MCV, MCH, MCHC, RDW-CV, RDW-SD and RET%, TBIL, γ-GT, lactate dehydrogenase (LDH) and hemolysis may be potential factors that influence hemolysis of ABO newborns. However, there was no statistical difference in MCH in blood routine between the two groups (P = 0.264, P > 0.05), leading to the conclusion that MCH was not a suspected influencing factor of hemolysis in ABO newborns (Table 2).
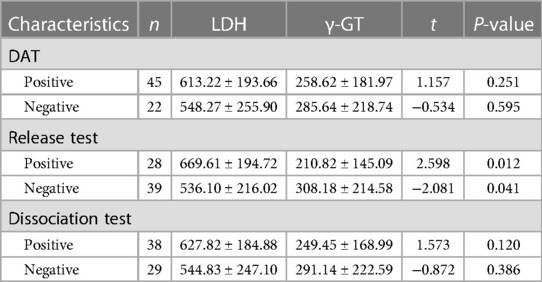
Within the ABO HDN cohort, a statistically significant difference was observed in LDH and γ-GT levels between the positive and negative release test groups across the three hemolysis tests (P < 0.05). In contrast, no statistically significant difference was observed in LDH and γ-GT levels among the three hemolysis tests (P > 0.05). These findings indicate a noteworthy association between higher LDH and γ-GT levels and increased positivity rates of the release test, as evidenced by Table 3.
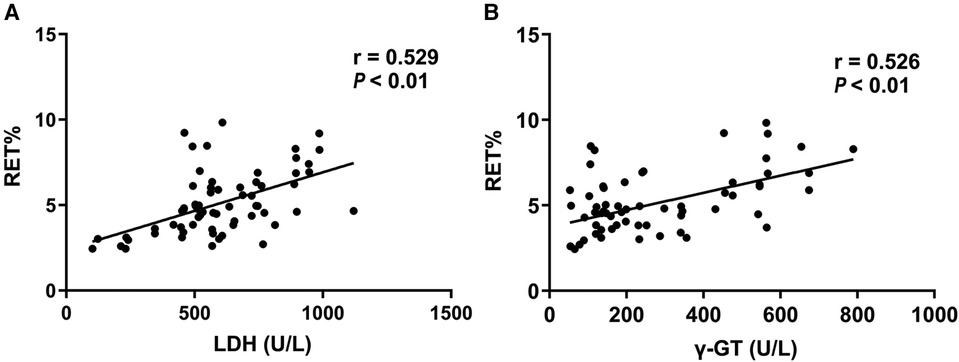
Pearson linear correlation analysis found that the level of LDH and γ-GT of 67 neonates in hemolytic group was linearly and positively correlated with RET% level (Figure 1, r = 0.529, P < 0.01; r = 0.526, P < 0.01).
In this study, the state variable analyzed was ABO hemolytic disease of newborn. Maternal pregnancy times, stool times, and various blood indicators such as WBC, N%, EO%, RBC, Hb, HCT, MCV, MCH, MCHC, RDW-CV, RDW-SD, RET, TBIL, γ-GT, LDH, and hemolysis were examined as independent variables. Binary logistic analysis revealed that RET%, LDH, and γ-GT were identified as independent risk factors for hemolysis (P < 0.05). The analysis further showed that the increase in the probability of hemolysis was associated with significant increases in RET%, LDH, and γ-GT, with an OR of 7.439, 1.006, and 1.019, respectively. To obtain a combined predictor of these variables, we used the formula for calculating variables in the transformation in SPSS. The resulting formulas for the combined predictors were L = RET% + β (LDH)/β (RET%) × LDH + β (γ-GT)/β (RET%) × γ-GT, L1 = RET% + β (LDH)/β (RET%) × LDH, and L2 = RET% + β (γ-GT)/β (RET% × γ-GT (Table 4).

Table 4. Binary logistic multivariate analysis of hemolytic group and non-hemolytic group of ABO newborns.
