- 1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Nutrition Research Center, Department of Community Nutrition, Faculty of Nutrition and Food Science, Tabriz University of Medical Sciences, Tabriz, Iran
Background: An infant's gut microbiome plays a vital role in their health, and various factors can impact their gut microbiota composition. This review aimed to summarize the current knowledge regarding the associations between maternal prenatal supplementation with vitamin D and the composition of infants' gut microbiota.
Method: A comprehensive systematic search was done on Web of Science, Scopus, PubMed, ScienceDirect, and Google Scholar databases without date restrictions until December 2022 using relevant keywords. All relevant original articles in English were eligible for the present review.
Results: Eight articles (two mice, three randomized clinical trials, and three cohort studies) were included in this review. The included mice studies reported that maternal prenatal vitamin D supplementation significantly affects the offspring's gut microbiome composition (such as enhancing the abundance of colonic Bacteroides). Moreover, the included cohort studies revealed a significant association between maternal supplementation with vitamin D during pregnancy and the infant's gut microbiome. However, one-third of clinical trials indicated that vitamin D levels in utero could influence the colonization of the microbial community in the infant's gut.
Conclusion: The findings of this review revealed that maternal vitamin D supplementation during pregnancy was linked to an infant's gut microbiome and could impact their gut microbiota composition. However, more studies are warranted to confirm these results.
Introduction
The human microbiota, especially the gut microbiota, is a diverse and rich community of microbes that plays an essential role in disease and health (1). The gut microbiota has critical roles in early postpartum immune development and in shaping the immune system functions throughout life (2). It has been demonstrated that the changes in microbiota are linked with various immune-mediated diseases (3). Obesity, inflammatory bowel disease, and other metabolic illnesses may result from an imbalance in the composition of the gut microbiome (4, 5).
The homeostasis of intestinal bacteria's important contribution to disease etiology emphasizes the need to recognize modifiable factors influencing the composition of intestinal bacteria (6). Several factors, such as diet, genetics, route of delivery, and antibiotic usage, can affect the diversity of gut microbiota (7, 8). The gut microbiota of an infant is initially seeded by the microbiota of the mother (9). The diet of pregnant and lactating women has been connected with the breastmilk microbiota changes and, as a result, in the gut microbiota of infants, with breastfed infants showing more significant effects than formula-fed infants (10). Recent research indicates that maternal nutrition could influence the shaping of the infant's gut microbiota, lending credence to the potential of maternal nutritional interventions to promote health outcomes and decrease disease risks among infants (11). Dietary nutrient intake, such as vitamin D [1,25(OH)2D], an essential fat-soluble vitamin, is required during pregnancy for the optimal health of the pregnant individual and fetus (12). In addition to the bone health of the offspring, maternal vitamin D levels could impact the other health outcomes of the child (13–15).
According to the current evidence, some of the vitamin D health benefits might be through affecting the gut flora (16–19). As a result, vitamin D may influence the function and structure of the gut microbiome (20, 21). Prenatal vitamin D levels may affect the infant gut microbiome by influencing the maternal microbiome (22). However, a clear overview of the existing evidence for this notion is lacking, and there is uncertainty about whether maternal prenatal supplementation with vitamin D could impact the composition of infants' gut flora. To address this question, a comprehensive systematic search was done to summarize the findings of studies investigating the association between maternal prenatal vitamin D supplementation and infants' gut microbial composition.
Methods
Search strategy
This systematic review was performed by considering the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (23) (Supplementary Table S1). The protocol of the current study was approved and registered by the Research Vice-Chancellor of Tabriz University of Medical Sciences (ethical code: IR.TBZMED.REC.1401.258). Electronic databases of the Web of Sciences, PubMed, ScienceDirect, Scopus, and Google Scholar were searched without date restrictions until December 2022 by the MESH terms and the following keywords: maternal OR pregnancy (mesh) OR “pregnant women” (mesh) OR prenatal OR growth OR mother AND “vitamin D” (mesh) OR Calciferol OR “1,25(OH)2D” OR “1,25-dihydroxyvitamin D” AND “gastrointestinal microbiome” (mesh) OR microbiota (mesh) OR “human microbiome” (mesh) OR microbiome (mesh) OR microflora OR “gut flora” AND infants OR infancy OR neonate OR newborn OR baby OR babies OR offspring. To ensure the inclusion of all eligible studies, a separate search was conducted through Google, and the references of included studies were reviewed. Supplementary Table S2 displays the strategy of search.
Articles screening and selection criteria
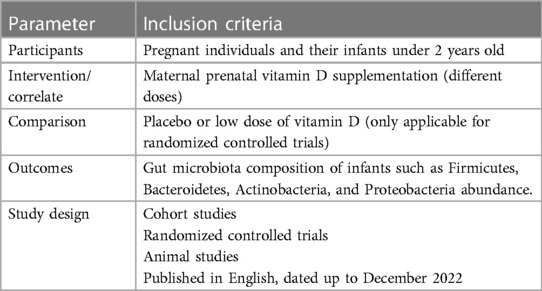
The extracted studies were saved in EndNote software, and duplicate studies were removed. The remaining titles and abstracts were screened to identify studies with the correct scope for the current review. Then, the original full-text English-language articles were selected among the screened articles and critically and separately analyzed for eligibility. All peer-reviewed animal and human studies that addressed the findings regarding the effects or association of maternal prenatal vitamin D supplementation on/with an infant's gut microbiota were eligible for inclusion in this study. Since routine maternal vitamin D supplementation is done during pregnancy and lactation, the cohort studies that evaluated the association between maternal vitamin D levels in pregnancy and the infant's microbiome met the eligibility criteria for inclusion in the present review. The PICOS (Population, Intervention, Comparison, Outcome, and Study Design) principles were used to structure the study's inclusion and exclusion criteria (Table 1), and discrepancies in selecting the included studies were resolved by discussion. PICO criteria for this review were as follows: Population: pregnant individuals and their infants under 2 years old; Intervention: maternal prenatal vitamin D supplementation (different doses); Comparison: placebo or low doses of vitamin D; and Outcome: gut microbiota composition of infants such as Firmicutes, Bacteroidetes, Clostridales, and Lachnobacterium abundance.
