- 1Department of Orthopedics, Fourth Medical Center of PLA General Hospital, Beijing, China
- 2Department of Orthopedics, Fuzhou No.2 General Hospital (Fuzhou No.2 Hospital), Fuzhou, China
Purpose: The purpose of this study was to observe whether developmental dysplasia of the hip (DDH) affects the development of the femoral head growth plate and to analyze the risk factors.
Methods: We selected female patients aged between 11 and 20 years with unilateral DDH and unclosed femoral head growth plate (s). The selected patients underwent anteroposterior radiography of the hip joint to compare the degree of development of the femoral head growth plate on both sides and to identify risk factors that affect the development of the growth plate in the femoral head.
Results: We included 48 female patients with unilateral DDH, with an average age of 14 years (range: 11.1–18.5 years) and an average BMI of 20.4 kg/m² (range: 15.5 kg/m²−27.9 kg/m²). Among them, 23 patients had earlier development of the femoral head growth plate on the affected side than on the healthy side, while the degree of development of the femoral head growth plate in 25 patients was the same as that on the contralateral side. When the Tönnis angle was greater than 29.5°C and/or the Reimers migration index was greater than 48.5%, there was a statistically significant difference in the acceleration of femoral head growth plate development.
Conclusion: An abnormal relative position of the acetabulum–femoral head caused by DDH can accelerate closure of the femoral head growth plate in immature female patients. The risk factors are a Tönnis angle greater than 29.5°C and/or Reimers migration index greater than 48.5%.
Introduction
The epiphyseal plate is a crucial structure during human growth and development, serving as the foundation of skeletal development (1). In long bones, it's a cartilaginous structure located between the epiphysis and diaphysis, regulating the growth of the bone shaft through endochondral ossification until the epiphysis and diaphysis are completely fused. Abnormal development or damage to the growth plate may lead to severe limb deformities and length discrepancies during bone growth. The growth of the epiphyseal plate primarily depends on the activity of cartilage cells (2). Genetics, race, nutritional status, hormone secretion, and external environmental factors are among the many factors that may affect the activity of epiphyseal plate cartilage cells. Mechanical loading is a significant external factor that can regulate the growth of epiphyseal plate cartilage. Usually, the growth plate can be affected by various complex forces, such as compression, tension, and shear forces. As stress increases, the shape of the epiphyseal plate changes as it adapts to the mechanical influences (3, 4).
The treatment of developmental dysplasia of the hip (DDH) is linked to the maturity of hip joint development (5–8). The degree of epiphyseal plate development is a marker of skeletal maturity. We can assess the maturity of the hip joint by the degree of development of the femoral head growth plate. The hip joint is a ball-and-socket joint composed of the femoral head and the acetabulum. The development of the femoral head growth plate is related to the normal head-acetabulum relationship. When the head-acetabulum relationship changes, the mechanical load on the femoral head will also change. Some studies have revealed that changes in hip joint mechanical loading patterns during growth can cause proximal femoral deformities (9, 10). This suggests that the development of the proximal femoral epiphysis is related to hip joint loading pattern. Hip joint loading pattern is related to hip joint structural development. DDH patients typically have an abnormal head-acetabulum relationship, resulting in a smaller contact area between the femoral head and the acetabulum, leading to abnormal stress on the femoral head growth plate. The mechanical regulation of endochondral ossification in the epiphyseal plate and the pathological mechanism of progressive skeletal deformities are closely related, especially when skeletal development is immature (11, 12).
Based on the above, we hypothesize that in DDH patients with immature skeletal development, the abnormal head-acetabulum relationship of the hip joint will cause changes in the development of the proximal femoral head growth plate.
Patients and methods
Patient selection
From January 2014 to December 2020, a total of 128 female DDH patients between the ages of 11 and 20 were selected from our center. The inclusion criteria were as follows: (1) unilateral hip dysplasia, the affected hip joints of the patients all met the criteria of Tönnis angle more than 10°, lateral center-edge angle less than 25°C, and incomplete dislocation; (2) all patients included in the study had standard pelvic radiographs, without obvious pelvic rotation; (3) the affected hip joints had either simple developmental dysplasia or subluxation, and at least one of the femoral head epiphyses had not yet closed.
The exclusion criteria were as follows: (1) patients with bilateral femoral head growth plate completely closed; (2) history of hip joint surgery; (3) other diseases affecting bone epiphyseal development, such as multiple epiphyseal dysplasia (MED) and Legg-Calvé-Perthes disease (LCPD); (4) endocrine disorders and neuromuscular dysplasia that affect bone epiphyseal development.
