- Department of the Cardiovascular Surgery, The Second Xiangya Hospital, Central South University, Changsha, China
Background: Tuberous Sclerosis Complex (TSC) is a hereditary condition that leads to the development of non-malignant neoplasms in various organs, including cardiac rhabdomyomas, which can cause significant complications.
Case presentation: This report describes the case of a 15-day-old male neonate who was hospitalized due to intracardiac masses and brain lesions, despite the absence of TSC gene mutations. The patient's mother exhibited facial angiofibromas, a common feature of TSC. Over a 2-year follow-up period, spontaneous regression of the cardiac tumor was observed.
Conclusions: This case illustrates that not all TSC cases exhibit detectable TSC gene mutations. Current treatment strategies, such as mTOR inhibitors, offer potential effectiveness in managing associated cardiac rhabdomyomas. Further research should focus on evaluating the therapeutic potential of these inhibitors.
Background
Tuberous sclerosis complex (TSC) is a rare and complex genetic disorder caused by mutations in the TSC1 or TSC2 genes (1). These genetic changes result in continuous hyperactivation of the mechanistic target of rapamycin (mTOR) pathway, leading to uncontrolled cell growth and proliferation (2). TSC manifests as hamartomas, benign growths, in various organs, including the heart, where they are referred to as cardiac rhabdomyomas (3).
Cardiac rhabdomyomas are the most common primary cardiac tumors in children, accounting for 45% of cases (4, 5). There is a strong association between cardiac rhabdomyomas and TSC, with approximately 70%–90% of children with cardiac rhabdomyomas also being diagnosed with TSC. Conversely, around 90% of TSC patients under the age of two exhibit either single or multiple cardiac rhabdomyomas (4, 6).
Although rhabdomyomas are typically benign, they can lead to serious complications in some cases, including arrhythmias and obstruction of heart chambers, resulting in heart failure (7). While most of these tumors regress naturally within the first few years of life, surgical intervention may be necessary for life-threatening hemodynamic complications (8). However, congenital cardiac rhabdomyomas are of particular interest due to the spontaneous regression observed in more than half of the cases (9).
In addition to the heart, TSC has a significant impact on the central nervous system, causing various structural and functional abnormalities. Structural manifestations include cortical tubers and subependymal nodules, while functional implications encompass seizures, cognitive impairment, and behavioral alterations (10). These cerebral abnormalities are typically detected using magnetic resonance imaging (11).
In addition to the heart and brain, TSC affects the skin, kidneys, lungs, and eyes, resulting in a wide range of phenotypic manifestations of varying severity (12). Skin features, in particular, are a common major feature, occurring in 70% of TSC patients (13).
Case presentation
This report presents the case of a 15-day-old male neonate with intracardiac masses. The neonate's initial medical history, including family history of cardiovascular disease, did not reveal any significant findings. On physical examination, a pale solid plaque measuring 2 × 2 cm was observed on the posterior lateral side of the neonate's right lower leg (Figure 1A). The patient's mother exhibited facial angiofibromas on both malar prominences and in the nasolabial folds (Figure 1B). Transthoracic echocardiography of the neonate confirmed the presence of multiple neoplasms within the interatrial septum, interventricular septum, right ventricular outflow tract, and left ventricle posterior wall (Figures 2A–D, see Supplementary Video S1). The largest nodule, measuring approximately 31 × 14 mm, was located in the inferolateral wall of the left ventricle, extending from the level of the mitral valve to the papillary muscle, with unclear borders and protrusion towards the pericardium (Figure 2C). Three-dimensional echocardiography (Figure 2E, see Supplementary Video S1) and Three-dimensional cardiac computed tomography angiography (Figure 2F, see Supplementary Video S2) further confirmed the presence of multiple tumorous masses in the heart. Three-dimensional cerebral computed tomography angiography revealed multiple nodular, high-density shadows with distinct borders in various regions, including the left fronto-parietal lobe, right frontotemporal lobe, and bilateral subependyma of the lateral ventricles. A prominent lesion measuring approximately 1.2 × 1.4 cm was observed adjacent to the frontal horn of the right lateral ventricle (Figure 3, see Supplementary Video S3). The 2018 World TSC Conference, hosted in Dallas, Texas, USA, introduced revisions to the clinical diagnostic criteria for TSC, as illustrated in Supplementary Table S1 (14). Based on the shagreen patche (Major Criteria 4), cardiac rhabdomyomas (Major Criteria 9) and multiple cortical tubers (Major Criteria 6), a confident diagnosis of TSC was established for this case, despite the absence of mutations in both the TSC1 and TSC2 genes (59 genes in total, with primers provided in Supplementary Table S2) of the neonate. As there was no significant influence on hemodynamics, close monitoring was recommended instead of open-heart surgery. During the 2-year follow-up period, the cardiac masses remained undetected, and the patient remained asymptomatic. No intellectual disability was detected.
