- 1Department of Pediatrics, Division of Nephrology, Duke University Medical Center, Durham, NC, United States
- 2Computational Biology and Bioinformatics Program, Duke Center for Statistical Genetics and Genomics, and Department of Biostatistics and Bioinformatics, Duke University, Durham, NC, United States
- 3Department of Surgery, Duke University Medical Center, Durham, NC, United States
Introduction: The etiology of most cases of nephrotic syndrome (NS) remains unknown, therefore patients are phenotypically categorized based on response to corticosteroid therapy as steroid sensitive NS (SSNS), or steroid resistant NS (SRNS). Genetic risk factors have been identified for SSNS from unbiased genome-wide association studies (GWAS), however it is unclear if these loci are disease risk loci in other forms of NS such as SRNS. Additionally, it remains unknown if these risk loci are associated with response to therapy. Thus, we investigated the association between SSNS risk loci and therapy response in a large, multi-race cohort of children along the entire spectrum of childhood-onset NS.
Methods: We enrolled 1,000 patients with childhood-onset NS comprised of SSNS and SRNS. Genotyping was done using TaqMan and Direct Sanger Sequencing for 9 previously reported childhood SSNS risk loci. We compared the allele frequencies (AF) and variant burden between NS vs. controls and SRNS vs. SSNS.
Results: All 9 risk loci were associated with NS compared with healthy controls (p = 3.5 × 10−3–<2.2 × 10−16). Variant burden greater than 7 was associated with risk of SRNS (OR 7.4, 95% CI 4.6–12.0, p = 8.2 × 10−16).
Conclusion: Our study showed that genetic risk loci for childhood SSNS are associated with pattern of therapy response, may help predict disease outcome, and set the stage for individualized treatment of NS.
Introduction
Childhood nephrotic syndrome (NS) is a common condition seen in pediatric nephrology clinics and is a major cause of chronic kidney disease (CKD) in children. It is characterized by massive proteinuria, hypoalbuminemia, edema, and dyslipidemia. The prevalence of NS differs by phenotype, race, and geographic location (1). Corticosteroids are the mainstay of treatment for NS, and response to corticosteroid therapy is an important predictor of disease outcome (2). Patients with steroid sensitive NS (SSNS) often have excellent outcomes and rarely progress to kidney failure (1). Conversely, patients with steroid resistant NS (SRNS) frequently require intensified immunosuppression with agents other than corticosteroids in order to delay disease progression. Despite intensive immunosuppression, 50% of patients with SRNS will develop kidney failure within 5–10 years of diagnosis (3).
The etiology of childhood NS remains unclear; however, our knowledge of disease pathogenesis continues to expand. Pathogenic variants in >80 genes that are enriched in the podocyte have been associated with SRNS in 10%–30% of cases (4). A majority of SSNS are presumed to be due to immune dysregulation, possibly mediated by environmental factors and genetic variations in adaptive immunity genes (5). Several studies have identified genetic variants in adaptive and non-adaptive immunity genes, including HLA-DQA1, BTNL2, HLA-DR/DQ, CALHM6, NPHS1/KIRREL, TNFSF15, and TNFRSF11A (6–10). In these studies, only children with SSNS were included and did not include patients with SRNS. Thus, it is unclear if these loci are disease risk loci in SRNS. Additionally, it remains unknown if these risk loci are also associated with corticosteroid therapy response. The objective of this study is to determine the association between known SSNS genetic risk loci and pattern of corticosteroid therapy response in a large cohort of patients with both SSNS and SRNS. Defining this association is an important step in individualizing therapy for NS to minimize the significant side effects associated with exposure to multiple immunomodulatory agents.
Methods and materials
Patients
We enrolled 1,000 patients in a multi-race cohort with childhood-onset NS comprised of patients with SSNS (n = 639), and SRNS (n = 236), and unknown final therapy response (n = 125). Inclusion criteria were: (1) diagnosis of NS as defined by ≥2 + on urine dipstick or spot urine protein-to-creatinine ratio of ≥2 mg/mg, hypoalbuminemia ≤2.5 mg/dl, and edema, (2) age at disease onset less than 21 years. Patients with known monogenic NS or secondary NS due to identifiable underlying cause including infection, malignancy, medication, or other disease associated with NS such as systemic lupus erythematosus were excluded. Possible disease courses were classified according to the Kidney Disease: Improving Global Outcomes criteria and included SSNS (remission induced by standard corticosteroid therapy) and SRNS (defined as failure to enter remission after at least four to 6 weeks of standard corticosteroid therapy) (11). Patients for whom corticosteroid response category was unknown at time of data analysis (n = 125) were excluded from subcategory comparison analyses (SSNS vs. SRNS). The protocol Pro00014951 complies with the Declaration of Helsinki and the Declaration of Istanbul. All participants or guardians signed a written informed consent approved by Duke University's Institutional Review Board.