According to the development of the femoral head growth plate on the affected side, the patients were divided into the group with earlier development of the femoral head growth plate on the affected side (i.e., the femoral head growth plate on the affected side developed earlier than that on the contralateral side) and the group with unaffected development of the femoral head growth plate on the affected side (i.e., the femoral head growth plate on the affected side had no change compared to that on the contralateral side).
Observation indicator
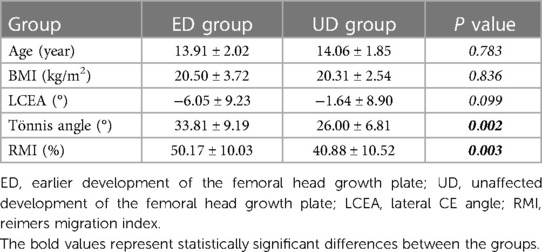
With reference to the female adolescents skeletal development indicators, based on the Tanner-Whitehouse 3 (TW3) method, the development of the femoral head growth plate was classified into four grades (see Figure 1) (13). Standard pelvic anterior-posterior radiographs were obtained using the Definium-6000 digital x-ray imaging system (GE-Healthcare) and standard x-ray imaging procedures. Two experienced pediatric orthopedic surgeons graded the degree of development of the femoral head growth plate on the PACS system, as well as measured the lateral CE angle, Tönnis angle, and Reimers migration index.
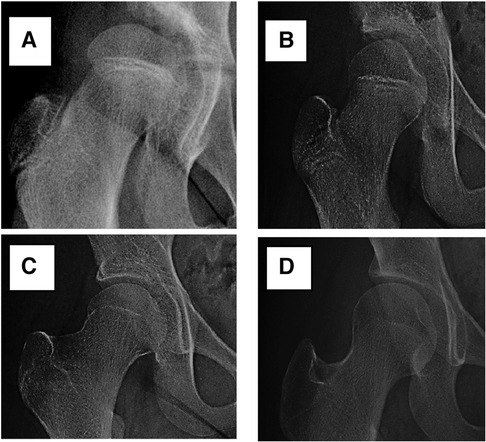
Figure 1. The developmental grades of the growth plate of the femoral head. Stage (A) (Grade 1): the beginning of the closure of the growth plate, with the chondrocyte gap narrowing and bone trabeculae visible. Stage (B) (Grade 2): partial closure of the growth plate (with less than 50% closure), usually starting from the middle and the chondrocyte gap between the trabeculae narrowing compared to Stage (A). Stage (C) (Grade 3): almost complete closure of the growth plate (with more than 50% closure), with some gaps still visible on one or both sides. Stage (D) (Grade 4): complete closure of the growth plate, with the residual or complete disappearance of the chondrocyte gap.
Statistical methods
SPSS 26 statistical software (IBM, Armonk, NY, USA) was used for statistical analysis. Independent sample t-tests were performed on the age, BMI, lateral CE angle, Tönnis angle, and Reimers migration index of the early-developing group and the unaffected group. P < 0.05 indicates statistical significance. The risk threshold value was calculated through the ROC curve.
Results
48 female patients with unilateral DDH were selected. Among them, 23 cases had earlier femoral head growth plate development on the affected side, while 25 patients had no affected femoral head growth plate development. The average age of the early femoral head growth plate development group was 13.9 years, while the average age of the unaffected femoral head growth plate group was 14.1 years (P = 0.783). The average BMI of the early femoral head growth plate development group was 20.5 kg/m², while that of the unaffected femoral head growth plate group was 20.3 kg/m² (P = 0.836).
In radiographic measurement, the average lateral CE angle of the early femoral head growth plate development group was −6.1°C, while that of the unaffected femoral head growth plate group was −1.6°C (P = 0.099). The average Tönnis angle of the early femoral head growth plate development group was 33.8°C, while that of the unaffected femoral head growth plate group was 26.0°C (P = 0.002). The average Reimers migration index of the early femoral head growth plate development group was 50%, while that of the unaffected femoral head growth plate group was 41% (P = 0.003) (Table 1). By calculating the risk threshold value through ROC curve (Figure 2), when the Tönnis angle is greater than 29.5°C and/or Reimers migration index is greater than 48.5%, accelerated the development of femoral head growth plate (see Figures 3, 4).
