- 1Pediatric Intensive Care Unit, Le Bonheur Children’s Hospital, Memphis, TN, United States
- 2Division of Pediatric Critical Care Medicine, University of Tennessee Health Science Center, Memphis, TN, United States
Introduction: The type of vascular access (central or peripheral) in pediatric critical care depends on several factors, including the duration of treatment, the properties of the medication (osmolarity or vesicant), and the need for central pressure monitoring. The utilization of peripheral intravascular catheters (PIVCs) has shown a notable increase in the number of patients being treated. Extended dwell or midline catheters are another peripheral access option in addition to PIVCs. However, there are currently no established guidelines on their placement.
Objectives: The aim of this study is to estimate the duration of dwell time for PIVCs, analyze the specific parameters affecting it, and develop recommendations for switching to extended dwell and midline catheter placement as an alternative to peripheral access.
Methods: The study enrolled patients aged 0–18 years admitted to the pediatric intensive care unit (PICU) for over 24 h and managed with peripheral access only over 2 years (2019–2021).
Measurements and main results: A total of 484 patients met the specified criteria. Patients who had peripheral access exhibited a lower PRISM score and a shorter length of stay in the PICU, with mean values of 18 (SD: 8.5) and 9.5 (SD: 6.4) days, respectively, compared with patients who had central access with mean values of 8.9 (SD: 5.9) and 5.7 (SD: 3.6) days, respectively. The PIVC dwell time was found to be 50.1 h (SD: 65.3) and required an average of 1.6 insertion attempts. Patients with three or more insertions exhibited an increased odds ratio of 5.2 (95% CI: 3.1–8.5) for receiving an extended dwell or midline insertion. Increased dwell time was associated with female gender, 59.5 h (P < 0.001), first attempt insertion, 53.5 h (P < 0.001), use of 24 Ga bore, 56.3 h (P = 0.04), left-sided insertions, 54.9 (P = 0.07), less agitation, 54.8 h (P = 0.02), and less edema, 61.6 (P < 0.001). Decreased dwell time was associated with the use of vancomycin infusion at 24.2 h (P < 0.001) and blood transfusions at 29.3 h (P < 0.001).
Conclusions: Extended catheters last longer than PIVCs in PICU patients. Extended catheter placement requires consideration of the length of treatment, as well as the overall body edema, the level of the patient's restlessness, and the need for vancomycin infusion or blood transfusions, as these factors reduce PIVC dwell time and expose the patients to painful insertions. For such cases, an extended dwell catheter may be a better option, even if the projected treatment time is less than 6 days.
Introduction
Majority of the patients in pediatric critical care require a form of vascular access (VA). In contemporary healthcare settings, the insertion of peripheral intravascular catheters (PIVCs) by bedside nurses remains the most commonly performed procedures. Many modern pediatric intensive care unit (PICUs) use ultrasound and near-infrared technologies combined with anxiolysis or anesthesia, interventional radiology, and specialized VA teams to facilitate vascular access (1, 2). However, access remains an invasive, painful, and complex procedure.
The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) is an expert consortium recommending the type of intravenous catheter (central or peripheral) for adult patients in critical care settings (3). Following the recommendations of MAGIC, the miniMAGIC consortium adjusted the guidelines for children (4). In their review, the duration of treatment and the need for hemodynamic monitoring are the most critical parameters. There are more factors to be considered since the dwell time of PIVCs depends additionally on other factors such as agitation, edema, or characteristics of the infusion, including vancomycin treatment or blood transfusion, as described in previous studies (5–7) (Figure 1).