Genotyping
Genotyping was performed for nine reported variants associated with childhood SSNS (6–10): HLA-DQA1 [rs1129740], BTNL2 [rs9348883], HLA-DR/DQ [rs4642516 & rs3134996], Intergenic (a locus between the genes HLA-DQA1 and HLA-DQB1) [rs9273371], CALHM6 [rs2637678], NPHS1/KIRREL [rs56117924], TNFSF15 [rs6478109], and TNFRSF11A [rs34213471]. Variant details are provided in Supplementary Table S1. Information was gathered from dbSNP database and original reporting articles (6–10, 12). DNA samples were brought to a concentration of 50 ng/ul and genotyped using TaqMan or Direct Sanger Sequencing. TaqMan allele discrimination assay (Applied Biosystems, Foster City, CA) was carried out on an ViiA7 sequencer detector system according to the manufacturer's protocol. Quality control measures for genotyping included genotyping of duplicate samples as well as Centre d'Etude du Polymorphism Humain (CEPH) controls. To pass quality control, all duplicated samples had a match of 100%.
Data analysis
Allele frequency
We compared the risk allele frequencies (AF) between all patients with NS vs. controls and SRNS vs. controls from public databases (Genome Aggregation Database [gnomAD], Trans-Omics for Precision Medicine [TOPMed], and Allele Frequency Aggregator [ALFA]) (13–15). As the data for our cases have not been deposited in any public database, to the best of our knowledge, there is no overlap between cases and publicly available controls. Additionally, we compared AF between SSNS vs. SRNS. The groups were compared using odds ratios and chi square test of independence with Bonferroni correction for the number of tests in each set using R Statistical Software (16, 17).
Variant burden analysis
To further characterize the role of these nine risk loci in pattern of therapy response, we investigated whether a higher burden of risk alleles was associated with increased odds of developing SSNS vs. SRNS. To determine the allele burden, the absolute number of risk alleles per patient was counted. Only variants with significant differences after Bonferroni correction in AF between therapy response groups were included in the variant burden analyses. For SSNS vs. SRNS, this included HLA-DQA1 [rs1129740], BTNL2 [rs9348883], HLA-DR/DQ [rs4642516 & rs3134996], Intergenic [rs9273371], and CALHM6 [rs2637678]. Additionally, only individuals with no missing genetic data (“missingness”) across all variants used were included. Patients with missingness were excluded: 113 patients with SRNS, and 47 patients with SSNS (Figure 1). The number of risk alleles (variant burden) was tested for association with SSNS vs. SRNS using odds ratios and Fisher's exact test with Bonferroni correction for the number of tests in each set (17). We also compared the frequency of homozygous genotype for each risk variants between SSNS and SRNS groups.
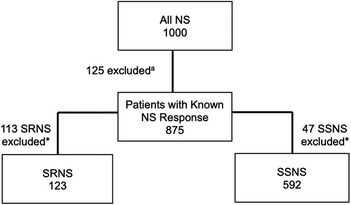
Figure 1. Flow diagram demonstrating exclusion of patients with missing phenotypea and genotype* data for the known SSNS risk alleles (termed “missingness”, based on significantly different AF for each comparison group) for variant burden analysis. Number of patients excluded due to missingness: 113 patients with SRNS and 47 patients with SSNS.