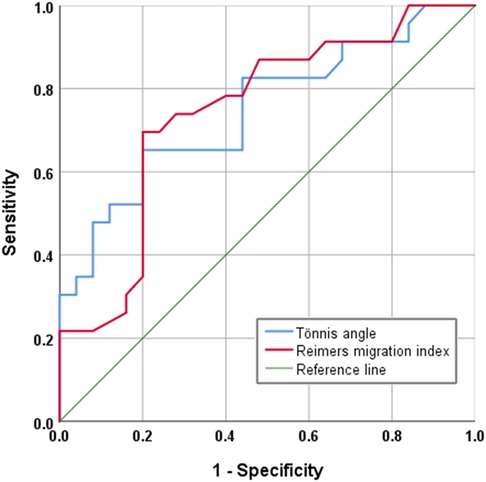
Figure 2. ROC curve. Tönnis angle AUC (Area Under Curve) is 0.748; Reimers migration index AUC (Area Under Curve) is 0.745.
Figure 3. A 12-year-old girl with hip dysplasia on the right side, where the tönnis angle is 41.2°, and the Reimers migration index is 53%. The growth plate on the affected side closed earlier than that on the healthy side.
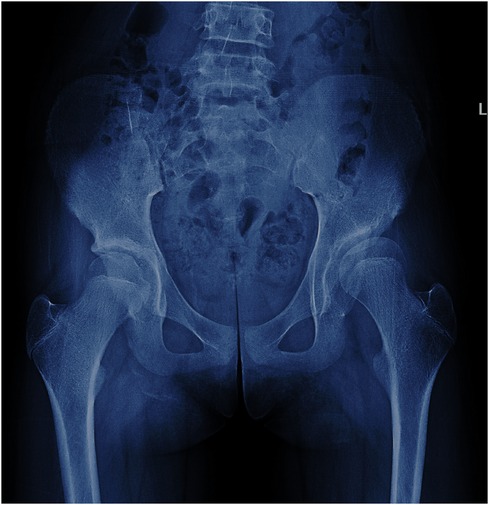
Figure 4. A 13-year-old girl with hip dysplasia on the left side, where the tönnis angle is 25.4°, and the Reimers migration index is 24%. The developmental status of the growth plate of the femoral head is consistent on both sides.
Discussion
The Hueter-Volkmann law (also known as the bone epiphysis pressure law) describes the relationship between bone epiphysis growth rate and the pressure to which they are subjected (14). Increased pressure on the epiphyses inhibits bone growth, while decreased pressure accelerates it. However, excessive pressure can impede normal epiphyseal growth. Mechanical stress can accelerate the formation of secondary ossification centers, regulate the entry of capillaries into cartilage ossification and growth plates, and affect the growth rate of growth plates at the cellular level. If the pressure on one side of the growth plate exceeds a certain level or with a high-pressure load, it can cause an increase in cell apoptosis. The abnormal mechanical environment affects the development of growth plates during immature skeletal development and is related to the pathological mechanism of progressive skeletal deformities (11, 12, 15–29).
Under normal circumstances, the hip joint only bears partial weight. However, when developmental dysplasia of the hip (DDH) occurs, the force surface can gradually shift to the edge of the acetabulum, and even show point-surface contact. This can significantly increase the pressure on the hip joint, up to 2–3 times that of normal. This change becomes more obvious as the Tönnis angle increases and the degree of lateral displacement of the femoral head increases. It can lead to occlusion of the lateral artery of the femoral head growth plate and affect the development of the growth plate. Clinical studies have also found that excessive stress can affect the growth and development of bone epiphyseal growth plates. Changes in hip joint load patterns during growth can cause proximal femoral deformities (30, 31). However, there were few studies on the development of growth plate of femoral head in DDH. Our research found that when the Tönnis angle was greater than 29.5°C and/or Reimers migration index was greater than 48.5%, the femoral head growth plate was in an unfavorable mechanical environment. This may cause accelerated closure of the femoral head growth plate in immature female DDH patients. This highlights the importance of early intervention.
The key to the treatment of children with DDH is to obtain concentric reduction (32). When the shape of the femoral head is not spherical, concentric reduction is impossible (33). The less circular the shape of the femoral head, the worse the treatment effect (34, 35). The shape of the femoral head is not only related to age but also to the growth environment around the femoral head growth plate. When the femoral head growth plate is in an unfavorable stimulation environment or there is a lesion, it can cause changes in the shape of the femoral head (35–38). This is also the principle of conservative treatment for DDH in infancy (39). In immature patients with skeletal development, when the mechanical environment of the femoral head growth plate improves, it can cause deformation of the femoral head shape (40–42). Therefore, early intervention is necessary when abnormal head-acetabulum relationships are occurred to prevent abnormal growth and development of the epiphysis and growth plates due to long-term abnormal mechanical stress, which can affect the postoperative effect. Early improvement of the mechanical environment of the femoral head growth plate can achieve good femoral head shape and good head-acetabulum matching.