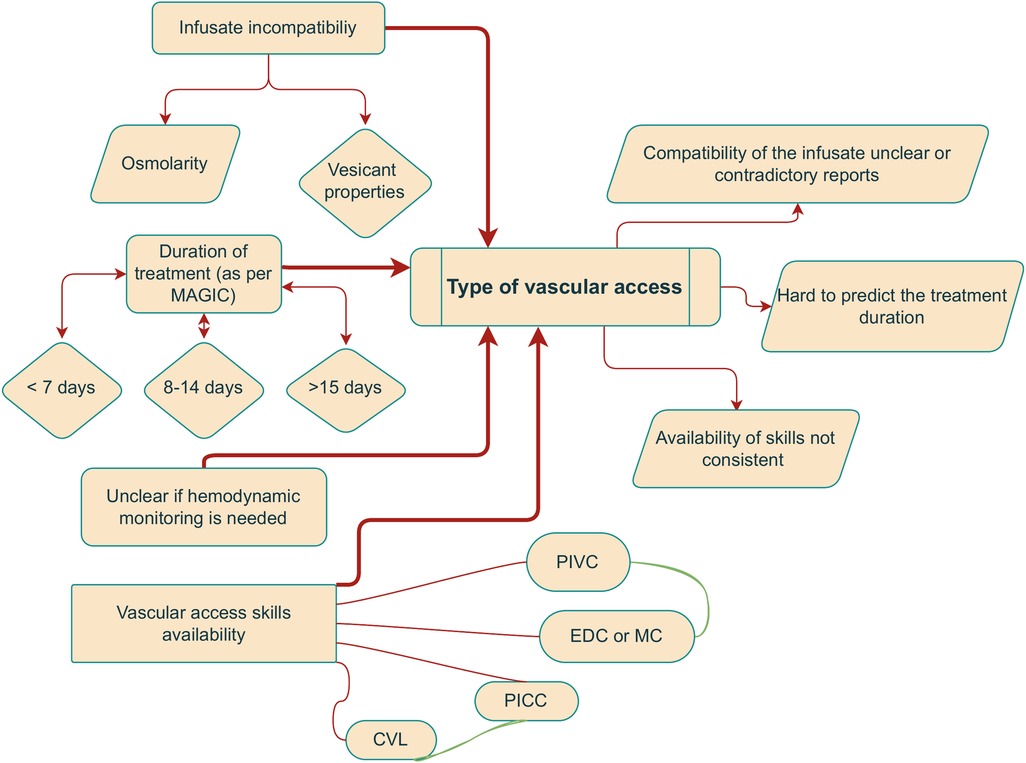
Figure 1. The decision for vascular access in the PICU is multifactorial and admittedly confusing. The flowchart shows that it gets more complicated: on the left are the most frequent “indications,” and on the right is the associated ambiguity.
Unstable critical care patients most often need a central catheter placement, and such central venous catheters (CVCs) are placed by the ICU team (8). In relatively less urgent situations, a peripherally inserted central catheter (PICC) line might be an option if there is sufficient time available. PICC can be used for administering intravenous medications with high osmolarity or vesicant infusions. However, PICC may not accurately transmit the central pressure (9). Midline catheters (MC) are often characterized by their shorter length compared with PICC, as well as their peripheral placement resulting in the tip not being positioned centrally (10). Extended dwell catheters (EDC) is of shorter length than MC, but longer than PIVC (11).
This study aims to describe the baseline dwell time of PIVCs and the factors that increase or decrease it. Knowing the average PIVC dwell time in an edematous or agitated patient or those requiring different treatment necessities can assist in determining whether placing a longer-lasting EDC or MC earlier is beneficial, thus providing more specific recommendations.
Materials and methods
The study included all patients admitted to the pediatric intensive care unit (PICU) between 1 January 2019 and 31 December 2021, who stayed at least 24 h and were managed without central access. Electronic medical records were retrospectively utilized to collect the data. The study titled “Longevity of Peripheral Intravascular Catheter” was approved by the University of Tennessee on 14 July 2022, 22-08810-XP. The need for informed consent was waived. The University adheres to the ethical standards governing human experimentation and the Helsinki Declaration of 1975.
The analysis encompassed patient information, including age, gender, race, weight, location of the access, and number of PIVCs, as well as factors that affected dwell time, such as generalized edema. Edema was assessed by employing skin indentations or with a fluid overload equation. The fluid overload equation is (current weight − admission weight)/admission weight (12, 13). Using PIVC for hydration, sedation, antibiotics, inotropes, and nutrition was recorded. The agitation (or hyperkinetic delirium) was also extracted.
Dwell time was computed from the documented insertion time until the recorded removal time of the PIVC. The reasoning for removal was noted as “end of treatment” or “failure” due to clotting or dislodgment (infiltration). In case of an infiltration or clotting, it needs to be reported to the institution's quality improvement monitoring. End-of-treatment removal might refer to a particular treatment conclusion and not necessarily that all intravascular treatments have finished: in case that PIVC was inserted to facilitate a transfusion and subsequently became clotted at the end, it can be removed and documented as the “end of treatment.” Thus, to avoid confusion with the uncertainty created by the definition of the “end of treatment,” dwell time was defined as the time from insertion to removal for any reason.
The functional dwell time was defined from insertion to removal because of a documented non-functioning, such as clotting or dislodgment (infiltration). It was computed with non-parametric survival Kaplan–Meier methods: it did not include PIVCs removed with documentation “end of treatment.”
The analysis was performed using IBM SPSS Statistics for Windows, version 29.0. Armonk, NY, USA. The data are presented as means (SD), and the associations are assessed using ANOVA for parametric and chi-square tests for non-parametric variables. The comparisons were performed under a priori assumption, and the significance α was set at .05. The BF10 and Kaplan–Meier were validated using JASP version 0.17.1 (2022) and Jamovi version 2.3 (2022).