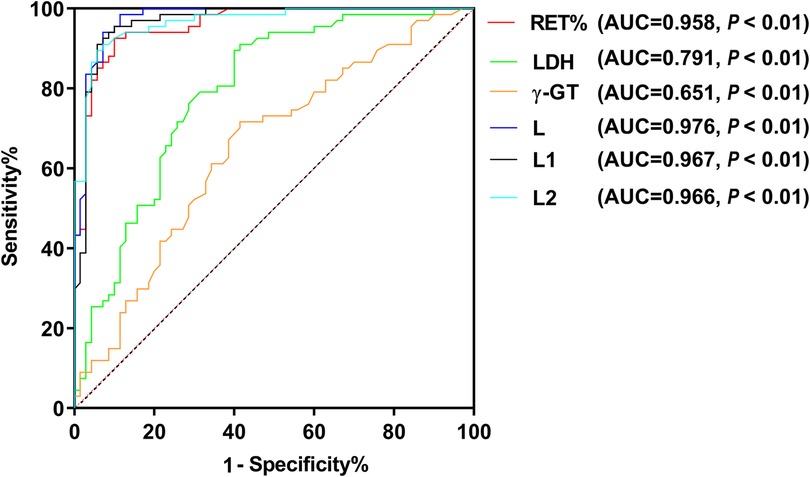
Based on the analysis of ABO hemolytic disease as the outcome variable, in comparison to the non-hemolytic group which served as the reference, the ROC analysis demonstrated that the AUC 95% CI of the combined predictors L, L1, L2 and RET%, LDH, γ-GT were all greater than 0.6. Additionally, the AUC 95% CI of the combined predictors L, L1, L2 were all greater than 0.9. These six variables, L > L1 > L2 > RET% > LDH > γ-GT, have significant value in predicting ABO hemolytic disease of newborn (Figure 2).
Discussion
Within cases of ABO blood group incompatibility between mothers and infants, approximately one-fifth of cases develop ABO hemolytic disease (17). The majority of affected children exhibit no additional abnormalities beyond jaundice. In most cases, jaundice is observed on the second or third day after birth, frequently accompanied by varying degrees of anemia (18). A subset of children may experience late anemia within three to six weeks post-birth as a result of antibody persistence, which can be easily overlooked (19). Furthermore, neonatal liver function often proves insufficient in metabolizing bilirubin, leading to its accumulation in the body. Additional symptoms such as hepatosplenomegaly may also present and can result in brain stem auditory conduction pathway damage, bilirubin encephalopathy, or even death in severe cases (20, 21). Our hospital serves as a neonatal critical care center within Baoshan District, Shanghai. Diagnostic tests for ABO blood group incompatibility are often not available at primary care facilities, leading to overlooked hemoglobin drops which can be misdiagnosed as physiological jaundice. Early detection of hemolysis through non-specific laboratory indicators and prompt treatment can prevent related complications and prove to be of great significance in safeguarding the health of newborns.
Reticulocytes present in the bloodstream of neonates serve as indicators not only of the level of hematopoietic compensation in children but also of the pathological processes that arise from erythrocyte decomposition and heme metabolism during hemolysis (22–24). Extensive research supports the usefulness of reticulocytes in assisting the diagnosis of hemolytic disease in newborns (25). In a study involving the carboxyhemoglobin (COHb) and reticulocyte count of hemolytic and non-hemolytic newborns, the reticulocyte count of the hemolytic group was found to be considerably higher than that of the non-hemolytic group (26). This suggests that hemolysis may be predicted by monitoring the levels of hemoglobin and reticulocytes during the early stages of hemolysis. Furthermore, the RET% was discovered to be an independent risk factor in the incidence of ABO hemolytic disease in newborns. Correspondingly, the probability of hemolysis increased by 7.439 times for every 1% increase in RET%. Based on the ROC curve, the critical value of RET% was established to be 3.015%, with a 92.5% sensitivity and a 90% specificity. The resulting AUC area was 0.958, indicating that when RET% is above 3.015%, the occurrence of hemolysis is more likely. These findings concur with analogous research outcomes obtained both locally and abroad.
The present study retrospectively analyzed 67 infants with ABO hemolytic disease of newborn and compared them with 70 infants in the non-hemolytic group. The results showed that γ-GT levels were significantly higher in the hemolytic group (P < 0.05). Additionally, LDH has been identified as a potential biomarker for evaluating hemolysis occurrence in children since it is widely distributed in all human tissues (27). Our study supports this notion and indicates that LDH was an independent risk factor for ABO hemolytic disease of the newborn, with a β coefficient of 0.006 > 0 or =1.006, indicating that the probability of hemolysis increases by 1.006 times for every 1 unit increase in LDH. Furthermore, the study found that γ-GT was positively correlated with neonatal serum total bilirubin, and it was identified as an independent risk factor for ABO hemolysis in neonates with a β coefficient of 0.019 > 0 or =1.019. Our study also demonstrated that the higher the γ-GT value, the higher the positive rate of the release test in the three hemolysis tests, and the observed difference was statistically significant (P < 0.05). These findings provide valuable insights to aid in clinical decision-making for the management of ABO hemolytic disease of the newborn.