Reviews, abstracts, conference papers, editorials, book chapters, posters, letters, theses, and generic studies were not included. Studies that measured the effects of infants' vitamin D supplementation on their gut microbiota were excluded. Studies that the full texts were not available were also excluded.
Data extraction
The extracted data from the eligible studies were the following: the authors' name, publication year, study design and location, the number of participants, mean age of infants and mothers, infant sex, maternal supplementation, breastfeeding status, confounders considered in the analysis, and findings concerning the effects of maternal supplementation with vitamin D during pregnancy on infant's gut microbiota (diversity and abundance of bacterial taxa such as Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria). Moreover, the method and duration of vitamin D treatment were extracted from the included studies.
Risk of bias assessment
The quality of the mice studies, cohort studies, and randomized controlled trials (RCTs) was evaluated by the Office of Health Assessment and Translation (OHAT) Handbook (24), the Newcastle–Ottawa scale, and the Cochrane Handbook, respectively. For each risk of bias question, the OHAT tool offers the following response options: definitely a high risk of bias (score = −2), probably a high risk of bias (score = −1), probably a low risk of bias (score = +1), or definitely a low risk of bias (score = +2). After answering questions, numerical values were given to each question from −2 to +2, and according to the calculated average for each included study, studies were tiered to three levels of high, moderate, and low risk of bias, using the OHAT recommendations. Based on Approach 1, Tier 1 studies had probably low or low risk of bias, Tier 3 studies had probably high or high risk of bias, and Tier 2 studies had none of Tier 1 or Tier 3 criteria. Approach 2 calculates the overall average ratings of all questions, then using a mean rating per the study, each study is assigned to one of the three tiers based on the cutoffs of more than 0.7, 0.7 to −0.6, and less than −0.6 for Tiers 1–3, respectively (25, 26).