Results
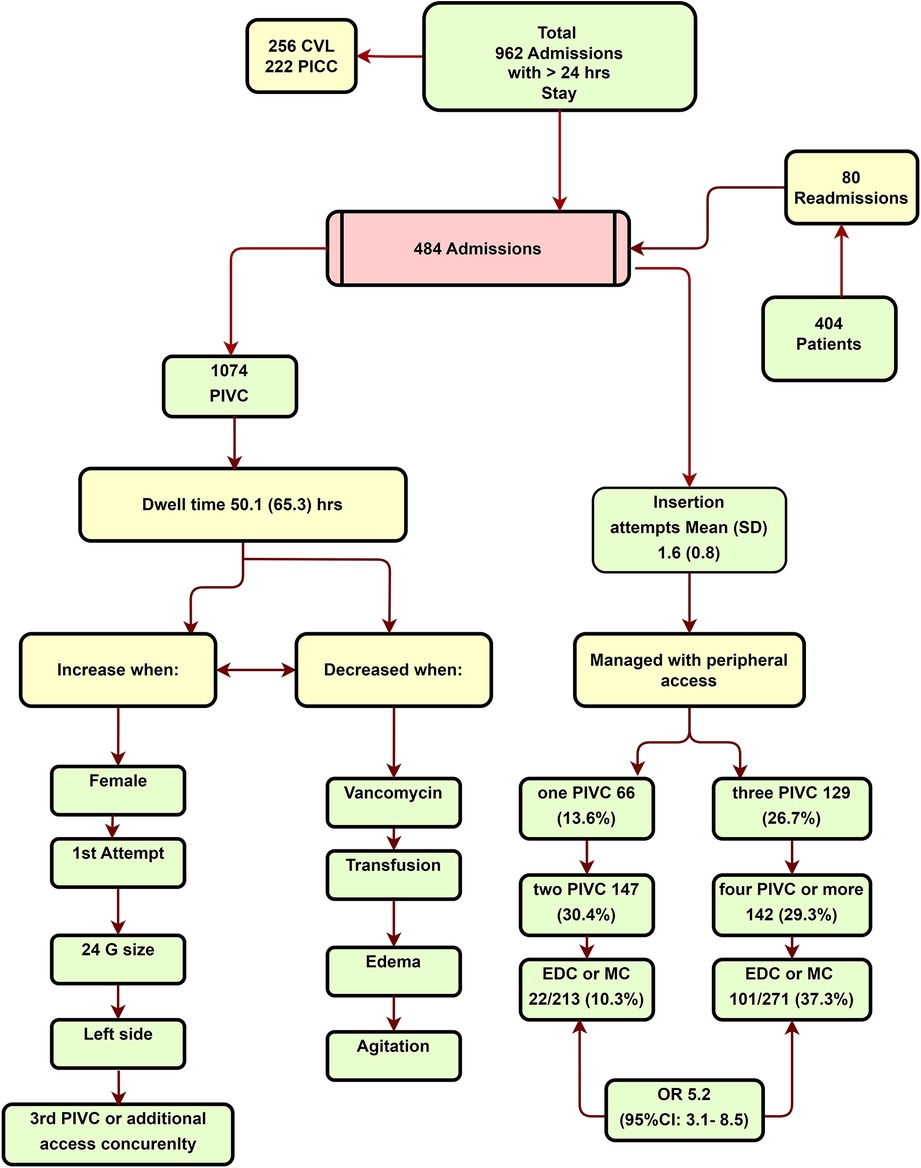
Figure 2 and Table 1 describe the characteristics of the enrolled patients. The study reviewed all the admissions in the PICU that lasted for 24 h or more. A total of 478 patients were excluded from the analysis, with 256 patients having central venous catheter (CVC), and 222 patients having PICC. The patients in the CVC and PICC group (central access group) had a higher level of sickness compared with those managed with peripheral access. The mean PRISM and PICU LOS for the central access group were 18 days (SD: 8.5) and 9.5 (SD: 6.4) days, respectively. In comparison, the peripheral-only access group had 8.9 (SD: 5.9) and 5.9 (SD: 3.6) days, P = 0.01 (BF10 = 4.25 × 10+66 = 0.001 (BF10 = 2.12 × 10+22). The central access placement in cases where there was only an indication of difficult vascular access was reported in 14.1% 36/256 (14.1%). The peripheral-only group included 484 admission records (404 patients) with 1,074 PIVC insertions (see Figure 2). In total, 80 patients were readmitted, with 22 readmissions occurring from home and 58 from the institution.
