- 1Department of Pediatrics, Medical College of Georgia, Augusta, GA, United States
- 2Department of Medicine, The George Washington University Hospital, Washington, DC, United States
- 3ALK, Bedminster, NJ, United States
- 4ALK-Abelló A/S, Hørsholm, Denmark
Allergic rhinoconjunctivitis (ARC) is a common disease that affects individuals of all ages. Pediatricians may be the first (and only) point of care for children with ARC. Sublingual immunotherapy (SLIT)-tablets are a convenient at-home, injection-free allergy immunotherapy option that can be used for the treatment of ARC. This paper provides a practical guide for pediatricians to aid in prescribing SLIT-tablets to children with ARC in North America. Topics include a summary of the available SLIT-tablets and their efficacy and safety, guidance on when SLIT-tablets are an appropriate option, and how to diagnose ARC and identify culprit allergens. Practical guidance is also provided through a proposed decision tree, a prescribing checklist and prescribing procedures, and suggested follow-up assessments.
1. Introduction
Allergic rhinoconjunctivitis (ARC) is a common disease that affects individuals of all ages around the world. The most common symptoms of ARC are runny nose, congestion, sneezing, and itchy, watery eyes. At times, the symptoms of ARC can be severe, disrupting sleep, interfering with the ability to perform daily activities, and inducing anxiety, irritability, fatigue, and frustration (1–4). The symptoms of ARC can even impair cognitive function and affect school performance (2, 5). High pollen levels or the presence of ARC symptoms are associated with poorer performance by adolescents on school exams (6, 7). Children with ARC also often have asthma and atopic dermatitis and are prone to ear infections and sinusitis, which may contribute to the burden of disease (2, 8, 9).
The symptoms of ARC are manifestations of an IgE-mediated response to seasonal allergens such as grass, ragweed, or tree pollen or perennial allergens such as house dust mites (HDM), molds, or animal dander. Pharmacotherapy options for ARC typically treat symptoms by targeting the histamine and other inflammatory mediators that are released or elevated after the IgE response is triggered by an allergen. These symptom-relieving medications include antihistamines, intranasal corticosteroids, and leukotriene inhibitors. Allergen immunotherapy (AIT) is another treatment option for ARC that effectively reduces symptoms by modifying the disease mechanisms that drive ARC (10). Three years of AIT is generally recommended to achieve a long-term sustained effect after treatment has been stopped (11, 12). Subcutaneous immunotherapy (SCIT; aka “allergy shots”) has been practiced for over 100 years and is the primary means of AIT used in the US (13, 14). Administration of SCIT is recommended in a clinical setting typically every 1–6 weeks with a 30 min wait period after the injection to monitor for severe systemic allergic reactions (15). Thus, the inconvenience and time needed for the frequent clinic visits are common reasons why patients decline SCIT or start SCIT but then stop (14). In addition, fear of needles or the prospect of repeated injections may make SCIT particularly unappealing for children. These factors likely contribute to the underutilization of SCIT. Many patients with ARC are simply unaware of AIT as a treatment option (3, 16).