ROC curve analysis indicates that the combination of RET%, LDH, and γ-GT in predicting hemolysis shows a significantly higher value than solely relying on ABO hemolytic disease in newborns or a combination of ABO and the mentioned predictors. Notably, these joint predictors demonstrate a greater predictive value when compared to the joint predictors of RET% + LDH (L1), RET% + γ-GT (L2), and independent predictions. Thus, it is recommended to conduct all three hemolysis tests for newborns suspected of this disease to predict the occurrence of ABO hemolytic disease comprehensively. Newborns with high suspicion of the disease should be promptly transferred to qualified superior hospitals to enhance the treatment success rate.
In conclusion, the percentages of reticulocytes, LDH and γ-GT serve as distinct prognostic indicators for early-onset ABO hemolytic disease in neonates. These biomarkers have been observed to be markedly elevated in affected neonates, and can be effectively utilized as predictors for the onset of ABO hemolytic disease during the early stages. Furthermore, the combination of these three predictors yields the most effective early prediction accuracy, highlighting its importance in the early prevention and treatment of ABO-HDN.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Shanghai Ninth People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the project is designed as a retrospective case-control study, and it will not pose any adverse effects on the participants.
Author contributions
XL: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YD: Data curation, Investigation, Writing – review & editing, Methodology, Software. YQ: Data curation, Investigation, Writing – review & editing. CX: Data curation, Investigation, Writing – review & editing. WL: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Yining He from the Biostatistics Research Laboratory of Shanghai Ninth People's Hospital for help with guiding statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jackson ME, Baker JM. Hemolytic disease of the fetus and newborn: historical and current state. Clin Lab Med. (2021) 41(1):133–51. doi: 10.1016/j.cll.2020.10.009
2. Metcalf RA, Khan J, Andrews J, Mayock D, Billimoria Z, Pagano MB. Severe ABO hemolytic disease of the newborn requiring exchange transfusion. J Pediatr Hematol Oncol. (2019) 41(8):632–4. doi: 10.1097/MPH.0000000000001248
3. Tormey CA, Hendrickson JE. Transfusion-related red blood cell alloantibodies: induction and consequences. Blood. (2019) 133(17):1821–30. doi: 10.1182/blood-2018-08-833962
4. Metcalf RA, Khan J, Andrews J, Mayock D, Billimoria Z, Pagano MB. Severe ABO hemolytic disease of the newborn requiring exchange transfusion. J Pediatr Hemato Oncol. (2019) 41(8):632–4. doi: 10.1097/MPH.0000000000001248
5. van der Geest BAM, de Mol MJS, Barendse ISA, de Graaf JP, Bertens LCM, Poley MJ, et al. Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study. Sci Rep. (2022) 12(1):14385. doi: 10.1038/s41598-022-17933-2
6. Lieberman L, Lopriore E, Baker JM, Bercovitz RS, Christensen RD, Crighton G, et al. International guidelines regarding the role of IVIG in the management of Rh- and ABO-mediated haemolytic disease of the newborn. Br J Haematol. (2022) 198(1):183–95. doi: 10.1111/bjh.18170
7. Watchko JF. ABO Hemolytic disease of the newborn: a need for clarity and consistency in diagnosis. J Perinatol. (2023) 43(2):242–7. doi: 10.1038/s41372-022-01556-6
8. Herschel M, Karrison T, Wen M, Caldarelli L, Baron B. Isoimmunization is unlikely to be the cause of hemolysis in ABO-incompatible but direct antiglobulin test-negative neonates. Pediatrics. (2002) 110(1 Pt 1):127–30. doi: 10.1542/peds.110.1.127
9. Wu Y, Liao L, Lin F. The diagnostic protocol for hereditary spherocytosis-2021 update. J Clin Lab Anal. (2021) 35(12):e24034. doi: 10.1002/jcla.24034
10. Okulu E, Erdeve O, Kilic I, Olukman O, Calkavur S, Buyukkale G, et al. Intravenous immunoglobulin use in hemolytic disease due to ABO incompatibility to prevent exchange transfusion. Front Pediatr. (2022) 10:864609. doi: 10.3389/fped.2022.864609