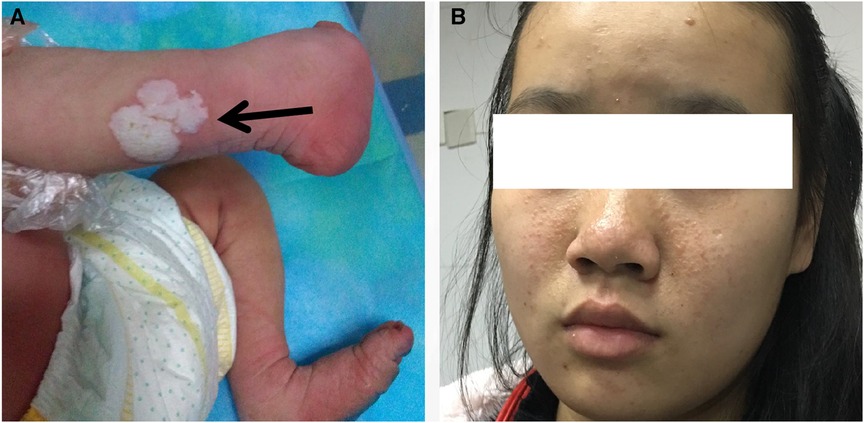
Figure 1. Images of the patient and the patient's mother. (A) On the posterior lateral side of the patient's right lower leg, a pale solid plaque measuring 2 × 2 cm in size was observed. (B) The patient's mother presented with facial angiofibromas on both malar prominences and in the nasolabial folds.
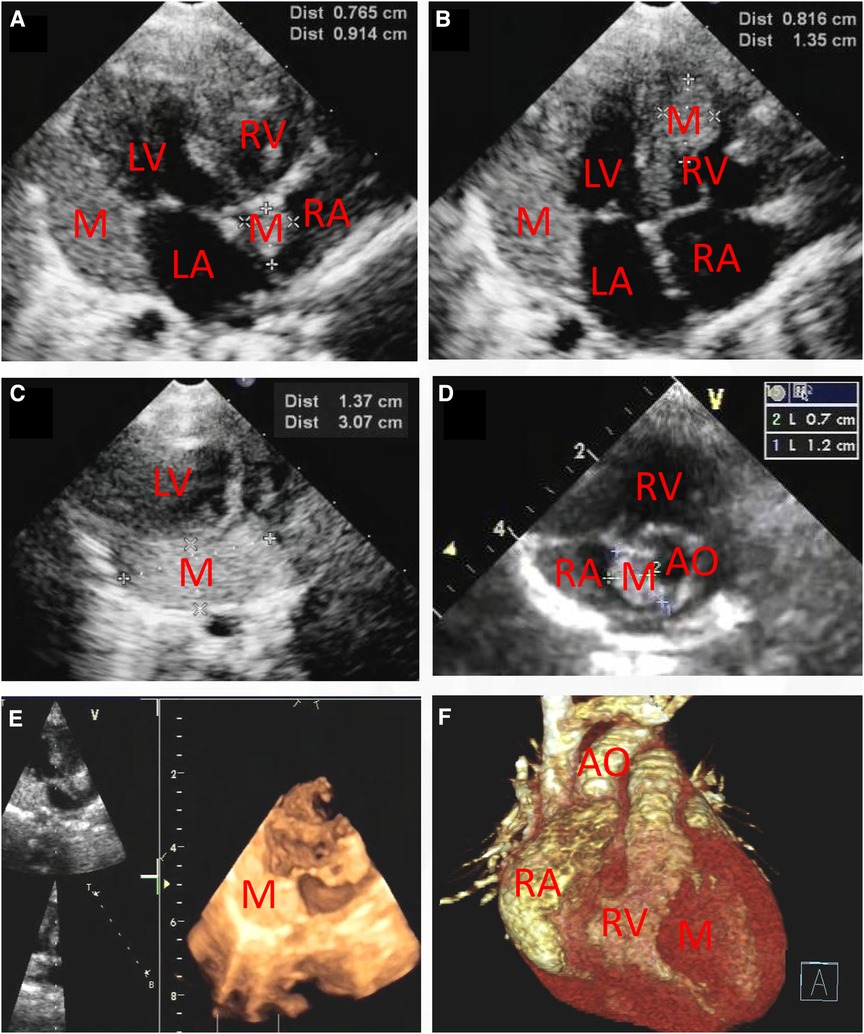
Figure 2. Transthoracic echocardiography (A–D) showing that there are multiple hyperechogenic masses within the atrial septum (0.765 cm × 0.914 cm and 0.7 cm × 1.2 cm), the interventricular septum (0.816 cm × 1.35 cm) and the inferolateral wall of the left ventricle (1.37 cm × 3.07 cm). Three-dimensional echocardiography (E) and Three-dimensional cardiac computed tomography angiography (F) confirmed the detection of cardiac multiple masses. M, mass; RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; AO, ascending aorta.