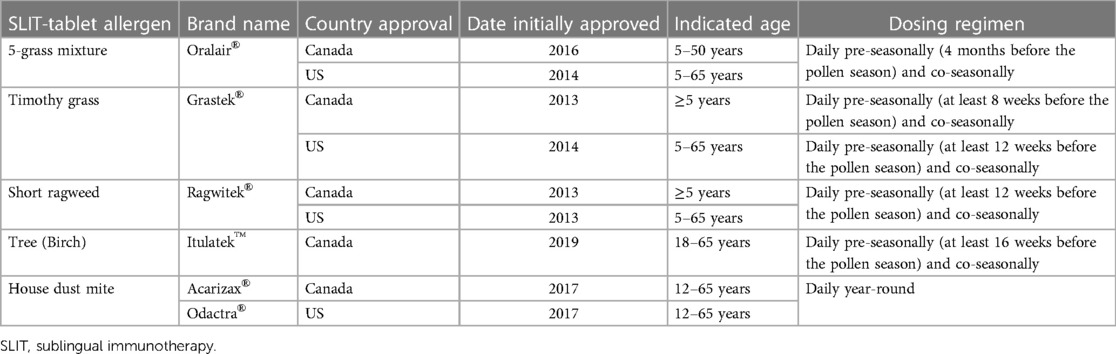
Sublingual immunotherapy (SLIT) is an injection-free form of AIT administered by drops or tablets with a safety profile that allows for at-home administration. Only the first dose needs to be administered in a clinical setting to ensure the treatment is tolerated (17). The convenience and injection-free nature of SLIT may be an appealing AIT option for children (18, 19). SLIT-drops are an off-label use of SCIT extracts in the US. There are little data on the efficacy and safety of SLIT-drops in North America, and they are not FDA approved or endorsed by the American Academy of Allergy, Asthma & Immunology (AAAAI) or the American College of Allergy, Asthma & Immunology (ACAAI) (17). In addition, development of eosinophilic esophagitis may be more likely with SLIT-drop use of North American SCIT extracts than tablets because of differences in contact of the released allergen with the lower esophagus (20). SLIT-tablets to treat grass, ragweed, and HDM-associated ARC are FDA approved in the US and a tree SLIT-tablet is also approved in Canada (Table 1). This paper provides a practical guide for pediatricians to aid in prescribing SLIT-tablets to children with ARC. The information presented is based on the authors’ clinical experience and non-systematic literature searches.
2. Role of pediatricians in ARC and AIT
An international survey found that the self-reported prevalence of ARC symptoms in the previous year was 13.3% for adolescents ages 13–14 years and 7.7% for children ages 6–7 years (1). Because of this high prevalence of ARC in children and an insufficient number of allergy specialists, primary care providers fulfill a critical need in the management of ARC (21). In a nationwide survey of individuals in the US who had ever been diagnosed with ARC, 41% of children were diagnosed by a pediatrician and 22% by a family medicine practitioner (22). Furthermore, the majority of patients reported seeking allergy care from a primary care provider rather than an allergist (22). Despite the importance of primary care providers in the management of ARC, surveys have identified that primary care providers may not feel adequate in understanding AIT, a critical aspect of ARC care. A survey of primary care providers across 5 countries in Europe found that only 10%–29% perceived their confidence level of AIT as “adequate” (23), although 37% of the pediatricians responding to the survey perceived themselves as having adequate knowledge of AIT (24). Thus, SCIT is generally prescribed by allergists or otolaryngologists because it requires knowledge of oftentimes complex extract preparation, space and personnel to prepare extracts, reimbursement procedures, clinic resources and procedures to manage potential anaphylactic reactions, and time, space, and administrative constraints to manage the recurring visits (15, 16, 25, 26). SLIT-tablets have simple daily dosing and no need for frequent clinic visits, which reduces the complexity and logistics that are associated with SCIT. As such, SLIT-tablets can be prescribed and managed in a primary care office provided the physician has experience in the assessment and management of allergic diseases (17).
3. SLIT-tablet efficacy and safety in children
There are currently 5 SLIT-tablets approved for the treatment of ARC in North America; 4 of the SLIT-tablets are approved for children or adolescents (Table 1). The ability of the SLIT-tablets to significantly reduce ARC symptoms and symptom-relieving medication use in children or adolescents has been demonstrated in randomized, double-blind, placebo-controlled trials (27–32). AIT may also have the added benefit of preventing progression to asthma in patients with ARC. In a 5-year prospective trial, children with ARC and no history of asthma who received the timothy grass SLIT-tablet for 3 years had a significantly lower risk of experiencing asthma symptoms or using asthma medication at year 5 compared with those who received placebo (odds ratio = 0.66, p = 0.04) (33). Real-world retrospective studies have also found a lower percentage of patients receiving timothy grass or 5-grass SLIT-tablets developed asthma compared with untreated patients (34, 35). Similar studies have yet to be conducted for the other SLIT-tablets. An additional potential benefit of AIT is prevention of new allergen sensitizations, although more data are needed on this topic (36).