The limitations of this study were that: (1) it was a preliminary study on the effect of abnormal head-acetabulum relationships on the development of the femoral head growth plate growth plate in female unilateral DDH; (2) the sample size was limited, and there was a lack of comparison between gender and normal adolescents. (3) Different rotational positions of the lower limbs maybe affect the the observation of growth plates. The subsequent studies were that: (1) determine whether a postoperative plasticity of the femoral head will occur when the Tönnis angle is greater than 29.5°C and/or the Reimers migration index is greater than 48.5%; (2) increase the sample size and comparisons between genders. (3) To compare the development of femoral head growth plate between DDH and normal adolescents.
In conclusion, our findings demonstrate that the development of femoral head growth plates in immature female DDH patients and found that DDH can cause adverse changes in the mechanical environment of the femoral head growth plate, which can lead to early closure and may have a negative impact on the later treatment. The risk factors are a Tönnis angle greater than 29.5°C and/or Reimers migration index greater than 48.5%.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by our hospital Ethics Committee review board (No. 2023KY002-KS001). Informed consent was obtained from all individual participants included in the study.
Author contributions
HC: conception and design, collection and assembly of data, analysis and interpretation of the data, drafting of the article. ZZ, YL, PZ, DL JZ, and HZ: statistical expertise, drafting of the article, collection and assembly of data, analysis and interpretation of the data. HC: conception and design, investigation, methodology, supervision, provision of study materials or patients. All authors had full access to all of the data in the study. All authors were involved in writing (review and editing). All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nguyen JC, Markhardt BK, Merrow AC, Dwek JR. Imaging of pediatric growth plate disturbances. Radiographics. (2017) 37:1791–812. doi: 10.1148/rg.2017170029
2. Canavese F, Castañeda P, Hui J, Li L, Li Y, Roposch A. Developmental dysplasia of the hip: promoting global exchanges to enable understanding the disease and improve patient care. Orthop Traumatol Surg Res. (2020) 106(7):1243–4. doi: 10.1016/j.otsr.2020.09.004
3. Foster AD. The impact of bipedal mechanical loading history on longitudinal long bone growth. PLoS One. (2019) 14:e0211692. doi: 10.1371/journal.pone.0211692
4. Castro-Abril HA, Gutiérrez ML, Garzón-Alvarado DA. Proximal femoral growth plate mechanical behavior: comparison between different developmental stages. Comput Biol Med. (2016) 76:192–201. doi: 10.1016/j.compbiomed.2016.07.011
5. Katsimbri P. The biology of normal bone remodelling. Eur J Cancer Care (Engl). (2017) 26(6):e12740. doi: 10.1111/ecc.12740
6. Fabry G. Clinical practice: the hip from birth to adolescence. Eur J Pediatr. (2010) 169:143–8. doi: 10.1007/s00431-009-1025-x
7. Reinhardt M, Stauner K, Schuh A, Steger W, Schraml A. Slipped capital femoral epiphysis: long-term outcome and remodelling after in situ fixation. Hip Int. (2016) 26:25–30. doi: 10.5301/hipint.5000298
8. Akiyama M, Nakashima Y, Kitano T, Nakamura T, Takamura K, Kohno Y, et al. Remodelling of femoral head-neck junction in slipped capital femoral epiphysis: a multicentre study. Int Orthop. (2013) 37:2331–6. doi: 10.1007/s00264-013-2047-6
9. Sadeghian SM, Lewis CL, Shefelbine SJ. Predicting growth plate orientation with altered hip loading: potential cause of cam morphology. Biomech Model Mechanobiol. (2020) 19:701–12. doi: 10.1007/s10237-019-01241-2