11. Ree IMC, Lopriore E, Zwiers C, Böhringer S, Janssen MWM, Oepkes D, et al. Suppression of compensatory erythropoiesis in hemolytic disease of the fetus and newborn due to intrauterine transfusions. Am J Obstet Gynecol. (2020) 223(1):119.e1–10. doi: 10.1016/j.ajog.2020.01.028
12. de Castro C, Grossi F, Weitz IC, Maciejewski J, Sharma V, Roman E, et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am J Hematol. (2020) 95(11):1334–43. doi: 10.1002/ajh.25960
13. Röth A, Fryzek J, Jiang X, Reichert H, Patel P, Su J, et al. Complement-mediated hemolysis persists year round in patients with cold agglutinin disease. Transfusion. (2022) 62(1):51–9. doi: 10.1111/trf.16745
14. Pham HP, Wilson A, Adeyemi A, Miles G, Kuang K, Carita P, et al. An observational analysis of disease burden in patients with cold agglutinin disease: results from a large US electronic health record database. J Manag Care Spec Pharm. (2022) 28(12):1419–28. doi: 10.18553/jmcp.2022.28.12.1419
15. Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. (2020) 7(6):1287–96. doi: 10.1016/j.kint.2020.01.035
16. Mohapatra E, Priya R, Nanda R, Patel S. Serum GGT and serum ferritin as early markers for metabolic syndrome. J Family Med Prim Care. (2020) 9(7):3458–63. doi: 10.4103/jfmpc.jfmpc_570_20
17. Páez M, Jiménez M, Corredor A. Hemolytic disease in fetuses and newborns due to antibodies against the M-antigen. Biomedica. (2021) 41(4):643–50. doi: 10.7705/biomedica.5930
18. Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med. (2017) 78(12):699–704. doi: 10.12968/hmed.2017.78.12.699
19. Dziegiel MH, Krog GR, Hansen AT, Olsen M, Lausen B, Nørgaard LN, et al. Laboratory monitoring of mother, fetus, and newborn in hemolytic disease of fetus and newborn. Transfus Med Hemother. (2021) 48(5):306–15. doi: 10.1159/000518782
20. Christensen RD, Baer VL, MacQueen BC, O'Brien EA, Ilstrup SJ. ABO hemolytic disease of the fetus and newborn: thirteen years of data after implementing a universal bilirubin screening and management program. J Perinatol. (2018) 38(5):517–25. doi: 10.1038/s41372-018-0048-4
21. Slaughter JL, Kemper AR, Newman TB. Technical report: diagnosis and management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. (2022) 150(3):e2022058865. doi: 10.1542/peds.2022-058865
22. Howard J, Ataga KI, Brown RC, Achebe M, Nduba V, El-Beshlawy A, et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. (2021) 8(5):e323–33. doi: 10.1016/S2352-3026(21)00059-4
23. Christensen RD, Yaish HM, Henry E, Bennett ST. Red blood cell distribution width: reference intervals for neonates. J Matern Fetal Neonatal Med. (2015) 28(8):883–8. doi: 10.3109/14767058.2014.938044
24. Delesderrier E, Curioni C, Omena J, Macedo CR, Cople-Rodrigues C, Citelli M. Antioxidant nutrients and hemolysis in sickle cell disease. Clin Chim Acta. (2020) 510:381–90. doi: 10.1016/j.cca.2020.07.020
25. Sarici SU, Yurdakök M, Serdar MA, Oran O, Erdem G, Tekinalp G, et al. An early (sixth-hour) serum bilirubin measurement is useful in predicting the development of significant hyperbilirubinemia and severe ABO hemolytic disease in a selective high-risk population of newborns with ABO incompatibility. Pediatrics. (2002) 109(4):e53. doi: 10.1542/peds.109.4.e53
26. Hayde M, Widness JA, Pollak A, Kohlhauser-Vollmuth C, Vreman HJ, Stevenson DK. Rhesus isoimmunization: increased hemolysis during early infancy. Pediatr Res. (1997) 41(5):716–21. doi: 10.1203/00006450-199705000-00018
Keywords: combined predictor, ABO hemolytic disease, newborn, lactate dehydrogenase (LDH), γ-glutamyltransferase (γ-GT)
Citation: Liu X, Dong Y, Qin Y, Xue C and Lyu W (2023) Clinical value of combined predictors of RET%, γ-GT, LDH in the ABO neonatal hemolytic disease. Front. Pediatr. 11:1265739. doi: 10.3389/fped.2023.1265739
Received: 23 July 2023; Accepted: 17 November 2023;
Published: 1 December 2023.
Edited by:
Fahri Ovalı, Istanbul Medeniyet University, TürkiyeReviewed by:
Debasish Mishra, All India Institute of Medical Sciences, IndiaSuman Routray, Kalinga Institute of Medical Sciences (KIMS), India
© 2023 Liu, Dong, Qin, Xue and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Lyu bHZhbmR5eWJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaoxiao Liu
Xiaoxiao Liu Yan Dong†
Yan Dong† Wei Lyu
Wei Lyu