Assessment using the Newcastle–Ottawa scale was divided into three sections: selection, comparability, and outcome or exposure evaluation. Its score for cohort studies had a maximum of 9 points, and the study with an overall score between 6 and 9 points (≥3 points for the selection, 1 point for the comparability, and ≥2 points for the outcome or exposure section) was of good quality (27, 28). The Cochrane Collaboration's tool includes six domains of bias and three risk-of-bias response options: “low,” “high,” and “unclear” (25). Each included study was considered to have a “low risk of bias” if it had a low risk of bias in all three key domains (selection, detection, and performance domains), an “unclear risk of bias” if it had an unclear risk of bias in one or more than one key domain, and a “high risk of bias” if it had a high risk of bias in one or more than one key domain (25, 29).
Results
Study selection
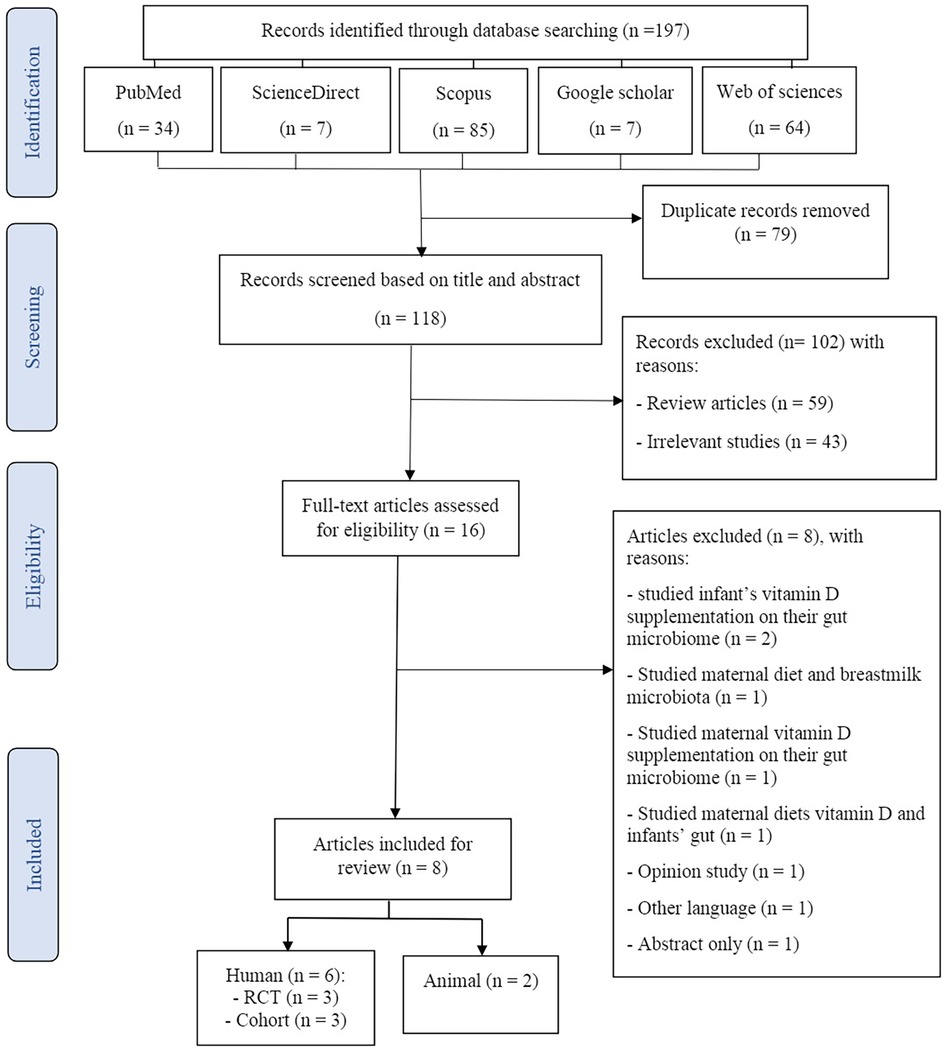
The process of search and study selection (PRISMA diagram) for this systematic review is shown in Figure 1. One hundred ninety-seven potential articles were retrieved through searching in PubMed (n = 34), Web of Science (n = 64), Scopus (n = 85), ScienceDirect (n = 7), and Google Scholar (n = 7) databases. After the elimination of duplicate articles, 118 studies remained for further screening. Of these, 104 studies were excluded based on the title and abstract screening of the articles in the first stage. During critical analysis, 16 articles were screened, of which 8 articles were excluded because these were studying the effects of infants' supplementation with vitamin D on their gut microbiome (n = 2) (30, 31), studying the association between maternal diet and breastmilk microbiota (n = 1) (32), studying the impacts of maternal vitamin D supplementation on their gut microbiome (n = 1) (33), studying the association of maternal diets' vitamin D and infants' gut (n = 1) (34), were opinion studies (n = 1) (35), were published in other languages (n = 1) (36), and were abstract only (n = 1) (37). Finally, eight articles (two mice, three RCTs, and two cohorts) were included in this systematic review.
Characterization of the included studies
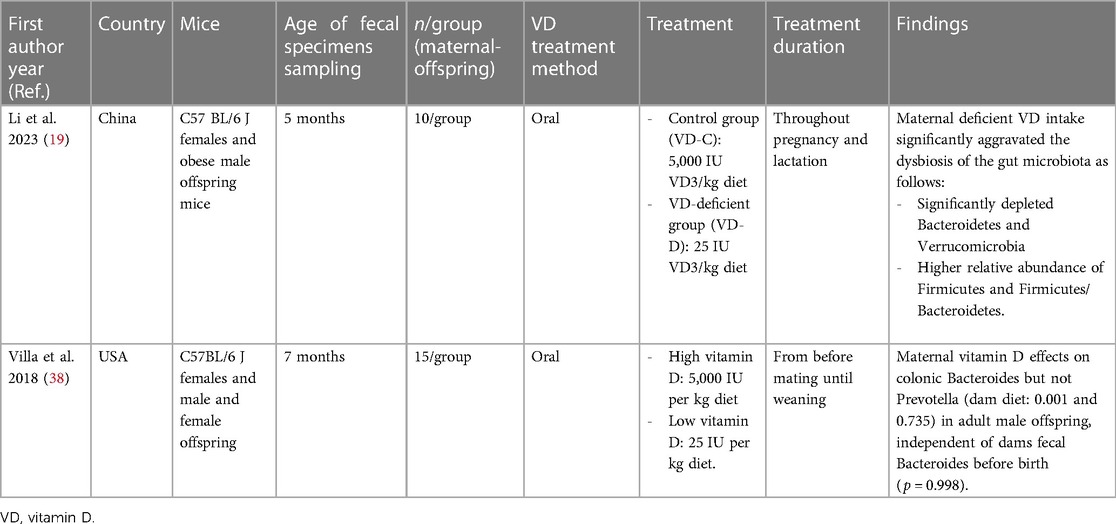
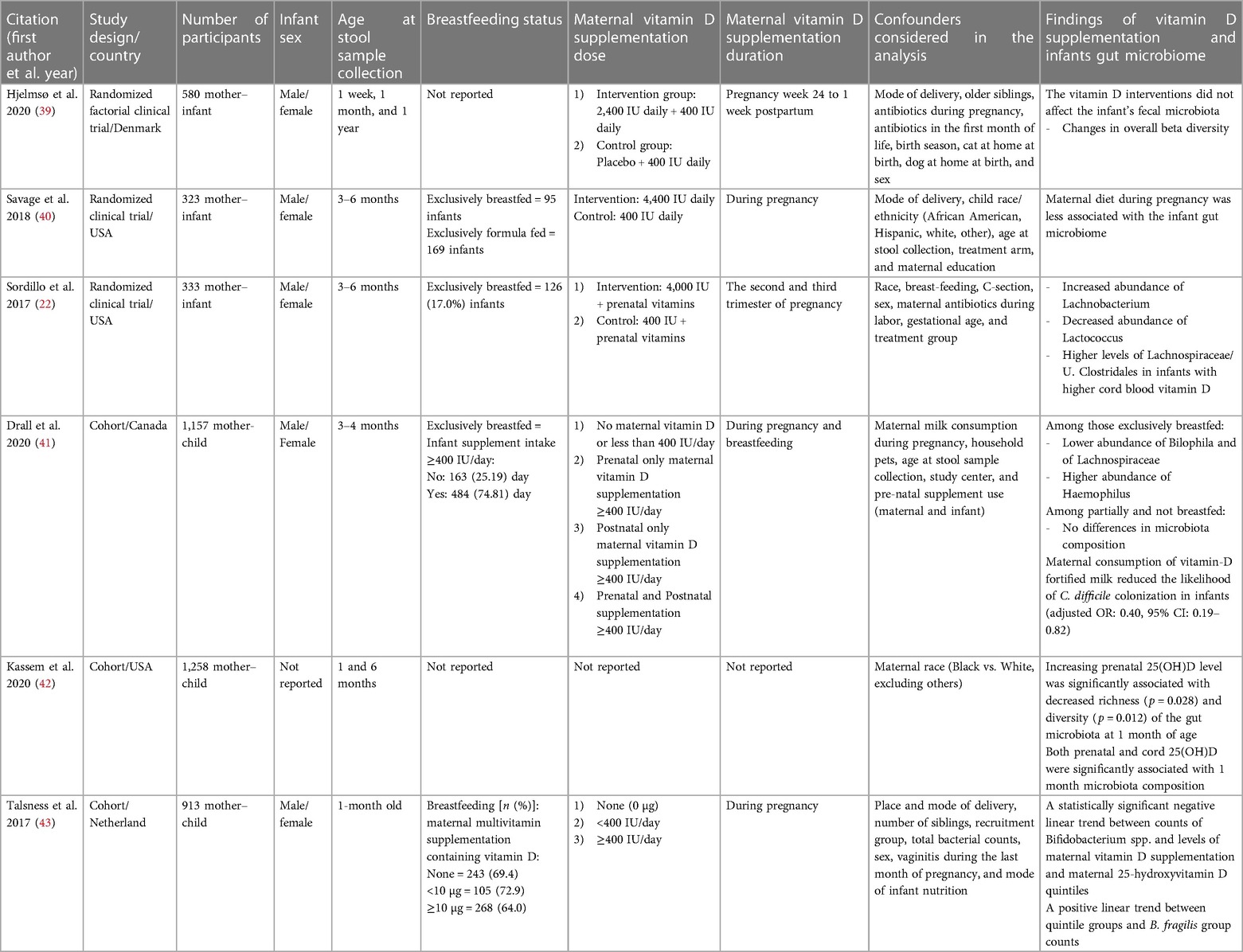
Tables 2, 3 present the main characteristics of the included studies. As shown in Table 2, two mice studies (19, 38) included 64 female C57BL/6 J mice that received 5,000 IU (high) and 25 IU (low) oral vitamin D during pregnancy. Three included RCTs were conducted from 2017 to 2020 and involved 1,236 mother–infant pairs. Pregnant individuals in intervention groups received 2,400, 4,000, and 4,400 IU vitamin D daily during week 24 