Results
Patients
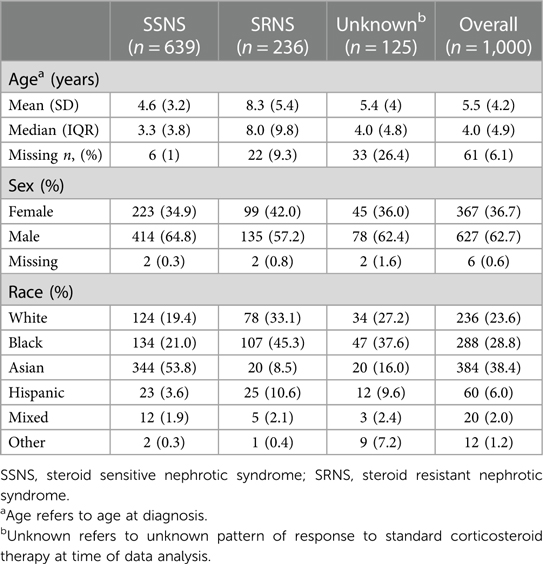
The total number of patients in our cohort was 1,000, which consisted of 639 patients with SSNS, 236 patients with SRNS, and 125 patients with unknown therapy response. Our cohort included self-reported race: Asian (38.4%), Black (28.8%), White (23.6%), Hispanic (6.0%), Mixed (2.0%), and Other (1.2%) (Table 1). The male-to-female ratio was 2:1 (62.7% vs. 36.7%). Median age at disease onset was 4.0 years (IQR 4.9). Almost half of the patients with SRNS were Black (45.3%) and SRNS patients were older at diagnosis (median age 8.0 years, IQR 9.8) (Table 1).
Frequency of NS variants
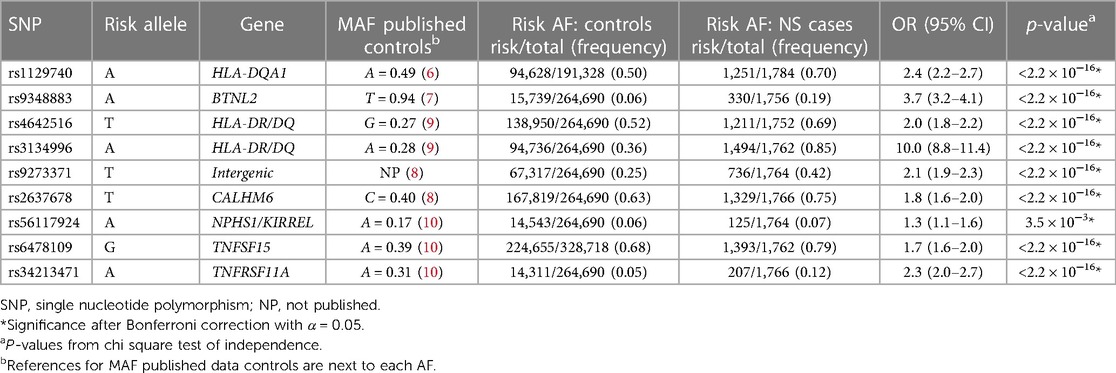
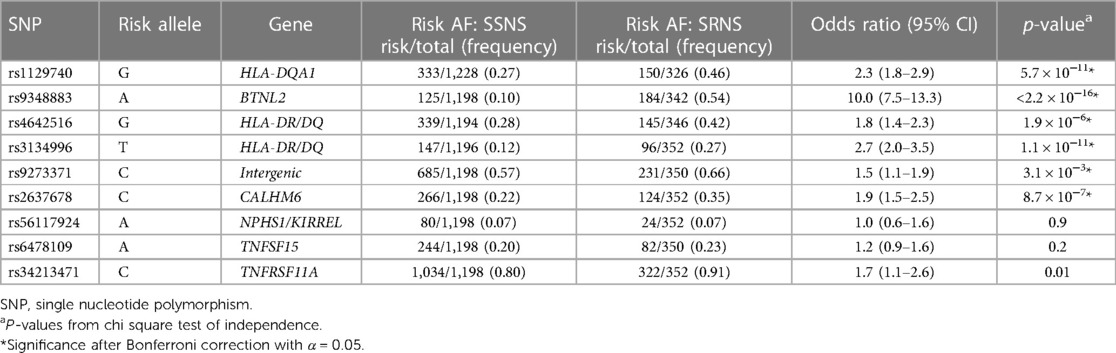
Risk AF was compared for each risk loci between NS vs. controls (Table 2) and SSNS vs. SRNS (Table 3).
AF comparison for NS vs. controls
To validate the association between the known SSNS risk loci and patients with childhood-onset NS, we compared AF between all patients in our cohort with NS to AF found in public databases as mentioned previously (Table 2). AF of all risk loci were significantly different between all patients with NS compared with controls with p < 3.5 × 10−3. When we specifically compared SRNS vs. controls, four risk alleles (BTNL2 [rs9348883], HLA-DR/DQ [rs3134996], intergenic [rs9273371], and TNFSF15 [rs6478109]) remained significantly different (Supplementary Table S2).