10. Siebenrock KA, Behning A, Mamisch TC, Schwab JM. Growth plate alteration precedes cam-type deformity in elite basketball players. Clin Orthop Relat Res. (2013) 471:1084–91. doi: 10.1007/s11999-012-2740-6
11. Stokes IA, Clark KC, Farnum CE, Aronsson DD. Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone. (2007) 41:197–205. doi: 10.1016/j.bone.2007.04.180
12. Stokes IA, Gwadera J, Dimock A, Farnum CE, Aronsson DD. Modulation of vertebral and tibial growth by compression loading: diurnal versus full-time loading. J Orthop Res. (2005) 23:188–95. doi: 10.1016/j.orthres.2004.06.012
13. Park KW, Kim JH, Sung S, Lee MY, Song HR. Assessment of skeletal age in multiple epiphyseal dysplasia. J Pediatr Orthop. (2014) 34(7):738–42. doi: 10.1097/BPO.0000000000000172
14. Zlotolow DA, Kozin SH. Hand and wrist injuries in the pediatric athlete. Clin Sports Med. (2020) 39:457–79. doi: 10.1016/j.csm.2020.01.001
15. Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. (2002) 17:284–92. doi: 10.1359/jbmr.2002.17.2.284
16. Sundaramurthy S, Mao JJ. Modulation of endochondral development of the distal femoral condyle by mechanical loading. J Orthop Res. (2006) 24:229–41. doi: 10.1002/jor.20024
17. Sergerie K, Parent S, Beauchemin PF, Londoño I, Moldovan F, Villemure I. Growth plate explants respond differently to in vitro static and dynamic loadings. J Orthop Res. (2011) 29:473–80. doi: 10.1002/jor.21282
18. Zimmermann EA, Bouguerra S, Londoño I, Moldovan F, Aubin CÉ, Villemure I. In situ deformation of growth plate chondrocytes in stress-controlled static vs dynamic compression. J Biomech. (2017) 56:76–82. doi: 10.1016/j.jbiomech.2017.03.008
19. Ménard AL, Grimard G, Valteau B, Londono I, Moldovan F, Villemure I. In vivo dynamic loading reduces bone growth without histomorphometric changes of the growth plate. J Orthop Res. (2014) 32:1129–36. doi: 10.1002/jor.22664
20. Killion CH, Mitchell EH, Duke CG, Serra R. Mechanical loading regulates organization of the actin cytoskeleton and column formation in postnatal growth plate. Mol Biol Cell. (2017) 28:1862–70. doi: 10.1091/mbc.e17-02-0084
21. Ariga K, Yonenobu K, Nakase T, Hosono N, Okuda S, Meng W, et al. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine (Phila Pa 1976). (2003) 28:1528–33. 12865839.12865839
22. Rais Y, Reich A, Simsa-Maziel S, Moshe M, Idelevich A, Kfir T, et al. The growth plate’s response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell Mol Life Sci. (2015) 72:597–615. doi: 10.1007/s00018-014-1690-4
23. Villemure I, Chung MA, Seck CS, Kimm MH, Matyas JR, Duncan NA. Static compressive loading reduces the mRNA expression of type II and X collagen in rat growth-plate chondrocytes during postnatal growth. Connect Tissue Res. (2005) 46:211–9. doi: 10.1080/03008200500344058
24. Ueki M, Tanaka N, Tanimoto K, Nishio C, Honda K, Lin YY, et al. The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann Biomed Eng. (2008) 36:793–800. doi: 10.1007/s10439-008-9462-7
25. Vendra BB, Roan E, Williams JL. Chondron curvature mapping in growth plate cartilage under compressive loading. J Mech Behav Biomed Mater. (2018) 84:168–77. doi: 10.1016/j.jmbbm.2018.05.015
26. Kaviani R, Londono I, Parent S, Moldovan F, Villemure I. Changes in growth plate extracellular matrix composition and biomechanics following in vitro static versus dynamic mechanical modulation. J Musculoskelet Neuronal Interact. (2018) 18:81–91.29504583
27. Kaviani R, Londono I, Parent S, Moldovan F, Villemure I. Growth plate cartilage shows different strain patterns in response to static versus dynamic mechanical modulation. Biomech Model Mechanobiol. (2016) 15:933–46. doi: 10.1007/s10237-015-0733-6
28. Gkiatas I, Lykissas M, Kostas-Agnantis I, Korompilias A, Batistatou A, Beris A. Factors affecting bone growth. Am J Orthop (Belle Mead NJ). (2015) 44:61–7.25658073