pregnancy to 1 week postpartum (39), second and third trimesters (22), and all pregnancy time (40), respectively, while pregnant individuals in other groups received 400 IU routine vitamin D intake during these times. All women were instructed to continue 400 IU of vitamin D supplementation during pregnancy. In two RCT infants, stool samples were obtained at age 3–6 months (22, 40), and in one case, stool samples were collected on 1 week, 1 month, and 1 year after birth (39). Both sexes had been included in both three RCTs. Exclusively breastfeeding percentage of infants was 17% and 29.41% in two RCTs, and one case has not reported the breastfeeding status of included infants.
A total of 3,328 mother–child pairs participated in three included cohort studies. All the cohort studies included both sexes of infants. In included cohort studies, infants' stool samples were obtained at ages 3–4 months (41), 1 and 6 months (42), and 1 month after birth (43). The breastfeeding percentage of infants was 74.81% (41) and 64% (43) in vitamin D-supplemented infants, and one case has not reported the percentage of infants’ breastfeeding. Drall et al. (41) created four categories for maternal supplementation with vitamin D according to the Dietary Reference Intakes and Recommended Dietary Allowances of 400 and 600 IU/day vitamin D supplementation during pregnancy and breastfeeding, respectively (44, 45). Their four maternal supplementations of vitamin D categories are the following: no or less than 400 IU/day supplementation, only prenatal supplementation of ≥400 IU/day, only postnatal supplementation of ≥400 IU/day, and prenatal and postnatal supplementation with ≥400 IU/day vitamin D. In another cohort study also based on maternal multivitamin supplementation containing vitamin D, three categories for maternal vitamin D supplementation were considered, including none supplementation, <400, and ≥400 IU/day (43). All RCTs and cohort studies considered some possible confounders in their analysis.
Risk of bias assessment
Supplementary Table S3 shows the mouse studies' risk of bias assessment and tier classifications. Based on the OHAT's tier system, two mouse studies were classified as tier 1. One of the mice studies has not reported using of randomization in the mice's allocation into the groups and the blindness of outcome assessors (19), and other mice studies provided unclear data about adequately concealed allocation to study groups (38).