AF comparison for SSNS vs. SRNS
To evaluate our hypothesis that known NS risk loci are associated with corticosteroid therapy response in patients with childhood-onset NS, we compared the AF between SSNS vs. SRNS (Table 3). Six of the reported risk alleles had significantly different AF when comparing SSNS vs. SRNS: HLA-DQA1 [rs1129740], BTNL2 [rs9348883], HLA-DR/DQ [rs4642516 & rs3134996], Intergenic [rs9273371], CALHM6 [rs2637678] (p < 3.1 × 10−3). Three risk alleles (NPHS1/KIRREL [rs56117924], TNFSF15 [rs6478109], and TNFRSF11A [rs34213471]) did not have significantly different AF between SSNS and SRNS after Bonferroni correction, indicating that NPHS1/KIRREL,TNFSF15 and TNFRSF11A are not associated with therapy response when comparing SSNS vs. SRNS.
Risk variant burden analysis
To assess whether risk variant burden is associated with pattern of corticosteroid therapy response in childhood NS, we included only those risk alleles found to have a significantly different AF after Bonferroni correction between SSNS vs. SRNS (Table 3). Additionally, we only included individuals with no missingness, thus, 47 and 113 patients were excluded from SSNS and SRNS group respectively (Figure 1). Patients with risk allele burden ≥7 had significantly greater odds of developing SRNS (OR 7.4, 95% CI 4.6–12.0; p = 8.2 × 10−16, Figure 2 and Supplementary Table S3). Allele burden was grouped based on patterns determined from ungrouped counts as shown in Supplementary Table S4 and Figure S1 for SSNS vs. SRNS. As expected, when comparing the frequency of homozygous variants between SSNS and SRNS group, we found that SRNS patients were enriched for homozygous variants (Supplementary Table S5).
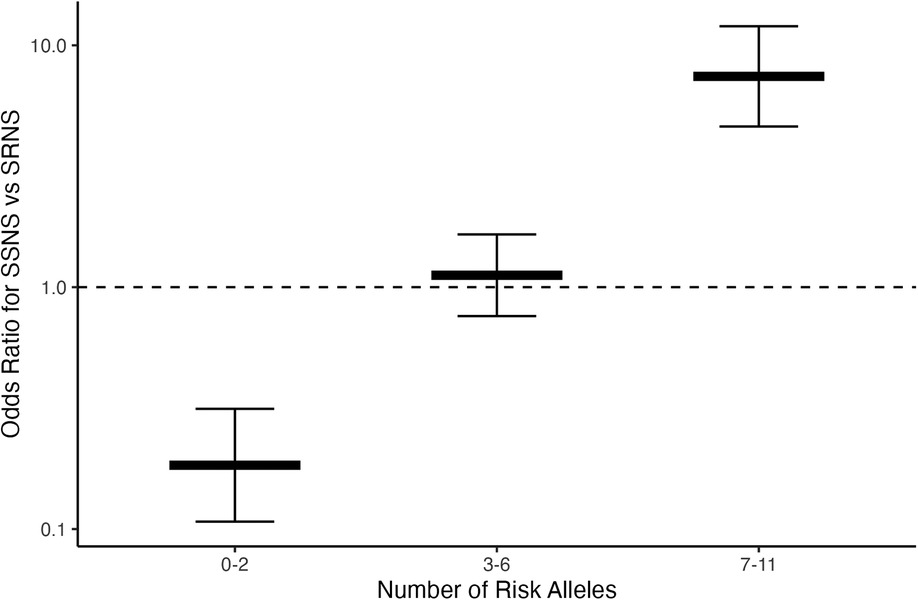
Figure 2. Risk loci burden for patients with SSNS vs. SRNS. NS patients with ≥7 risk alleles have significantly increased odds of developing SRNS (OR 7.4, 95% CI 4.6–12.0; p = 8.2 × 10−16). Note, there were no patients with 12 alleles.