29. Pichler K, Herbert V, Schmidt B, Fischerauer EE, Leithner A, Weinberg AM. Expression of matrix metalloproteinases in human growth plate chondrocytes is enhanced at high levels of mechanical loading: a possible explanation for overuse injuries in children. Bone Joint J. (2013) 95:568–73. doi: 10.1302/0301-620X.95B4.30639
30. Papavasiliou A, Siatras T, Bintoudi A, Milosis D, Lallas V, Sykaras E, et al. The gymnasts’ hip and groin: a magnetic resonance imaging study in asymptomatic elite athletes. Skeletal Radiol. (2014) 43:1071–7. doi: 10.1007/s00256-014-1885-7
31. Harsanyi S, Zamborsky R, Krajciova L, Kokavec M, Danisovic L. Developmental dysplasia of the hip: a review of etiopathogenesis, risk factors, and genetic aspects. Medicina (Kaunas). (2020) 56(4):153. doi: 10.3390/medicina56040153
32. Fu Z, Zhang Z, Deng S, Yang J, Li B, Zhang H, et al. MRI Assessment of femoral head docking following closed reduction of developmental dysplasia of the hip. Bone Joint J. (2023) 105:140–7. doi: 10.1302/0301-620X.105B2.BJJ-2022-0547.R2
33. Rosenberg MR, Walton R, Rae EA, Bailey S, Nicol RO. Intra-articular dysplasia of the femoral head in developmental dysplasia of the hip. J Pediatr Orthop B. (2017) 26:298–302. doi: 10.1097/BPB.0000000000000356
34. Sankar WN, Neubuerger CO, Moseley CF. Femoral head sphericity in untreated developmental dislocation of the hip. J Pediatr Orthop. (2010) 30:558–61. doi: 10.1097/BPO.0b013e3181e4f53e
35. Chang CH, Yang WE, Kao HK, Shih CH, Kuo KN. Predictive value for femoral head sphericity from early radiographic signs in surgery for developmental dysplasia of the hip. J Pediatr Orthop. (2011) 31:240–5. doi: 10.1097/BPO.0b013e31820fc895
36. Vo A, Beaule PE, Sampaio ML, Rotaru C, Rakhra KS. The femoral head-neck contour varies as a function of physeal development. Bone Joint Res. (2015) 4:17–22. doi: 10.1302/2046-3758.42.2000356
37. Wanner MR, Loder RT, Jennings SG, Ouyang F, Karmazyn B. Changes in femoral head size and growth rate in young children with severe developmental dysplasia of the hip. Pediatr Radiol. (2017) 47:1787–92. doi: 10.1007/s00247-017-3938-2
38. Terjesen T, Wiig O, Svenningsen S. Varus femoral osteotomy improves sphericity of the femoral head in older children with severe form of legg-calvé-perthes disease. Clin Orthop Relat Res. (2012) 470:2394–401. doi: 10.1007/s11999-011-2181-7
39. Pavone V, de Cristo C, Vescio A, Lucenti L, Sapienza M, Sessa G, et al. Dynamic and static splinting for treatment of developmental dysplasia of the hip: a systematic review. Children (Basel). (2021) 8(2):104. doi: 10.3390/children8020104
40. Min JJ, Kwon SS, Sung KH, Lee KM, Chung CY, Park MS. Remodelling of femoral head deformity after hip reconstructive surgery in patients with cerebral palsy. Bone Joint J. (2021) 103:198–203. doi: 10.1302/0301-620X.103B1.BJJ-2020-1339.R1
41. Kim HT, Gu JK, Bae SH, Jang JH, Lee JS. Does valgus femoral osteotomy improve femoral head roundness in severe legg-calvé-perthes disease. Clin Orthop Relat Res. (2013) 471:1021–7. doi: 10.1007/s11999-012-2606-y
Keywords: developmental dysplasia of the hip, femoral patient, skeletal immaturity, femoral head, growth plate
Citation: Ren N, Zhang Z, Li Y, Zheng P, Cheng H, Luo D, Zhang J and Zhang H (2023) Effect of hip dysplasia on the development of the femoral head growth plate. Front. Pediatr. 11:1247455. doi: 10.3389/fped.2023.1247455
Received: 26 June 2023; Accepted: 28 September 2023;
Published: 16 October 2023.
Edited by:
Federico Canavese, Centre Hospitalier Regional et Universitaire de Lille, FranceReviewed by:
Marco Sapienza, University of Catania, ItalyCaleb Grote, Children's Mercy Kansas City, United States
Zhongli Zhang, Tianjin Hospital, China
© 2023 Ren, Zhang, Li, Zheng, Cheng, Luo, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Cheng c2hlbnpoZW50aWVAMTYzLmNvbQ==
 Ningtao Ren1
Ningtao Ren1 Yong Li
Yong Li Hui Cheng
Hui Cheng Hong Zhang
Hong Zhang