The included RCTs' risk of bias assessment details are described in Supplementary Table S4. Based on the Cochrane collaboration's criteria, all included RCTs had a low risk of bias. The personnel and participants were not blind in one of the RCTs (40), and others have not reported clear data about allocation concealment (39) and blinding of the outcome's assessor (22). The quality assessment of cohort studies resulted in the mean scores of 8 (41, 42) and 9 (43) as good quality (Supplementary Tables S5).
Outcomes
Table 3 summarizes the findings about vitamin D supplementation effects on offspring's gut flora composition. Moreover, a synoptic view of the main changes in the offspring's gut microbiome after maternal vitamin D administration is presented in Supplementary Table S6. Of eight included studies (two mice, three RCTs, and three cohorts), two mice and four humans (one RCT and three cohorts) studies (75%) demonstrated that maternal vitamin D supplementation during pregnancy was associated with the infant's gut microbiome and could impact gut microbiota composition such as Firmicutes and Bacteroidetes phyla. The description of the included studies is as follows.
Four included studies (one micee, one RCT, and two cohorts) revealed that maternal prenatal supplementation with vitamin D was associated with Firmicutes phylum abundance (19, 22, 41, 42). Li et al. indicated that vitamin D deficiency in the maternal significantly aggravated the dysbiosis of the mice's gut microbiota, with a relatively high abundance of Firmicutes (Lachnoclostridium, Lactobacillus, Ruminiclostridium_9, and Romboutsia), and high Firmicutes/Bacteroidetes ratio (19). Moreover, Sordillo et al. suggested that vitamin D levels in utero could impact the colonized microbial community of the infant's gut. They observed that the levels of Lachnospiraceae/U. Clostridales were higher in infants with higher vitamin D levels in cord blood, and multivariate analysis showed an increased Lachnobacterium abundance and a decreased Lactococcus abundance (22). Drall et al. also showed that maternal prenatal supplementation with vitamin D was related to lower Bilophila and Lachnospiraceae abundance but increased Haemophilus abundance in exclusively breastfed offspring (41). Another cohort study reported that prenatal 25(OH)D levels were positively associated with the microbiota composition of 1-month-aged infants including Acinetobacter, Corynebacterium, and Ruminococcus gnavus (42).
Two mouse studies mentioned above also reported that maternal prenatal vitamin D supplementation had an effect on the Bacteroidetes (Bacteroides, Alliprevotella, and Akkermansia) abundant in offspring mice gut microbiome (19, 38). Moreover, Talsness et al., in a cohort study, revealed similar findings. They reported a positive relationship between maternal 25(OH)D quintiles and Bacteroides fragilis group counts in the gut microbiota of 1-month-old infants. In addition, they indicated a negative linear trend between maternal supplementation of vitamin D and maternal 25(OH)D quintiles with Bifidobacterium spp. counts as a genus of Actinobacteria phylum. Their results suggested that vitamin D was associated with several key bacterial taxa abundance within the infants' microbiota (43).
Finally, two RCT studies reported no significant findings (39, 40). Hjelmsø et al. (39) demonstrated that the vitamin D interventions did not affect the infant's fecal microbiota, and Savage et al. (40) indicated a week association between the diet of maternal during pregnancy and the composition of the infant's gut microbiome. Overall, the included RCTs revealed mixed results for the effect of maternal prenatal vitamin D supplementation on the gut microbiome of infants (Table 3).
Discussion
Gut microbiota has a significant role in health and disease, and it is now recognized as a human organ that influences other organs (46). Several factors, such as dietary nutrients, could change the balance of gut microbiota (47). The present systematic review, using published animal, RCT, and cohort data, provides a comprehensive assessment of the association between maternal supplementation with vitamin D during pregnancy and the infants' gut microbiome. All the included studies, except two cases, demonstrated that maternal vitamin D supplementation during pregnancy was linked with the infants' gut microbiome and could impact the infant's gut microbiota composition.
The typical microbiota involves four main phyla (Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria) (48), and the two major bacterial phyla in human feces are Firmicutes and Bacteroidetes (48, 49). Li et al. reported that maternal deficiency in vitamin D intake during pregnancy results in aggravated dysbiosis of the gut microbiota including the depletion of Bacteroidetes and Verrucomicrobia, and enhancement of the Firmicutes abundance and Firmicutes/Bacteroidetes ratio in the offspring's gut microbiota (19). Villa et al. (38) also showed that maternal vitamin D enhanced the abundance of colonic Bacteroides in infants. Thus, maternal vitamin D supplementation could change the infant's microbiota toward an increase in Bacteroidetes and a decrease in Firmicutes. Many Bacteroidota are symbiotic organisms that have adapted well to the digestive tract. Bacteroidota colonizes the infant's gastrointestinal system because nondigestible oligosaccharides in the mother's milk promote the growth of Bacteroides and Bifidobacterium spp. (50). A change in the gut flora composition to higher Bacteroidetes levels and lower Firmicutes levels may benefit the host, whereas an increase in Firmicutes may contribute to gut barrier failure (51, 52).