Discussion
There are currently no robust clinical, laboratory, or genetic predictors of pattern of therapy response in patients with NS. Factors such as age at presentation, serum albumin, hematuria, and total duration of corticosteroid therapy have been studied, but the results are often inconsistent and not reproducible (18–20). Therefore, in this study, we investigated the relationship between known SSNS risk alleles from unbiased GWAS studies and therapy response. Our study confirmed that the known SSNS risk loci are indeed associated with all types of NS compared with historical controls. Importantly, we are the first to demonstrate that the variant burden of six of these nine risk alleles is associated with increased odds of having SRNS vs. SSNS, the most severe form of NS. Thus, these six risk loci may be helpful tools to add to the clinical armamentarium for predicting pattern of response.
Herein, we validated nine previously identified genetic risk variants for the development of childhood-onset NS (6–10).These risk variants are in genes associated with adaptive immunity (HLA-DQA1, BTNL2, HLA-DR/DQ, CALHM6, TNFSF15, and TNFRSF11A) and maintenance of podocyte structure and function (NPHS1/KIRREL). While these variants have been associated with childhood SSNS and were individually identified through GWAS studies, they have not yet been studied as a group of risk factors in patients with all types of childhood NS (SSNS and SRNS). Importantly, we showed that a subgroup of variants in BTNL2, HLA-DR/DQ, Intergenic, and TNFSF15 were also associated with SRNS compared to controls and may be able to differentiate SSNS from SRNS. Prospective studies, however, are required prior to their widespread use in clinical practice.
In our study, the HLA-DQA1 [rs1129740], HLA-DR/DQ [rs3134996], and an Intergenic locus between HLA-DQA1 and HLA-DQB1 [rs9273371] had significantly different AF in NS vs. controls, confirming that these alleles are associated with childhood NS. It has been proposed that variants in the HLA genes lead to inappropriate antigen stimulation and results in abnormal T-lymphocyte function, possibly leading to podocyte injury (6).
Additionally, we studied BTLN2 risk locus in our multi-race cohort and found the AF was significantly different in patients with NS vs. controls as well as when comparing SSNS vs. SRNS patients, suggesting it is associated with disease and therapy response. The BTNL2 protein is a type-1 membrane protein which provides co-inhibitory signals to T-lymphocytes (21). BTNL2 also induces FoxP3 expression and development of regulatory T-cells (22).
Furthermore, the CALHM6 risk locus [rs2637678] had a significantly different AF in NS vs. controls and SSNS vs. SRNS, suggesting that this allele is associated with both disease and therapy response. CALHM6 is typically expressed in immune cells, including B- and T-lymphocytes, NK cells, and macrophages and encodes for a cation channel important in the transport of various molecules between cells, signifying that CALHM6 proteins are important for immune cell interactions (23). It is speculated that some members of the CALHM6 family regulate apoptosis of immune cells, and dysfunction of these proteins leads to the impaired immune regulation through multiple pathways leading to development of NS (8).
We also demonstrated that the TNF gene family yet again played an important role in NS. Jia et al. previously identified a risk variant in the TNFSF15 gene [rs6478109] in their Japanese cohort which was replicated in Korean, South Asian, and African populations (10). The TNFSF15 (Tumor Necrosis Factor Super-Family Member 15) gene encodes for the ligand TL1A (Tumor Necrosis Factor-Like Cytokine 1A), which is expressed on antigen presenting cells and a variety of lymphocyte subtypes (24). The activation of TL1A plays important roles in chronic immune-mediated diseases and has been demonstrated in kidney-specific disease such as ANCA-associated renal vasculitis (24, 25). Interestingly, the risk allele at the TNFSF15 locus [rs6478109] had significantly different AF in patients with NS vs. cases in our cohort but did not have a significantly different AF when comparing corticosteroid therapy response (SSNS vs. SRNS), suggesting this allele is associated with NS but not a predictor of therapy response.
A second variant was identified by Jia et al. in the Tumor Necrosis Factor Receptor Super-Family Member 11A (TNFRSF11A) region (10). T-lymphocytes are known to express RANKL (Receptor Activator of NF- κB Ligand) and to act on dendritic cells which are a major antigen presenting cell (26). In addition, the Receptor Activator of NF- κB (RANK) + RANKL signaling is important in the negative selection during T-lymphocyte development (27). In the present study, the risk allele for this locus was found to have a significantly different AF in NS cases vs. controls, indicating association with disease.