Anaphylaxis is a possibility with AIT in general since treatment exposes a patient to the allergen to which they are allergic, however, anaphylaxis is rare with SLIT. Across the entire clinical development program of the timothy grass, ragweed, tree, and HDM SLIT-tablets comprising over 14,000 subjects, the anaphylaxis rate was 0.03% with active treatment (compared with 0.02% with placebo) (37). By comparison, the rate of anaphylaxis to penicillin is estimated to be between 0.015% to 0.04% (38). There have been no reported deaths related to SLIT-tablets.
The most common adverse events with SLIT-tablets are local allergic reactions that occur at or near the site of tablet administration (e.g., throat irritation, oral pruritus, ear pruritus; Table 2) (39, 40). These reactions are expected since the patient is being exposed to the allergen that causes their allergic symptoms. The vast majority of the adverse events related to SLIT-tablets are mild or moderate; in a pooled analysis of pediatric data from timothy grass SLIT-tablet trials, only 3% of adverse events related to the grass SLIT-tablet were severe (41). Severe local allergic reactions are not a major safety issue unless they compromise the airway. To date, there have been no such events reported in SLIT-tablet clinical trials (38, 40). However, the local allergic reactions are a tolerance issue that can be bothersome for patients and lead to treatment discontinuation. Data from clinical trials show that the local allergic reactions typically resolve within 30–60 min after SLIT-tablet administration and recur for less than 2 weeks (39). Caregivers considering SLIT-tablets for their child need to be informed during the shared decision making process about the potential local allergic reactions that can occur with the SLIT-tablets, the expected duration of the reactions, and guidance on how to manage them to help children maintain treatment. This information is important to encourage adherence.
4. When is SLIT-tablet an appropriate option?
According to SLIT guidelines from the World Allergy Organization, patients with a history of ARC symptoms related to allergen exposure and with documented allergen-specific IgE (sIgE) are eligible for SLIT (Table 3) (19). The FDA prescribing information for the SLIT-tablets indicate that they should be used for the treatment of ARC confirmed by positive skin test or in vitro testing for sIgE antibodies (42–45). Despite intensive research efforts, no validated biomarkers have been identified that can predict which patients will successfully respond to AIT. Essentially, SLIT-tablet treatment can be considered for any child (within the approved age range) with a positive sIgE and a clinical history that suggests ARC relevant to the allergen. SLIT-tablets do not need to be reserved only for children whose symptoms are not well-controlled by symptom-relieving medication or who have undesirable side effects to pharmacotherapies, although greater consideration may be given for children with these particular situations (Table 3) (19). In addition, children who are at high risk for developing asthma (i.e., atopic parents; obesity; past history of food allergy or atopic dermatitis) (46, 47) may be good candidates for AIT because of its disease-modifying aspects. There are some children for whom SLIT-tablets may not be a good option, including those who are taking certain medications that may enhance the likelihood or interfere with treatment of a severe allergic reaction to the SLIT-tablets (e.g., antidepressants), or those who are receiving other AIT (Table 3). Some patients and caregivers may choose not to start SLIT-tablets since they require adherence to daily dosing for three years.