The association between vitamin D levels and human gut microbiota composition is sufficiently evident (18). The maternal gut microbiota is also linked to maternal dietary vitamin D consumption (33). However, only a few studies have investigated the effects of maternal prenatal vitamin D supplementation on the infants' gut microbiome and the potential links between maternal vitamin D levels and gut flora in early life. Maternal supplementation with vitamin D during pregnancy was negatively associated with Bifidobacterium species counts of 1-month-old infants' gut microbiota in the KOALA birth cohort, while the counts of Clostridioides difficile were reduced in 1-month-old infants' gut flora following prenatal vitamin D supplementation of pregnant individuals (43). Although asymptomatic Clostridioides difficile colonizes approximately 30% of newborns, it has been related to a disruption in gut microbiota composition and later pediatric allergy illness (53). The 25(OH)D levels of cord blood were linked with higher Lachnobacterium levels and lower Lactococcus levels in the Trial of Vitamin D Antenatal Asthma Reduction (22). Drall et al. also showed an association between maternal prenatal and postnatal vitamin D supplementation (≥400 IU/day) and lower Bilophila spp. abundance (41). Bilophila has been associated with colitis and inflammation in mice (54, 55) and colic in infants (56). These results suggest that maternal vitamin D levels could affect several key bacterial taxa in the gut microbiome of infants.
Vitamin D is involved in several physiological processes, including gut microbiota preservation and the exclusion of opportunistic bacteria. The receptors of vitamin D are highly expressed in the intestinal enterocytes, in particular, the proximal colon, and contribute to the production of antimicrobial peptides such as cathelicidins, defensins, claudins, and zonulin occludes (57–59). In other words, vitamin D is a well-known upregulating factor for the gene expression of antimicrobial peptides in various cell types, such as colonic cells (60). Selective killing of pathogenic bacteria would result in greater opportunity for colonization with healthy bacteria. Fermentation products of symbiotic bacteria could upregulate intestinal expression of the vitamin D receptor (VDR) and downregulate inflammation (61). Vitamin D also plays a role in maintaining the mucosal barrier function by upregulating the expression of tight junction and adherent junction proteins and suppressing epithelial cell apoptosis that maintains the integrity and function of the gut barrier (57–59). Furthermore, Li et al. (19) found that proinflammatory cytokine and lipid transport molecule gene expression was greater in the vitamin D deficient group, while intestinal barrier function was lower in their offspring than in the vitamin D control group. There were strong connections between intestinal microbial community dysbiosis and gene expression in the ileum and colon linked with inflammation, barrier function, and lipid transport (19). Because inflammation helps harmful bacteria overcome resident bacteria for colonization resistance, reducing intestinal inflammation promotes microbiota balance (61). Vitamin D is well known to have a pivotal role in intestinal homeostasis by binding with the VDR and subsequently affecting the expressions of relevant genes on the intestinal epithelial barrier and chronic inflammatory state (20). Vitamin D by reinforcing intercellular junctions contributes to maintaining the mucosal barrier function. Disrupting the gut barrier function exacerbates infection with pathogenic bacteria and enhances inflammation, which then has downstream influences on the gut microbiota (18–20). Overall, vitamin D status can alter the gut microbiota because it stimulates anti-inflammatory responses and prevention of infections by the immune system. As a result, vitamin D status is a modifiable factor that is important in the prevention of immune-mediated illnesses by influencing the maintenance of gut microbiota homeostasis (43).
Strengths and limitations
The inclusion of both animal and human studies in this review makes this study a comprehensive systematic review. However, there were some limitations to this study. The small number of included animal, RCT, and cohort studies was the main limitation of this systematic review. The included RCTs also used different doses of vitamin D. Furthermore, due to the heterogeneity of the results reported among the included studies, it was not possible to perform a meta-analysis. In addition, stool samples were collected at different time points; future studies should collect more samples over the first year of life. In the included studies, infant microbiota was not sampled right after birth. This would have allowed us to detect a possible fecal bacterial transfer event during birth.
Conclusion
The findings of the present systematic review suggest a significant association between maternal supplementation with vitamin D during pregnancy and the infant's gut microbiota composition. The included animal studies also reported that maternal prenatal vitamin D supplementation can influence the gut microbiota composition of offspring. However, the included clinical trials revealed mixed results. It seems that adequate maternal vitamin D intake during the prenatal period is an important factor in the maintenance of the infant's gut microbiota hemostasis. Further, well-designed RCTs are warranted to confirm the possible effects of maternal prenatal vitamin D supplementation on the infants' gut microbiota. Prospective cohort studies with a large number of infants are needed to examine the association between routine maternal vitamin D treatment during pregnancy and the infant's gut microbiota. Furthermore, mechanistic examinations on the effect of maternal vitamin D levels on the infants' gut microbiome are also suggested.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Both authors have made substantial contributions to the conception and design of this study. RM-G and MR conducted the search and data extraction, and the first draft of the manuscript was written by RM-G, and MR commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The current study was approved and supported by the Nutrition Research Center and the Research Vice-Chancellor of Tabriz University of Medical Sciences, Tabriz, Iran (Grant number: 69697).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1248517/full#supplementary-material