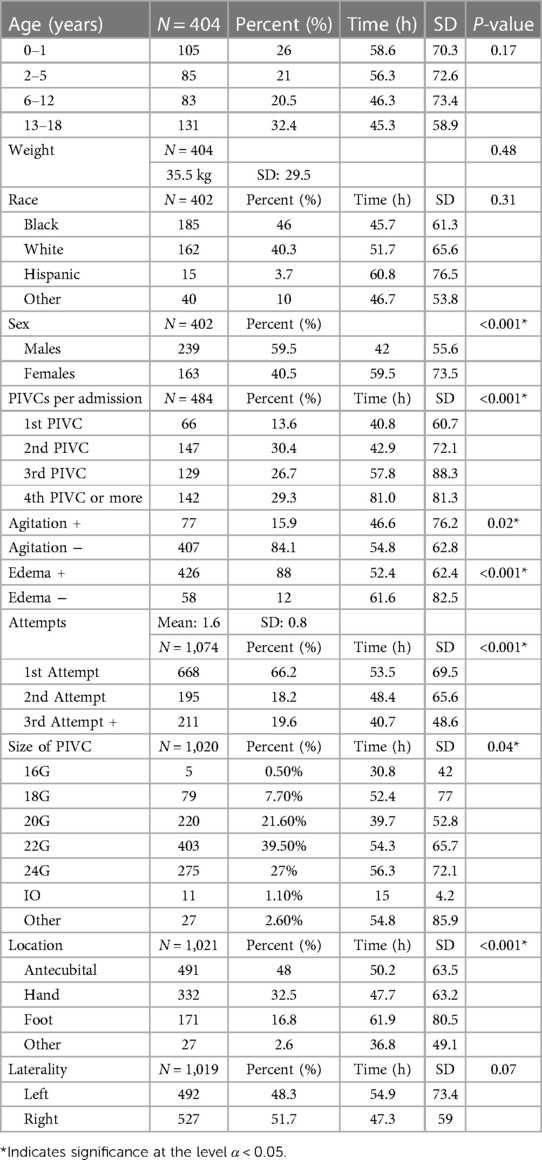
The mean age of the patients included in the study was 4.52 years (SD: 4.16). In particular, the study included a total of 105 (26%) patients, 0–1 year of age (YOA), 85 (21%) patients, 2–5 YOA, 83 (20.5%) patients, 6–12 YOA, and 131 (32.4%) patients, 13–18 YOA. The dwell times were not statistically different between the age subgroups (P = 0.17), Table 1. The age distribution of the study sample was similar to the age distribution of patients admitted to the PICU at the institution.
The mean weight was 35.5 kg (SD: 29.5). A multiple linear regression was used to test if Age and Weight significantly predicted the Dwell time. The overall regression was not statistically significant [R2 = 0.01, F(df1 = 2, df2 = 481) = 2.36, P = 0.09].
The PIVCs lasted an average of 50.1 h (SD: 65.3), while female patients showed a slightly longer dwell time over males 59.5 vs. 42 h, P = 0.01 (Table 1).
There was no significant difference found between races, P = 0.31. In total, 66 (13.7%) admissions were managed with one PIVC insertion. However, 129 (26.7%) admissions needed three PIVCs, and 142 (29.3%) of the admissions required four or more PIVCs. There were 1.6 insertion attempts per patient (SD: 0.80). PIVCs placed on the first attempt lasted longer than those required multiple attempts, with a mean of 53.5 h (SD: 69.5) (P = 0.01), as well as the 24 Ga bore than larger bore catheters, lasting 56.3 h (P = 0.01). The incidence of reported infiltration was rare, accounting for just six out of the 1,074 (.5%) PIVC insertions. The potential under-reporting of the incidence could be attributed to the laborious process of the reporting system.
Among the admissions that required three or more PIVC insertions, 101 out of 271 (37.3%) required EDC or MC in comparison with patients who had one or two PIVC insertions [(22/213 (10.3%), P < 0.001, odds ratio (OR) 5.2 (95% CI: 3.1–8.5), BF10 = 4.92 × 10+8].
PIVCs that were inserted later in the admission course had a longer duration (Table 1) and were highly correlated with EDC or MC insertion at Pearson ρ = 0.89.
PIVC inserted on the left side exhibited longer dwell times. In addition, there was a trend for the foot PIVCs to demonstrate a statistical significance, with a mean dwell time of 61.9 h (SD: 80.5), P = 0.08 (trend). Edema was reported in 58 out of 484 (12%) admissions, and the mean dwell times were found to be worst for this group at 46.6 h (SD: 76.2) compared with those without edema at 54.8 h (SD: 62.8), P = 0.01.
PIVCs were utilized predominantly for fluid administration (432, 89.3%), sedation (219, 45.3%), and administration of antibiotics (193, 39.9%). The use of sedation medications was associated with a prolonged mean dwell time of 58.5 h (SD: 47.70), P = 0.01. The group did not include patients who were administered total parenteral nutrition (TPN) with dextrose concentration higher than 12.5% or hypertonic saline solutions with higher than 2% concentration via the PIVC, while inotropes were infused in a few cases. The total number is small, 32/484 (6.6%), and the duration of these cases is less than 24 h. The study found no difference in the dwell time of the PIVCs in any of the above categories.