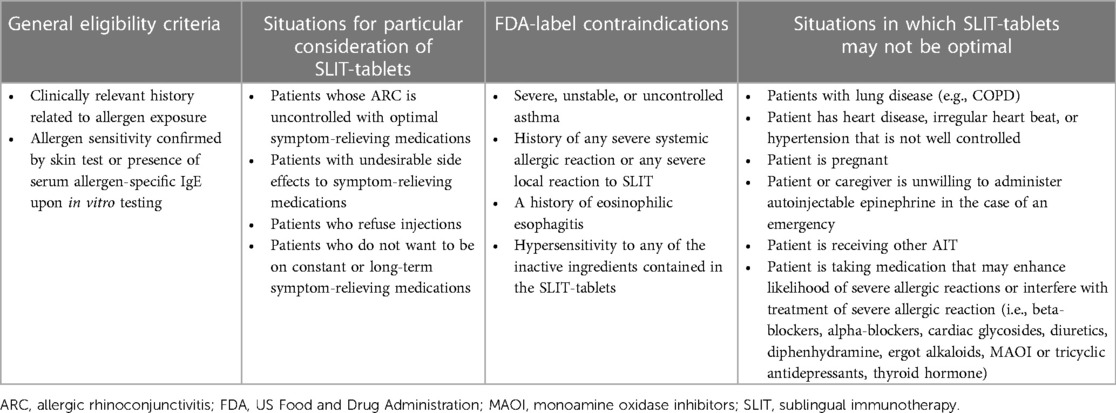
Table 3. Eligibility criteria for treatment of ARC with SLIT-tablets and FDA-label contraindications (19, 42–45).
5. Diagnosis of ARC and identification of culprit allergens
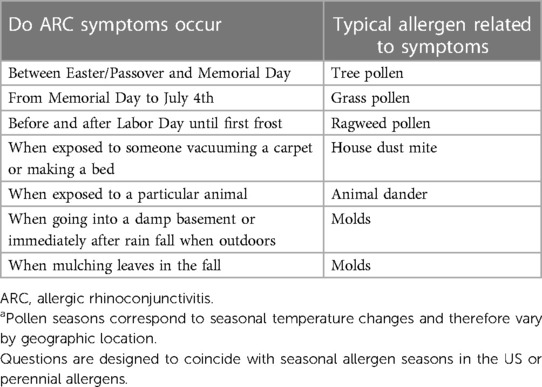
Diagnosis of ARC is determined from clinical history, physical examination, and serum sIgE or skin testing. Detailed guidance on AR diagnosis has been provided by an ACAAI/AAAAI Joint Task Force (48), and simplified guidance pathways for the diagnosis of ARC in primary care have been published (49). Basic guidance to help determine if a child's rhinitis symptoms are attributed to ARC instead of a viral cold is shown in Table 4 (50). In addition, Table 5 provides a list of simple and quick questions that link exposure to prominent, easily remembered US holidays that can help determine clinically relevant allergens associated with symptoms. However, pollen seasons correspond to seasonal temperature changes and therefore vary by geographic location. Pediatricians should verify the typical pollen times in their region. To determine the presence of serum sIgE antibodies toward a suspected culprit allergen that can be treated with the SLIT-tablets available in the US, a limited diagnostic panel is available with a US LabCorp procedure code of 607706. The panel measures sIgE towards the HDM allergens Der p and Der f, the grass allergen Phl p 5, and the short ragweed allergen Amb a 1. If the results do not indicate positive sIgE to the 4 allergens but the child remains symptomatic, referral to an allergist is recommended.
6. Decision tree for SLIT-tablets
Figure 1 is a decision tree to help pediatricians decide between offering SLIT-tablets to their patient or referring the patient to an allergy specialist. Primarily the decision comes down to the asthma status of the patient, whether the patient is monosensitized or polysensitized, and the availability of a SLIT-tablet applicable to the patient's allergy. Patients with severe, uncontrolled, or unstable asthma, or multiple atopic comorbidities, should automatically be referred to an allergy specialist. If the patient does not have asthma or has well-controlled asthma, has an established clinically relevant allergy to only one allergen (monoallergic), and a SLIT-tablet is available that is applicable to the patient's allergy, the SLIT-tablet can be offered. Positive sIgE to multiple allergens may or may not indicate polyallergy and depends on a corresponding clinical history. For example, a patient may have a positive sIgE for ragweed and grass but only have symptoms during ragweed season. Such a patient would be considered polysensitized, but monoallergic, and ragweed SLIT-tablet alone would be appropriate.