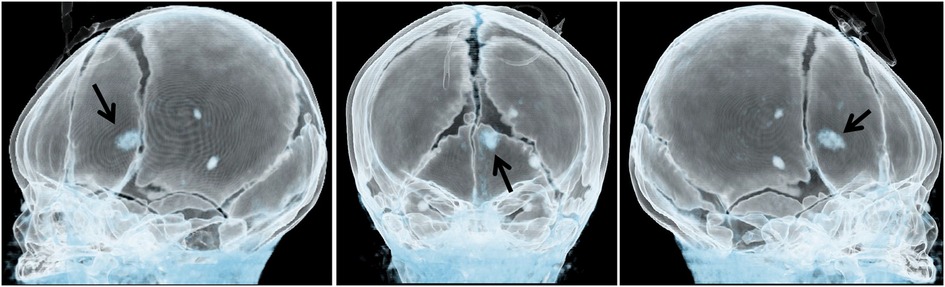
Figure 3. Three-dimensional cerebral computed tomography angiography showing multiple nodular high-density shadows with distinct borders. These shadows were distributed across several regions, including the left fronto-parietal lobe, right frontotemporal lobe, and bilateral subependyma of the lateral ventricles. A prominent lesion, measuring approximately 1.2 × 1.4 cm, was observed adjacent to the frontal horn of the right lateral ventricle (arrow).
Discussion
Primary pediatric cardiac tumors are predominantly benign, with rhabdomyoma, fibroma, and teratoma being the most common types (15, 16). The association between rhabdomyoma and TSC, an autosomal dominant neuroectodermal disorder, is particularly strong, affecting approximately 1 in 5,000–10,000 live births (1). TSC and related rhabdomyomas result from mutations in the tumor suppressor genes TSC1 or TSC2, leading to a deficiency of hamartin or tuberin proteins (17). The loss of these proteins dysregulates the mTOR pathway, promoting uncontrolled cell proliferation and contributing to the development of hamartomatous growth in multiple organs (2).
Cardiac rhabdomyomas are generally benign and tend to regress naturally (4). The regression of these tumors, particularly in younger patients and smaller-sized tumors, is attributed to the withdrawal of maternal estrogen post-birth (18). The decline in maternal hormone support leads to the degradation of myofilaments, cytoplasmic glycogen vacuolization, and apoptosis, resulting in tumor regression (18). Therefore, asymptomatic rhabdomyomas often require only close surveillance (19). Clinical intervention is only necessary in cases of hemodynamic impairment and intractable arrhythmia (19).
Genetic testing for TSC is recommended for patients diagnosed with multiple cardiac rhabdomyomas or those exhibiting potential rhabdomyomas, as well as their family members, to evaluate familial or sporadic occurrence (20). However, genetic testing is not mandatory for TSC diagnosis, as approximately 10%–15% of clinically defined TSC cases lack detectable TSC1 or TSC2 mutations (20), as observed in this case report. De novo mutation other than TSC1 or TSC2 mutations may be the reason. Next-generation sequencing (NGS) is now preferred over previous DNA detection techniques due to its improved sensitivity and diagnostic yield (21). The patient's parent declined further diagnostic procedures, including a skin biopsy, as they believed that the diagnosis could be made through observation.
Regarding treatment options, mTOR inhibitors have shown promise in managing various tumor types associated with TSC, such as refractory epilepsy, renal angiomyolipomas, pulmonary lymphangioleiomyomatosis, and facial angiofibromas (22–25). Current research primarily focuses on targeted therapies that aim to inhibit specific pathways involved in tumorigenesis, including the epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), mTOR, and vascular endothelial growth factor (VEGF) pathways. These targeted agents have demonstrated promising evidence of disease activity (21). For example, imatinib, a tyrosine-kinase inhibitor, has shown potential in clinical trials and is being studied in combination with everolimus for potential synergistic effects (26).