Vancomycin infusion and blood transfusion significantly decreased the mean dwell time to 24.2 h (SD: 34.7) and 29.3 h (SD: 41.7), respectively, at P < 0.001. Inconsolable agitation or delirious behavior was documented in 77 patients. The mean dwell times were shorter at 46.6 h (SD: 76.2) than at 54.8 h (SD: 62.8).
Kaplan–Meier curves are used to compare the PIVC with EDC or MD, as shown in Figure 3. The long rank value is X2 = 205 (P = 0.01). There is a 460/1,074 (42.8%) PIVC failure rate (reported as occluded or infiltrated) vs. 11/123 (8.9%) for EDC or MC (BF10 = 2.96 × 10−13). The median survival time for the PIVC until failure (functional dwell time) is 82 h (67.9–97.2). However, it is not possible to estimate the EDC or MD dwell time as they remain in place longer than the patient’s PICU stay (the patients were discharged to the pediatric floor with a functional catheter).
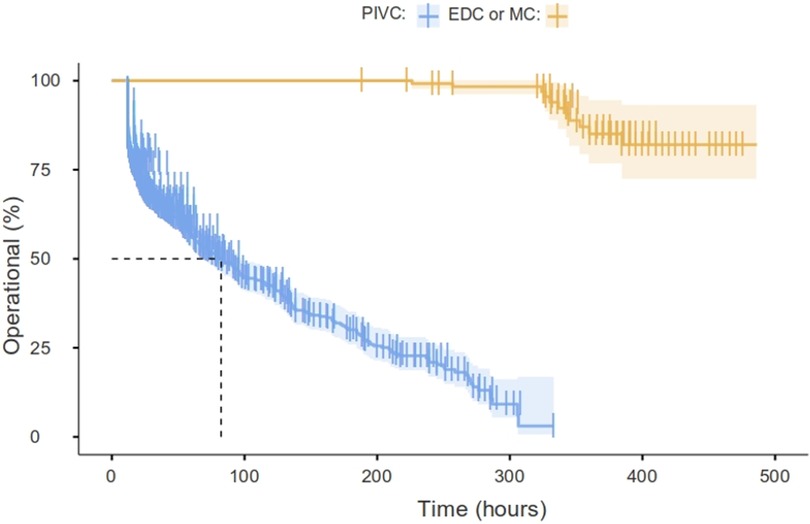
Figure 3. Percent operational PIVC vs. EDC or MD overtime (Kaplan–Meier). The median of the PIVC survival to failure time is 82 h (67.9–97.2), and it is not possible to estimate the EDC or MD dwell time as they remain in place longer than the patient’s PICU stay (the patients were discharged to the pediatric floor with a functional catheter). This time is called “functional dwell time” (insertion to not functioning) to distinguish it from the dwell time computed from insertion to removal for any reason (not functioning or reported as end of treatment).
Discussion
Despite the significant number of critically sick children managed in the PICU with peripheral access, the dwell times and the modifying parameters have not been reported. The mean dwell time was found to be 50.1 h (measured from insertion to removal) that aligns well with the mean dwell time previously reported for hospitalized children (55.6–67 h) (5, 14). The median duration of functional dwell time from insertion to documented failure computed using non-parametric “survival” analysis was 82 h (IQR: 67.7–97.2). The potential for inflation may arise due to delays in discovery or documentation issues.
Increased dwell time was associated with female gender 59.5 h (P < 0.001), first attempt insertion 53.5 h (P < 0.001), use of 24 Ga bore 56.3 h (P = 0.04), left-sided insertions 54.9 h (P = 0.07), less patient agitation 54.8 h (P = 0.02), and less edema 61.6 h (P < 0.001). None of the observed increases exceeded 1.5 times the average dwell time (1.5 × 50.1 h = 75.2 h). Therefore, even under favorable conditions that allow for longer durations, the dwell time remains shorter according to the practitioner’s preferences. A decreased dwell time was associated with the use of vancomycin infusion at 24.2 h (P < 0.001) and blood transfusions at 29.3 h (P < 0.001).
It was observed that if a patient had repeated insertions, the subsequent insertion of PIVCs in the admission course lasted longer. The number of repeated insertions is correlated strongly (Pearson ρ = 0.89) with EDC and MC insertion. Following the insertion of another type of vascular access, the PIVC may be used less frequently. For example, a patient with a midline may require a PIVC for occasional additional medications. The PIVC will last longer than when utilizing it for all the infusions. A third insertion increases the odds of vascular access team consultation and EDC or MC placement to 5.2.
A recent literature showed that PIVCs can be used for inotropes, 3% saline, and TPN solutions (15–18). The data presented in this study could not confirm or reject the hypothesis, as it did not include an evaluation of such a hypothesis. However, it was observed that occasional PIVCs (6.6% of the total) were utilized for inotropes, hypertonic saline, and TPN solutions for a duration of less than 24 h. It is important to note that the dopamine was diluted for peripheral use, the hypertonic saline was administered at a concentration of only 2%, and the TPN solution contained dextrose up to a 12.5% concentration as per institutional policy.