Finally, highly penetrant pathogenic variants in NPHS1 are known causes of monogenic nephrotic syndrome. This is interesting, as previously reported and in the present study, common variants in this same gene are risk factors for NS. More recently, autoantibodies to nephrin have been reported to be associated with minimal change disease (MCD), the clinicopathologic correlate of SSNS. It is unclear if the variants in NPHS1 in the presence of permissive HLA variants are the drivers of autoantibody production against nephrin (28). Further studies to examine the relationship between NPHS1 and HLA variants with nephrin autoantibodies are warranted, in light of our findings.
Beside validating these nine genetic risk variants, we more importantly are the first to demonstrate the variant burden for corticosteroid therapy response in a large, multi-race cohort. As the variant burden increased, the severity of NS potentially increased. Our findings validate and expand on the work of Debiec and colleagues who found that having a higher variant burden, specifically having three or four of HLA-D risk alleles (rs1063348 and rs28366266) was associated with disease course in patients with NS. Variant burden has been studied in several other immune-mediated diseases in both additive and non-additive manners and have been found to have similar results with variant burden explaining phenotypic variance (29, 30).
Beyond predicting disease development, identifying ways to predict response to traditional therapies is important. The use of immunomodulatory therapy should not be taken lightly as these medications have potentially serious side effects. For NS in particular, corticosteroid therapy is the treatment of choice for the initial presentation (31). Therefore, children are begun on this therapy and response is monitored (namely, resolution of proteinuria). Most patients respond well to corticosteroids (32). However, a subset of patients will not respond to corticosteroid therapy (i.e., will not experience remission of proteinuria, termed SRNS), therefore identification of predictors of therapy response will avoid unnecessary exposure of this subgroup to major side effects of corticosteroid.
While clinical factors have not been shown to definitively predict disease course or development, risk prediction scores similar to polygenic risk scores for cardiovascular disease have been proposed for childhood SSNS (33–35). Ideally, combining clinical factors at diagnosis with genetic testing for both risk variants burden and monogenic causes of NS could be developed into a similar risk score to predict corticosteroid therapy response in patients with childhood-onset NS in order to mitigate harmful side effects of prolonged corticosteroid exposure and streamline to more targeted B and T cell therapies.
Although our study demonstrated promising findings, limitations of this study exist. One such limitation included a lack of racially matched controls. We attempted to mitigate this effect by choosing control data from public databases containing genetic information from a multitude of studies conducted worldwide (13–15). Additionally, we relied on self-reported race and our sample size was suboptimal for risk stratification by race. Moreover, as this was a retrospective analysis, we did not have intermediate outcomes such as frequent relapsing, steroid dependent, and late steroid responsiveness. Finally, genotyping for APOL1 CKD risk genotype was not available for our cohort. However, previous studies have shown that the APOL1 high-risk genotype is strongly associated with SRNS but not SSNS in individuals of African ancestry (36).
In conclusion, our study confirmed that certain genetic risk variants are associated with NS as well as pattern of corticosteroid therapy response in patients with childhood-onset NS. We determined that variant burden of these risk loci is associated with pattern of therapy response. This data suggests that this six variant panel may be a promising tool to determine corticosteroid response and guide NS management. Future prospective studies are needed to confirm these observations and investigate the mechanisms by which each risk loci contributes to disease development and therapy response.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to the risk of patient identification through genotype level data. However, data are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Duke University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
RC, EC, AO, and AJ, and RG contributed to conception and design of the study. RC, RG, MC, KH and BL performed genotyping and organized the database. RC, TT, and AO performed the statistical analysis. RC wrote the first draft of the manuscript. RC, EC, TT, AO, and RG wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1248733/full#supplementary-material
References
1. Veltkamp F, Rensma LR, Bouts AHM. Incidence and relapse of idiopathic nephrotic syndrome: meta-analysis. Pediatrics. (2021) 148(1):e2020029249. doi: 10.1542/peds.2020-029249
2. Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A report of the international study of kidney disease in children. Kidney Int. (1981) 20(6):765–71. doi: 10.1038/ki.1981.209
3. Trautmann A, Schnaidt S, Lipska-Ziętkiewicz BS, Bodria M, Ozaltin F, Emma F, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. (2017) 28(10):3055–65. doi: 10.1681/asn.2016101121
4. Lipska-Ziętkiewicz BS. Genetic steroid-resistant nephrotic syndrome overview. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, editors. Genereviews(®). Seattle, WA: University of Washington. (1993). Copyright © 1993–2023, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.