The upcoming randomized trial (ORACLE) holds the promise of providing more robust and evidence-based outcomes regarding the effectiveness of mTOR inhibitors in the treatment of cardiac rhabdomyomas in children with TSC (27). This trial aims to provide valuable insights into the therapeutic potential of mTOR inhibitors and further advance our understanding of their role in managing cardiac rhabdomyomas associated with TSC (27).
Conclusion
The presented case highlights that cardiac rhabdomyomas are part of TSC, and surgical removal is not always necessary. Close monitoring is the recommended approach for asymptomatic cardiac rhabdomyomas due to their characteristic of spontaneous regression.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
HZ: Data curation, Writing – original draft. ZZ: Data curation, Writing – review & editing. ZT: Writing – review & editing. YL: Writing – review & editing. JY: Writing – review & editing. CF: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1263631/full#supplementary-material
Supplementary Video S1
Transthoracic echocardiography and three-dimensional echocardiography showing multiple cardiac masses.
Supplementary Video S2
Three-dimensional cardiac computed tomography angiography showing multiple cardiac masses.
Supplementary Video S3
Three-dimensional cerebral computed tomography angiography showing multiple nodular high-density shadows with distinct borders.
References
1. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. (2008) 372:657–68. doi: 10.1016/S0140-6736(08)61279-9
2. Peron A, Au KS, Northrup H. Genetics, genomics, and genotype-phenotype correlations of TSC: insights for clinical practice. Am J Med Genet C Semin Med Genet. (2018) 178:281–90. doi: 10.1002/ajmg.c.31651
3. Bosi G, Lintermans JP, Pellegrino PA, Svaluto-Moreolo G, Vliers A. The natural history of cardiac rhabdomyoma with and without tuberous sclerosis. Acta Paediatr. (1996) 85:928–31. doi: 10.1111/j.1651-2227.1996.tb14188.x
4. Hinton RB, Prakash A, Romp RL, Krueger DA, Knilans TK. Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the international tuberous sclerosis consensus group. J Am Heart Assoc. (2014) 3:e001493. doi: 10.1161/JAHA.114.001493
5. Chepurnenko SA, Nasytko AD, Shavkuta GV. Asymptomatic course of rhabdomyoma of the heart. Kardiologiia. (2023) 63:68–72. doi: 10.18087/cardio.2023.8.n1821
6. Davis PE, Filip-Dhima R, Sideridis G, Peters JM, Au KS, Northrup H, et al. Presentation and diagnosis of tuberous sclerosis Complex in infants. Pediatrics. (2017) 140(6):e20164040. doi: 10.1542/peds.2016-4040
7. Castro-Monsalve J, Alvarado-Socarras JL, Mantilla KA, Forero L, Moreno A, Prada CE. Cardiac rhabdomyomas in tuberous sclerosis complex. J Pediatr. (2018) 192:264–264.e1. doi: 10.1016/j.jpeds.2017.09.050
8. Gamboa VRL, Giovo M, Francucci V. Cardiac rhabdomyomas as prenatal diagnostic markers of tuberous sclerosis complex. An Bras Dermatol. (2023):S0365-0596(23)00144-7. doi: 10.1016/j.abd.2021.10.019. [Epub ahead of print].37481376
9. Myers KA, Wong KK, Tipple M, Sanatani S. Benign cardiac tumours, malignant arrhythmias. Can J Cardiol. (2010) 26:e58–61. doi: 10.1016/S0828-282X(10)70009-X
10. Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 iinternational tuberous sclerosis complex consensus conference. Pediatr Neurol. (2013) 49:243–54. doi: 10.1016/j.pediatrneurol.2013.08.001
11. Coban G, Gumeler E, Parlak S, Konuskan B, Karakaya J, Yalnizoglu D, et al. Synthetic MRI in children with tuberous sclerosis complex. Insights Imaging. (2022) 13:115. doi: 10.1186/s13244-022-01219-2
12. Tiberio D, Franz DN, Phillips JR. Regression of a cardiac rhabdomyoma in a patient receiving everolimus. Pediatrics. (2011) 127:e1335–7. doi: 10.1542/peds.2010-2910
13. Alshoabi SA, Hamid AM, Alhazmi FH, Qurashi AA, Abdulaal OM, Aloufi KM, et al. Diagnostic features of tuberous sclerosis complex: case report and literature review. Quant Imaging Med Surg. (2022) 12(1):846–61. doi: 10.21037/qims-21-412
14. Northrup H, Aronow ME, Bebin EM, Bissler J, Darling TN, de Vries PJ, et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr Neurol. (2021) 123:50–66. doi: 10.1016/j.pediatrneurol.2021.07.011
15. Isaacs H Jr. Fetal and neonatal cardiac tumors. Pediatr Cardiol. (2004) 25:252–73. doi: 10.1007/s00246-003-0590-4
16. Fan C, Gao Z, Yin NI, Yang J. Surgical treatment of primary cardiac tumors in children: experience of a single institute. Oncol Lett. (2015) 10:2071–4. doi: 10.3892/ol.2015.3529
17. Henske EP, Jóźwiak S, Kingswood JC, Sampson JR, Thiele EA. Tuberous sclerosis complex. Nat Rev Dis Primers. (2016) 2:16035. doi: 10.1038/nrdp.2016.35
18. Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss & Adams’ heart disease in infants, children, and adolescents: Including the fetus and young adult. Philadelphia, PA: Lippincott Williams & Wilkins (2013).