It is still unclear whether the PIVCs might be equally responsible for thrombosis and catheter infections; some reports already implicate midlines. In a published study, the rate of EDC-associated infection varied from 0 to 1.07/1,000 midline days (19–22). The studies supported that “silicone-base” catheters such as PICC have a lower incidence of infection (21). CVCs were associated with a higher risk of central line-associated bloodstream infection (CLABSI) (RR = 2.20; 95% CI: 1.05–4.61; P = 0.04) compared with PICCs. The incidence of thrombosis was reported to be higher in midline catheters than in PICCs (7.04% MCs and 4.72% PICCs; OR: 1.53; P = 0.01), although the incidence is low and requires further studies to be validated (22). Currently, there is a lack of available data regarding the incidence of thrombosis or infection associated with EDCs.
Uncomplicated vascular access is an illusion. A fine-tuning of the treatment options is required in order to achieve a balance between complications. According to the Centers for Disease Control and Prevention (CDC), it is recommended to utilize an MC or PICC catheter when administering therapy that extends beyond 60 days (https://www.cdc.gov/infectioncontrol/guidelines/bsi/recommendations.html). The miniMAGIC recommendations offer guidelines based on the expected duration of the therapy (7 days). PIVC-based treatment for 6–7 days can potentially result in several painful insertion attempts due to their relatively short dwelling time, and the infectious complications (if any, since they have not been reported for EDCs) should be carefully evaluated in relation to the recurring adverse effects of PIVCs. Multiple attempts increase the chances of infections, phlebitis, and thrombosis risks. Repeated attempts can potentially cause infiltration/extravasation from the previously injured vessel (22–24). Since the odds of EDC/MC increase after the third insertion, it is advisable to prioritize their early consideration and subsequent insertion without exposing the patient to the discomfort of repeated insertions (23).
This study did not specifically evaluate the success rate of insertion using different methods—the vascular access team using ultrasounds to implant all the EDC, MD, and PICC in the authors’ institution. Vascular access teams have been associated with a reduction in CLABSI and an improvement in patient satisfaction (25, 26). A 2018 Cochrane review suggested that there is a need for further research on the utilization of vascular access teams (27, 28).
A comprehensive analysis of 3,700 US-based hospitals provided a detailed causal-comparative design that addressed the difference in the reported CLABSI rates based on hospital type (teaching and non-teaching) for hospitals with a vascular access team and whether there was a difference in the reported CLABSI rates based on the presence or absence of a vascular access team (29). The results demonstrated there were significant differences in the reported CLABSI rates by hospital type (P < 0.001), and the presence or absence of a VA team was also of consequence, indicating that there were significant differences in reported CLABSI rates (P = 0.004) based on the presence or absence of a VA team (29).
The results suggested that a dedicated VA team is associated with a lower incidence of infection, regardless of hospital type. This finding is consistent with the results of previous studies showing that VA teams can improve patient outcomes by reducing the risk of infection, complications, and costs associated with vascular access devices. The findings of this study will guide healthcare leaders in their efforts to implement evidence-based guidelines and infection risk reduction strategies throughout healthcare organizations. Establishing VA teams inside healthcare organizations has the potential to improve patient safety and increase the quality of care provided to those in need of vascular access.
Accurately monitoring peripheral vascular access, dwell time, and complication is a vital source of information, and it has to be communicated in a non-penalizing way to encourage accurate reporting. The authors do not support the practice of utilizing peripheral access in critical care patients as a means of reducing the incidence of central access complications. Instead, they advocate for an evidence-based evaluation of vascular access that takes into account the specific requirements for each patient. Educational competency in peripheral vascular accesses is also essential. and performing an ultrasound-guided placement of central catheter is a crucial aspect of this competency (30).
The recommended duration of treatment was outlined in the miniMAGIC guidelines. This study suggests using EDC in cases when there is edema, agitation, or a need for vancomycin infusion, transfusion, or prolonged treatment. The scope of this study is limited to a retrospective, single-center analysis. The ability of the PIVC placement varies with the experience, care ratio, and specific infusion policies. The limitations of dwell time reporting have already been highlighted. The actual dwell times observed in the sample depend on the specific characteristics of the PICU, but the underlying relations are expected to remain similar. The experience of the team and various protocols for early insertion will increase the dwell time. The miniMAGIC system covers specifically PICU patients only, but it is particularly beneficial for pediatric patients with congenital heart disease who often require femoral access for catheterization; in these cases, the EDC option is even more advantageous.
Conclusions
EDC/MC is a superior alternative to PIVCs for peripheral access in a considerable number of PICU patients. The need for vascular access consultation and more stable insertion increased following the third PIVC. The absence of a skilled clinician, resulting in the occurrence of multiple punctures, has the potential to cause injury to patients. A third insertion might be a simple bedside criterion to alert the team to request a consultation for an overdue EDC or MC. However, early evaluation and utilization of vascular access teams are considered the optimal clinical approach.