5. Horinouchi T, Nozu K, Iijima K. An updated view of the pathogenesis of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. (2022) 37(9):1957–65. doi: 10.1007/s00467-021-05401-4
6. Gbadegesin RA, Adeyemo A, Webb NJ, Greenbaum LA, Abeyagunawardena A, Thalgahagoda S, et al. Hla-Dqa1 and Plcg2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. (2015) 26(7):1701–10. doi: 10.1681/asn.2014030247
7. Debiec H, Dossier C, Letouzé E, Gillies CE, Vivarelli M, Putler RK, et al. Transethnic, genome-wide analysis reveals immune-related risk alleles and phenotypic correlates in pediatric steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. (2018) 29(7):2000–13. doi: 10.1681/asn.2017111185
8. Dufek S, Cheshire C, Levine AP, Trompeter RS, Issler N, Stubbs M, et al. Genetic identification of two novel loci associated with steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. (2019) 30(8):1375–84. doi: 10.1681/asn.2018101054
9. Jia X, Horinouchi T, Hitomi Y, Shono A, Khor SS, Omae Y, et al. Strong association of the hla-dr/dq locus with childhood steroid-sensitive nephrotic syndrome in the Japanese population. J Am Soc Nephrol. (2018) 29(8):2189–99. doi: 10.1681/asn.2017080859
10. Jia X, Yamamura T, Gbadegesin R, McNulty MT, Song K, Nagano C, et al. Common risk variants in Nphs1 and Tnfsf15 are associated with childhood steroid-sensitive nephrotic syndrome. Kidney Int. (2020) 98(5):1308–22. doi: 10.1016/j.kint.2020.05.029
11. Kdigo 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100(4s):S1–s276. doi: 10.1016/j.kint.2021.05.021
12. Sherry ST, Ward M, Sirotkin K. Dbsnp-database for single nucleotide polymorphisms and other classes of Minor genetic variation. Genome Res. (1999) 9(8):677–9. doi: 10.1101/gr.9.8.677
13. Phan L JY, Zhang H, Qiang W, Shekhtman E, Shao D, Revoe D, et al. Alfa: allele frequency aggregator.: national center for biotechnology information, U.S. National Library of Medicine. (2020). Available from: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (Cited 2022).
14. Taliun D, Harris DN, Kessler MD, Carlson J, Szpiech ZA, Torres R, et al. Sequencing of 53,831 diverse genomes from the nhlbi topmed program. Nature. (2021) 590(7845):290–9. doi: 10.1038/s41586-021-03205-y
15. Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint Spectrum quantified from variation in 141,456 humans. Nature. (2020) 581(7809):434–43. doi: 10.1038/s41586-020-2308-7
18. Fan A, Jiang X, Mo Y, Tan H, Jiang M, Li J. Plasma levels of oxidative stress in children with steroid-sensitive nephrotic syndrome and their predictive value for relapse frequency. Pediatr Nephrol. (2016) 31(1):83–8. doi: 10.1007/s00467-015-3195-2
19. Abdel-Hafez MA, Abou-El-Hana NM, Erfan AA, El-Gamasy M, Abdel-Nabi H. Predictive risk factors of steroid dependent nephrotic syndrome in children. J Nephropathol. (2017) 6(3):180–6. doi: 10.15171/jnp.2017.31
20. Dakshayani B, Lakshmanna M, Premalatha R. Predictors of frequent relapsing and steroid-dependent nephrotic syndrome in children. Turk Pediatri Ars. (2018) 53(1):24–30. doi: 10.5152/TurkPediatriArs.2018.5749
21. Basak AJ, Maiti S, Hansda A, Mahata D, Duraivelan K, Kundapura SV, et al. Structural insights into N-terminal IgV domain of BTNL2, a T cell inhibitory molecule, suggests a non-canonical binding interface for its putative receptors. J Mol Biol. (2020) 432(22):5938–50. doi: 10.1016/j.jmb.2020.09.013
22. Swanson RM, Gavin MA, Escobar SS, Rottman JB, Lipsky BP, Dube S, et al. Butyrophilin-Like 2 modulates B7 costimulation to induce Foxp3 expression and regulatory T cell development in mature T cells. J Immunol. (2013) 190(5):2027–35. doi: 10.4049/jimmunol.1201760