19. Mohammed F, Tan GC, Hor KN, Arnold M, Wong YP. A case of surgically resected cardiac rhabdomyoma with progressive left ventricular outflow tract obstruction. Cardiovasc Pathol. (2020) 49:107226. doi: 10.1016/j.carpath.2020.107226
20. Curatolo P, Specchio N, Aronica E. Advances in the genetics and neuropathology of tuberous sclerosis complex: edging closer to targeted therapy. Lancet Neurol. (2022) 21:843–56. doi: 10.1016/S1474-4422(22)00213-7
21. Lam HC, Nijmeh J, Henske EP. New developments in the genetics and pathogenesis of tumours in tuberous sclerosis complex. J Pathol. (2017) 241:219–25. doi: 10.1002/path.4827
22. French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. (2016) 388:2153–63. doi: 10.1016/S0140-6736(16)31419-2
23. Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. (2013) 381:125–32. doi: 10.1016/S0140-6736(12)61134-9
24. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2013) 381:817–24. doi: 10.1016/S0140-6736(12)61767-X
25. McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. (2011) 364:1595–606. doi: 10.1056/NEJMoa1100391
26. Di Maio S, Yip S, Al Zhrani GA, Alotaibi FE, Al Turki A, Kong E, et al. Novel targeted therapies in chordoma: an update. Ther Clin Risk Manag. (2015) 11:873–83. doi: 10.2147/TCRM.S50526
27. Stelmaszewski EV, Parente DB, Farina A, Stein A, Gutierrez A, Raquelo-Menegassio AF, et al. Everolimus for cardiac rhabdomyomas in children with tuberous sclerosis. The ORACLE study protocol (everOlimus for caRdiac rhAbdomyomas in tuberous sCLErosis): a randomised, multicentre, placebo-controlled, double-blind phase II trial. Cardiol Young. (2020) 30:337–45. doi: 10.1017/S1047951119003147
Keywords: tuberous sclerosis complex (TSC), cardiac tumor, rhabdomyomas, subependymal nodules, mTOR inhibitor
Citation: Zhou H, Zheng Z, Tu Z, Li Y, Yang J and Fan C (2023) Cardiac self-limiting rhabdomyomas in a neonatal patient with tuberous sclerosis complex: a case report with negative genetic testing. Front. Pediatr. 11:1263631. doi: 10.3389/fped.2023.1263631
Received: 20 July 2023; Accepted: 27 September 2023;
Published: 10 October 2023.
Edited by:
Shuai Ren, Affiliated Hospital of Nanjing University of Chinese Medicine, ChinaReviewed by:
Chuan Wang, Capital Medical University, ChinaXingli Xu, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, China
Zhiguo Zou, Shanghai Jiao Tong University, China
© 2023 Zhou, Zheng, Tu, Li, Yang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengming Fan ZmFuY2hlbmdtaW5nQGNzdS5lZHUuY24=
Abbreviations TSC, tuberous sclerosis complex; mTOR, mechanistic target of rapamycin; LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle; AO, aorta; NGS, next-generation sequencing; EGFR, epidermal growth factor receptor; PDGFR, platelet-derived growth factor receptor; VEGF, vascular endothelial growth factor.
 Huatao Zhou
Huatao Zhou Zilong Zheng
Zilong Zheng Zhi Tu
Zhi Tu Yichen Li
Yichen Li Jinfu Yang
Jinfu Yang Chengming Fan
Chengming Fan