The recommendation of miniMAGIC protocols is sufficient for central vascular access. For peripheral access, the duration of the treatment should also be considered. Comfort, fewer painful punctures, and preventing complication from vancomycin and transfusions are essential. Extended dwell catheters and vascular team consultation offer a great alternative to PIVCs. The study recommends that healthcare providers should take into account the patient's edema, the reported or estimated insertion difficulty, the level of agitation, and the need for transfusion or vancomycin infusion for an early call for EDC in PICU patients. This approach may prove advantageous even for patients with a treatment duration of less than 6 days.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SA: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – Original draft, Writing – Review & editing. SG: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – Original draft, Writing – Review & editing, Formal analysis, Funding acquisition, Supervision, Validation. AW: Investigation, Methodology, Resources, Writing – Original draft, Writing – Review & editing, Validation. TS: Investigation, Project administration, Software, Supervision, Visualization, Methodology, Resources, Validation, Writing – Original draft, Writing – Review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CVC, central venous catheter; CICC, centrally inserted central catheter; CLABSI, central line-associated bloodstream infection; CVAD, central venous access device; EDC, extended dwell catheters; MAGIC, Michigan appropriateness guide for intravenous catheters; MC, midline catheters; PIVC, peripheral intravenous catheter; PICC, peripherally inserted central catheter; PICU, pediatric intensive care unit; TPN, total parenteral nutrition.
References
1. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound: peripheral intravenous catheter insertion. Chest. (2020) 157(2):369–75. doi: 10.1016/j.chest.2019.04.139
2. Hackett A, Wells C, Zhang Z, Kero J, Soriano J, Rivera J, et al. Development of a peripheral intravenous access training program for nurses in the pediatric intensive care units. J Pediatr Nurs. (2021) 61:394–403. doi: 10.1016/j.pedn.2021.09.017
3. Chopra V, Flanders SA, Saint S, Woller SC, O'Grady NP, Safdar N, et al. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. (2015) 163(6 Suppl):S1–40. doi: 10.7326/M15-0744
4. Ullman AJ, Bernstein SJ, Brown E, Aiyagari R, Doellman D, Faustino VS, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. (2020) 145(Suppl 3):S269–84. doi: 10.1542/peds.2019-3474I
5. Tripathi S, Gladfelter T. Peripheral intravenous catheters in hospitalized patients: practice, dwell times, and factors impacting the dwell times: a single center retrospective study. J Vasc Access. (2022) 23(4):581–8. doi: 10.1177/11297298211000874
6. Ansel B, Boyce M, Embree JL. Extending short peripheral catheter dwell time: a best practice discussion. J Infus Nurs. (2017) 40(3):143–6.28419010
7. Shenoy S, Karunakar BP. Factors influencing the peripheral venous catheter survival in critically ill children in a pediatric intensive care unit. Indian J Pediatr. (2014) 81(12):1293–6. doi: 10.1007/s12098-014-1430-7
8. Ares G, Hunter CJ. Central venous access in children: indications, devices, and risks. Curr Opin Pediatr. (2017) 29(3):340–6. doi: 10.1097/MOP.0000000000000485
9. Khan A, Laing E, Beaumont A, Wong J, Warrier S, Heriot A. Peripheral parenteral nutrition in surgery—a systematic review and meta-analysis. Clin Nutr ESPEN. (2023) 54:337–48. doi: 10.1016/j.clnesp.2023.02.004
10. Bing S, Smotherman C, Rodriguez RG, Skarupa DJ, Ra JH, Crandall ML. PICC vs. midlines: comparison of peripherally inserted central catheters and midline catheters with respect to incidence of thromboembolic and infectious complications. Am J Surg. (2022) 223(5):983–7. doi: 10.1016/j.amjsurg.2021.09.029
11. Chen I-L, Ou-Yang M-C, Chen F-S, Chung M-Y, Chen C-C, Liu Y-C, et al. The equations of the inserted length of percutaneous central venous catheters on neonates in NICU. Pediatr Neonatol. (2019) 60(3):305–10. doi: 10.1016/j.pedneo.2018.07.011
12. Brodovicz KG, McNaughton K, Uemura N, Meininger G, Girman CJ, Yale SH. Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin Med Res. (2009) 7(1–2):21–31. doi: 10.3121/cmr.2009.819