23. Malik U, Javed A, Ali A, Asghar K. Structural and functional annotation of human FAM26F: a multifaceted protein having a critical role in the immune system. Gene. (2017) 597:66–75. doi: 10.1016/j.gene.2016.10.029
24. Valatas V, Kolios G, Bamias G. Tl1a (Tnfsf15) and Dr3 (Tnfrsf25): a co-stimulatory system of cytokines with diverse functions in gut mucosal immunity. Front Immunol. (2019) 10:583. doi: 10.3389/fimmu.2019.00583
25. Maruyama H, Hirayama K, Nagai M, Ebihara I, Shimohata H, Kobayashi M. Serum decoy receptor 3 levels are associated with the disease activity of MPO-ANCA-associated renal vasculitis. Clin Rheumatol. (2016) 35(10):2469–76. doi: 10.1007/s10067-016-3321-y
26. Walsh MC, Choi Y. Regulation of T cell-associated tissues and T cell activation by RANKL-RANK-OPG. J Bone Miner Metab. (2021) 39(1):54–63. doi: 10.1007/s00774-020-01178-y
27. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. (2014) 5:511. doi: 10.3389/fimmu.2014.00511
28. Watts AJB, Keller KH, Lerner G, Rosales I, Collins AB, Sekulic M, et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol. (2022) 33(1):238–52. doi: 10.1681/asn.2021060794
29. Lenz TL, Deutsch AJ, Han B, Hu X, Okada Y, Eyre S, et al. Widespread non-additive and interaction effects within hla loci modulate the risk of autoimmune diseases. Nat Genet. (2015) 47(9):1085–90. doi: 10.1038/ng.3379
30. Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM, et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet. (2015) 47(8):898–905. doi: 10.1038/ng.3353
31. Trautmann A, Vivarelli M, Samuel S, Gipson D, Sinha A, Schaefer F, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. (2020) 35(8):1529–61. doi: 10.1007/s00467-020-04519-1
32. Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child. (1982) 57(7):544–8. doi: 10.1136/adc.57.7.544
33. Barry A, McNulty MT, Jia X, Gupta Y, Debiec H, Luo Y, et al. Multi-population genome-wide association study implicates immune and non-immune factors in pediatric steroid-sensitive nephrotic syndrome. Nat Commun. (2023) 14(1):2481. doi: 10.1038/s41467-023-37985-w
34. Downie ML, Gupta S, Chan MMY, Sadeghi-Alavijeh O, Cao J, Parekh RS, et al. Shared genetic risk across different presentations of gene test-negative idiopathic nephrotic syndrome. Pediatr Nephrol. (2023) 38(6):1793–800. doi: 10.1007/s00467-022-05789-7
35. Abraham G, Rutten-Jacobs L, Inouye M. Risk prediction using polygenic risk scores for prevention of stroke and other cardiovascular diseases. Stroke. (2021) 52(9):2983–91. doi: 10.1161/strokeaha.120.032619
Keywords: nephrotic syndrome, SRNS, SSNS, risk factors, genetics
Citation: Cason RK, Chambers E, Tu T, Chryst-Stangl M, Huggins K, Lane BM, Ochoa A, Jackson AM and Gbadegesin RA (2023) Genetic risk variants for childhood nephrotic syndrome and corticosteroid response. Front. Pediatr. 11:1248733. doi: 10.3389/fped.2023.1248733
Received: 27 June 2023; Accepted: 22 September 2023;
Published: 6 October 2023.
Edited by:
Aditi Sinha, All India Institute of Medical Sciences, IndiaReviewed by:
Andrew Mallett, Townsville University Hospital, AustraliaDaw-Yang Hwang, National Health Research Institutes, Taiwan
© 2023 Cason, Chambers, Tu, Chryst-Stangl, Huggins, Lane, Ochoa, Jackson and Gbadegesin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rasheed A. Gbadegesin cmFzaGVlZC5nYmFkZWdlc2luQGR1a2UuZWR1
†These authors have contributed equally to this work
 Rachel K. Cason
Rachel K. Cason Eileen Chambers
Eileen Chambers Tiffany Tu2
Tiffany Tu2 Megan Chryst-Stangl
Megan Chryst-Stangl Brandon M. Lane
Brandon M. Lane Annette M. Jackson
Annette M. Jackson Rasheed A. Gbadegesin
Rasheed A. Gbadegesin