References
1. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. (2012) 70(Suppl_1):S38–44. doi: 10.1111/j.1753-4887.2012.00493.x
2. Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. (2011) 69(6):465–72. doi: 10.1203/PDR.0b013e318217638a
3. Kosiewicz MM, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol. (2011) 2:180. doi: 10.3389/fmicb.2011.00180
4. Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. (2014) 588(22):4223–33. doi: 10.1016/j.febslet.2014.09.039
5. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. (2006) 444(7122):1022–3. doi: 10.1038/4441022a
6. Bischoff SC. ‘Gut health’: a new objective in medicine? BMC Med. (2011) 9:24. doi: 10.1186/1741-7015-9-24
7. Martin R, Nauta A, Ben Amor K, Knippels L, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. (2010) 1(4):367–82. doi: 10.3920/BM2010.0027
8. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107(26):11971–5. doi: 10.1073/pnas.1002601107
9. Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. (2018) 24(1):133–45.e5. doi: 10.1016/j.chom.2018.06.005
10. Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care. (2011) 41(6):158–76. doi: 10.1016/j.cppeds.2011.01.001
11. Zaidi AZ, Moore SE, Okala SG. Impact of maternal nutritional supplementation during pregnancy and lactation on the infant gut or breastmilk microbiota: a systematic review. Nutrients. (2021) 13(4):1137. doi: 10.3390/nu13041137
12. Wagner CL, Hollis BW, Kotsa K, Fakhoury H, Karras SN. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord. (2017) 18:307–22. doi: 10.1007/s11154-017-9414-3
13. Wegienka G, Havstad S, Zoratti EM, Kim H, Ownby DR, Johnson CC, et al. Association between vitamin D levels and allergy-related outcomes vary by race and other factors. J Allergy Clin Immunol. (2015) 136(5):1309–14.e4. doi: 10.1016/j.jaci.2015.04.017
14. Daraki V, Roumeliotaki T, Chalkiadaki G, Katrinaki M, Karachaliou M, Leventakou V, et al. Low maternal vitamin D status in pregnancy increases the risk of childhood obesity. Pediatr Obes. (2018) 13(8):467–75. doi: 10.1111/ijpo.12267
15. Antonucci R, Locci C, Clemente MG, Chicconi E, Antonucci L. Vitamin D deficiency in childhood: old lessons and current challenges. J Pediatr Endocrinol Metab. (2018) 31(3):247–60. doi: 10.1515/jpem-2017-0391
16. Wongwiwatthananukit S, Sansanayudh N, Phetkrajaysang N, Krittiyanunt S. Effects of vitamin D 2 supplementation on insulin sensitivity and metabolic parameters in metabolic syndrome patients. J Endocrinol Invest. (2013) 36:558–63. doi: 10.3275/8817
17. Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol Biol. (2016) 164:36–9. doi: 10.1016/j.jsbmb.2015.09.013
18. Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, et al. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. (2019) 58:2895–910. doi: 10.1007/s00394-018-1842-7
19. Li P, Wang Y, Li P, Chen X, Liu Y, Zha L, et al. Maternal vitamin D deficiency aggravates the dysbiosis of gut microbiota by affecting intestinal barrier function and inflammation in obese male offspring mice. Nutrition. (2023) 105:111837. doi: 10.1016/j.nut.2022.111837
20. Jin D, Wu S, Zhang Y-G, Lu R, Xia Y, Dong H, et al. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther. (2015) 37(5):996–1009.e7. doi: 10.1016/j.clinthera.2015.04.004
21. Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate–induced colitis. J Nutr. (2013) 143(10):1679–86. doi: 10.3945/jn.113.180794
22. Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3–6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol. (2017) 139(2):482–91.e14. doi: 10.1016/j.jaci.2016.08.045
23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151(4):W-65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
24. Ohat N. Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. US National Toxicology Program Office of Health Assessment and Translation. Available at: https://ntp.niehs.nih.gov/pubhealth/hat/review/index-2.html 2019;5(8): checked on March 4, 2019.
25. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. London, United Kingdom: John Wiley & Sons (2019).
26. Suh M, Wikoff D, Lipworth L, Goodman M, Fitch S, Mittal L, et al. Hexavalent chromium and stomach cancer: a systematic review and meta-analysis. Crit Rev Toxicol. (2019) 49(2):140–59. doi: 10.1080/10408444.2019.1578730
27. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hosp Res Inst. (2011) 2(1):1–12.
29. White CM, Pasupuleti V, Roman YM, Li Y, Hernandez AV. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2019) 146:104280. doi: 10.1016/j.phrs.2019.104280
30. Ma TF, Bu SH, Paneth N, Kerver JM, Comstock SS. Vitamin D supplementation in exclusively breastfed infants is associated with alterations in the fecal microbiome. Nutrients. (2022) 14(1):202–15. doi: 10.3390/nu14010202
31. Lei WT, Huang KY, Jhong JH, Chen CH, Weng SL. Metagenomic analysis of the gut microbiome composition associated with vitamin D supplementation in Taiwanese infants. Sci Rep. (2021) 11(1):2856-61. doi: 10.1038/s41598-021-82584-8
32. Cortes-Macías E, Selma-Royo M, García-Mantrana I, Calatayud M, González S, Martínez-Costa C, et al. Maternal diet shapes the breast milk microbiota composition and diversity: impact of mode of delivery and antibiotic exposure. J Nutr. (2021) 151(2):330–40. doi: 10.1093/jn/nxaa310
33. Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. (2016) 4(1):55. doi: 10.1186/s40168-016-0200-3
34. Villa CR, Chen J, Wen B, Sacco SM, Taibi A, Ward WE, et al. Maternal vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int J Obes. (2016) 40(12):1875–83. doi: 10.1038/ijo.2016.177
35. Rigon G, Vallone C, Lucantoni V, Signore F. Maternal factors pre- and during delivery contribute to gut microbiota shaping in newborns. Front Cell Infect Microbiol. (2012) 2:93. doi: 10.3389/fcimb.2012.00093
36. Chang XL, Shang Y, Liu YJ, Li P, Wang YY, Liang AM, et al. Effects of calcium supplementation during the pregnancy and early infancy stage on the body mass index and gut microbiota in the infants. Zhonghua yu fang yi xue za zhi [Chinese Journal of Preventive Medicine]. (2018) 52(6):642–6. doi: 10.3760/cma.j.issn.0253-9624.2018.06.014
37. Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, et al. Factors influencing the infant gut microbiome at age 3–6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol. (2017) 139(2):482–91.e14. doi: 10.1016/j.jaci.2016.08.045
38. Villa CR, Taibi A, Chen J, Ward WE, Comelli EM. Colonic Bacteroides are positively associated with trabecular bone structure and programmed by maternal vitamin D in male but not female offspring in an obesogenic environment. Int J Obes. (2018) 42(4):696–703. doi: 10.1038/ijo.2017.294
39. Hjelmsø MH, Shah SA, Thorsen J, Rasmussen M, Vestergaard G, Mortensen MS, et al. Prenatal dietary supplements influence the infant airway microbiota in a randomized factorial clinical trial. Nat Commun. (2020) 11(1):426. doi: 10.1038/s41467-020-14308-x
40. Savage JH, Lee-Sarwar KA, Sordillo JE, Lange NE, Zhou Y, O’Connor GT, et al. Diet during pregnancy and infancy and the infant intestinal microbiome. J Pediatr. (2018) 203:47–54.e4. doi: 10.1016/j.jpeds.2018.07.066
41. Drall KM, Field CJ, Haqq AM, de Souza RJ, Tun HM, Morales-Lizcano NP, et al. Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: implications for viral respiratory infections. Gut Microbes. (2020) 12(1):1799734. doi: 10.1080/19490976.2020.1799734
42. Kassem Z, Sitarik A, Levin AM, Lynch SV, Havstad S, Fujimura K, et al. Maternal and cord blood vitamin D level and the infant gut microbiota in a birth cohort study. Matern Health Neonatol Perinatol. (2020) 6:5. doi: 10.1186/s40748-020-00119-x
43. Talsness CE, Penders J, Jansen E, Damoiseaux J, Thijs C, Mommers M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA birth cohort study. PLoS One. (2017) 12(11):e0188011. doi: 10.1371/journal.pone.0188011
44. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press (1997).
45. Canada H. Vitamin D and calcium: updated dietary reference intakes. Health Canada Ottawa (Canada). Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/tables.html (Accessed May 1, 2019).
46. Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. (2015) 73(Suppl_1):32–40. doi: 10.1093/nutrit/nuv039
47. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505(7484):559–63. doi: 10.1038/nature12820
48. Gominak S. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med Hypotheses. (2016) 94:103–7. doi: 10.1016/j.mehy.2016.07.007
49. The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. (2012) 486:207–14. doi: 10.1038/nature11234
50. Rajilić-Stojanović M, De Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. (2014) 38(5):996–1047. doi: 10.1111/1574-6976.12075
51. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. (2010) 107(33):14691–6. doi: 10.1073/pnas.1005963107
52. Schippa S, Conte MP. Dysbiotic events in gut microbiota: impact on human health. Nutrients. (2014) 6(12):5786–805. doi: 10.3390/nu6125786
53. Gilca R, Fortin É, Frenette C, Longtin Y, Gourdeau M. Seasonal variations in Clostridium difficile infections are associated with influenza and respiratory syncytial virus activity independently of antibiotic prescriptions: a time series analysis in Quebec, Canada. Antimicrob Agents Chemother. (2012) 56(2):639–46. doi: 10.1128/AAC.05411-11
54. Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’hara R, et al. Community ecology package. R Package Version. (2013) 2(0):321–6.
55. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. (2011) 5(2):169–72. doi: 10.1038/ismej.2010.133
56. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B: Stat. (1995) 57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
57. Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front Immunol. (2016) 7:627. doi: 10.3389/fimmu.2016.00627
58. Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med. (2012) 33(1):77–82. doi: 10.1016/j.mam.2011.10.014
59. Wu S, Liao AP, Xia Y, Li YC, Li J-D, Sartor RB, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-κB activity in intestine. Am J Pathol. (2010) 177(2):686–97. doi: 10.2353/ajpath.2010.090998
60. Lagishetty V, Chun RF, Liu NQ, Lisse TS, Adams JS, Hewison M, et al. 1α-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol. (2010) 121(1–2):228–33. doi: 10.1016/j.jsbmb.2010.02.004
Keywords: vitamin D, maternal, pregnancy, gut microbiota, infant
Citation: Molani-Gol R and Rafraf M (2023) Maternal vitamin D in pregnancy and infant's gut microbiota: a systematic review. Front. Pediatr. 11:1248517. doi: 10.3389/fped.2023.1248517
Received: 27 June 2023; Accepted: 26 September 2023;
Published: 16 October 2023.
Edited by:
Pietro Vajro, University of Salerno, ItalyReviewed by:
Sarah Comstock, Michigan State University, United StatesDebora Porri, University of Pavia, Italy
© 2023 Molani-Gol and Rafraf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryam Rafraf cmFmcmFmbUB0YnptZWQuYWMuaXI=; cmFmcmFmbUB5YWhvby5jb20=
 Roghayeh Molani-Gol
Roghayeh Molani-Gol Maryam Rafraf
Maryam Rafraf