13. Shields BA, Fossati SO, Cole RE, Kieffer AJ, Vega SJ, Aden JK, et al. Adjusting body weight for edema in severely burned patients. Burns J Int Soc Burn Inj. (2023) 49(3):562–5. doi: 10.1016/j.burns.2023.01.008
14. Jeong IS, Jeon GR, Lee MS, Shin BJ, Kim Y-J, Park SM, et al. Intravenous infiltration risk by catheter dwell time among hospitalized children. J Pediatr Nurs. (2017) 32:47–51. doi: 10.1016/j.pedn.2016.08.008
15. Mesghali E, Fitter S, Bahjri K, Moussavi K. Safety of peripheral line administration of 3% hypertonic saline and mannitol in the emergency department. J Emerg Med. (2019) 56(4):431–6. doi: 10.1016/j.jemermed.2018.12.046
16. Brown CS, Rabinstein AA, Zhao Y, Wieruszewski ED. Safety of peripheral 3% hypertonic saline bolus administration for neurologic emergency. Am J Emerg Med. (2023) 69:83–6. doi: 10.1016/j.ajem.2023.04.007
17. Fessler AG, Rejrat CE. Re-evaluating safe osmolarity for peripheral parenteral nutrition in neonatal intensive care patients. J Pediatr Pharmacol Ther. (2021) 26(6):632–7.34421414
18. Abrar S, Abbas Q, Inam M, Khan I, Khalid F, Raza S. Safety of vasopressor medications through peripheral line in pediatric patients in PICU in a resource-limited setting. Crit Care Res Pract. (2022) 2022:6160563.35402044
19. DeVries M, Lee J, Hoffman L. Infection free midline catheter implementation at a community hospital (2 years). Am J Infect Control. (2019) 47(9):1118–21. doi: 10.1016/j.ajic.2019.03.001
20. Nickel B. Does the midline peripheral intravenous catheter have a place in critical care? Crit Care Nurse. (2021) 41(6):e1–21. doi: 10.4037/ccn2021818
21. Bahl A, Karabon P, Chu D. Comparison of venous thrombosis complications in midlines vs. peripherally inserted central catheters: are midlines the safer option? Clin Appl Thromb Hemost. (2019) 25:1076029619839150.30909723
22. Swaminathan L, Flanders S, Horowitz J, Zhang Q, O'Malley M, Chopra V. Safety and outcomes of midline catheters vs peripherally inserted central catheters for patients with short-term indications: a multicenter study. JAMA Intern Med. (2022) 182(1):50–8. doi: 10.1001/jamainternmed.2021.6844
23. Prasad RO, Chew T, Giri JR, Hoerauf K. Patient experience with vascular access management informs satisfaction with overall hospitalization experience. J Infus Nurs. (2022) 45(2):95–103.35272306
24. Larsen E, Keogh S, Marsh N, Rickard C. Experiences of peripheral IV insertion in hospital: a qualitative study. Br J Nurs Mark Allen Publ. (2017) 26(19):S18–25. doi: 10.12968/bjon.2017.26.19.S18
25. Hunter MR. Development of a vascular access team in an acute care setting. J Infus Nurs. (2003) 26(2):86–91.12642796
26. Carr PJ, Higgins NS, Cooke ML, Mihala G, Rickard CM. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Database Syst Rev. (2018) 3(3):CD011429.29558570
27. Vascular access specialist teams for device insertion and prevention of failure—PubMed. Available at: https://pubmed-ncbi-nlm-nih-gov.ezproxy.uthsc.edu/29558570/ (Accessed August 31, 2023).
28. Martínez MIC, Torres MA, Revilla AMD, Centeno SM, Oria AM, Roncal IE, et al. Impact assessment following implementation of a vascular access team. J Vasc Access. (2022) 23(1):135–44. doi: 10.1177/1129729820984284
29. Bardin-Spencer AJ. A causal-comparative examination of CLABSI, vascular access teams and hospital types. PhD-Thesis, Grand Canyon University (2021).
Keywords: central venous catheter, central line-associated bloodstream infection, central venous access device, extended dwell catheters, Michigan appropriateness guide for intravenous catheters, midline catheters, peripheral intravenous catheter, peripherally inserted central catheter
Citation: Armstrong SH, Gangu S, West AN and Spentzas T (2023) Peripheral vascular access as exclusive access mode in pediatric intensive care unit. Front. Pediatr. 11:1259395. doi: 10.3389/fped.2023.1259395
Received: 15 July 2023; Accepted: 20 September 2023;
Published: 9 October 2023.
Edited by:
Dincer Riza Yildizdas, Çukurova University, TürkiyeReviewed by:
Timothy R. Spencer, Global Vascular Access, LLC, United StatesFaruk Ekinci, Çukurova University, Türkiye
© 2023 Armstrong, Gangu, West and Spentzas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Spentzas dG9tLnNwZW50emFzQGdtYWlsLmNvbQ==; dHNwZW50emFAdXRoc2MuZWR1
 Sonya Hayes Armstrong1
Sonya Hayes Armstrong1 Shantaveer Gangu
Shantaveer Gangu Alina Nico West
Alina Nico West Thomas Spentzas
Thomas Spentzas