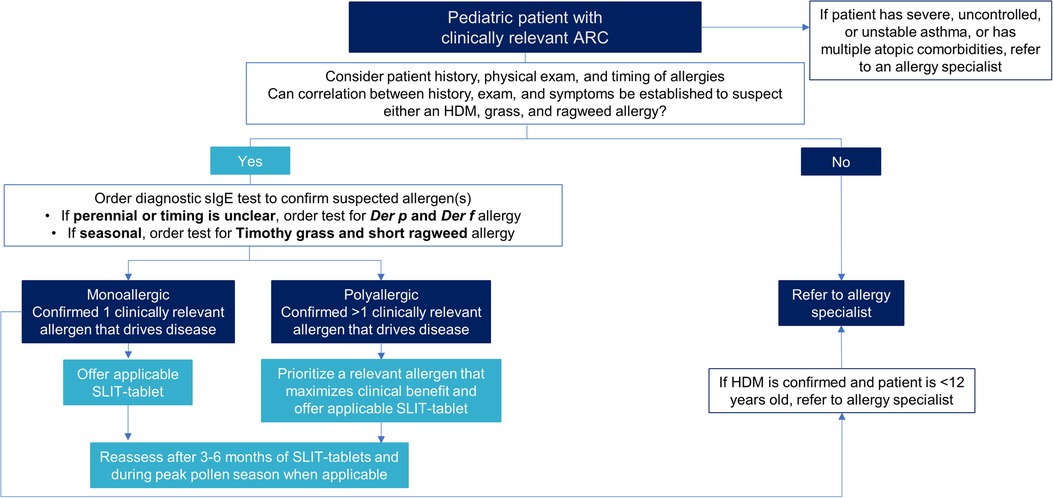
Figure 1. Decision tree for pediatricians between offering SLIT-tablets or referring to an allergy specialist. ARC, allergic rhinoconjunctivitis; Der f, Dermatophagoides farinae; Der p, Dermatophagoides pteronyssinus; HDM, house dust mite; sIgE, allergen-specific immunoglobulin E; SLIT, sublingual immunotherapy.
The decision to offer SLIT-tablets to patients who are polyallergic is more complicated. Polyallergy is quite common at approximately 50% in children with ARC (51). Some of the polyallergies are among related highly cross-reactive pollen allergens and can be treated with AIT against one of the allergens within the cross-reactive group. The AAAAI/ACAAI Joint Task Force on AIT and the Canadian Society of Allergy and Clinical Immunology endorse this approach (15, 52). For example, the Northern grasses (timothy, sweet vernal, orchard, perennial rye, and meadow) are highly cross-reactive, and allergy to any of these grasses can be treated with the timothy grass SLIT-tablet. Trees are another example. The tree SLIT-tablet that contains a birch extract is effective in reducing symptoms and symptom-relieving medication use during other tree pollen seasons that are related to birch (e.g., hazel, alder, and oak) (53, 54).
If the patient is polyallergic to unrelated allergens, typical practice in the US by allergy specialists is to administer SCIT with multiple extracts that target several of the clinically relevant allergens. In contrast, the selection of one clinically relevant allergen for SCIT or SLIT is standard practice in Europe and Asia. Similarly, to offer a SLIT-tablet for polyallergic patients, the pediatrician needs to decide if it is possible to prioritize one particular allergen to target. Treating for HDM allergy with or without seasonal polyallergy is the first recommended prioritization, followed by treating the seasonal pollen allergen that would maximize clinical benefit. Coadministration of 2 different SLIT-tablets after sequential initiation periods has been shown to be well-tolerated in randomized, double-blind, placebo-controlled safety studies, but efficacy data with coadministration are not yet available (55, 56).
7. Prescribing procedures for SLIT-tablets
Each SLIT-tablet has one daily dose that has been determined to be effective and safe in randomized clinical trials. There is no difference in dosing between adults and children, with the exception of the 5-grass SLIT-tablet that requires the dose to be increased over the first 2 days (100 index of reactivity [IR] on day 1, 2× 100 IR on day 2) in children ages 5–17 years before reaching the maintenance dose of 300 IR on day 3 (45). The HDM SLIT-tablet should be taken daily year-round, and seasonal SLIT-tablets should be started at least 8–16 weeks before the expected start of the pollen season to ensure the onset of effect before the season (Table 1). The timothy grass SLIT-tablet can also be taken daily year-round. Data from SLIT trials indicate that initiation of treatment during the pollen season is well tolerated (57). The first dose of any SLIT-tablet must be administered under the supervision of a physician with experience in the diagnosis and treatment of allergic diseases (42–45). The patient then needs to be observed in the office for 30 min to watch for signs and symptoms of anaphylaxis.
There are some important safety instructions that need to be given to caregivers and patients when prescribing SLIT-tablets, most of which are covered in the FDA-approved patient Medication Guides for each SLIT-tablet that should be given to the caregiver. First, caregivers and patients need to be informed of the signs and symptoms of serious allergic reactions (i.e., dyspnea, throat swelling that causes trouble speaking, breathing, or swallowing, dizziness, etc) and instructed to seek immediate medical care and discontinue therapy should any of these occur. In the US, an epinephrine autoinjector is a required co-prescription for SLIT-tablets. Caregivers and patients need to be instructed regarding the appropriate use of the autoinjector and trained in its use. In addition, caregivers and patients also need to be informed that treatment should be stopped if they experience severe or persistent symptoms of esophagitis, if they have persistent or escalating side effects in the mouth or throat, or if patients with asthma have difficulty breathing or their asthma becomes difficult to control. If the patient disrupts treatment for any of these reasons, they need to contact the prescribing physician. Treatment should also be temporarily stopped in case of oral inflammation or wounds (i.e., dental surgery) to allow the oral cavity to heal. Data with the HDM SLIT-tablet suggest that there is no safety issue if treatment is reinitiated after a treatment interruption of approximately 2 weeks (58). If a dose is accidentally missed, the patient should not take two tablets but should wait and take one tablet at their next usually scheduled time. Caregivers and patients also need to be informed about the most common side effects (Table 2) and how often these may last (30–60 min) and recur (about 2 weeks). Caregivers and patients need to be prepared to expect these local allergic reactions and be informed that by themselves these reactions are not dangerous. Otherwise, they may become nervous and stop treatment.
A checklist of steps for prescribing SLIT-tablets to children with ARC is shown in Table 6.
8. Role of the caregiver in administration and monitoring of SLIT-tablet treatment
For younger children, the caregiver should place the SLIT-tablet under the child's tongue to ensure proper sublingual placement. The SLIT-tablets are manufactured using the same technology as other quick dissolving tablets used sublingually in children as young as 1 month of age (e.g., ondansetron orally disintegrating tablets) and will stick to the wet mucosa and dissolve immediately. The child should not swallow for at least 1 min or eat or drink for 5 min. The appropriate age at which a child can self-administer the SLIT-tablet will vary depending on the child, but whomever administers the SLIT-tablet should wash their hands afterward. The child should be observed by the caregiver for at least 5 min after each administration to watch for signs of SLIT-tablet aspiration or an allergic reaction.
9. Follow-up visits
Follow-up visits for children prescribed SLIT-tablets are important to assess efficacy, safety, and adherence. Numerous immunologic changes have been characterized in response to AIT in accordance with the disease modifying activity (59), but monitoring of these changes is not practical in the clinic setting (12). Assessment of efficacy relies on the perception of symptoms by patients and caregivers. Clinical improvement with seasonal SLIT-tablets is expected during the first pollen season if treatment is initiated within the recommended preseasonal time period (60). An onset of effect with the HDM SLIT-tablet can be expected in as early as 8 weeks (60). An improvement in symptoms supports continuation for the full recommended 3-year course. If there is no improvement in symptoms after the first year, there is no indication to continue treatment (15). For pediatricians who wish to take a measurement-based care approach, short and simple tools such as a visual analog scale or the validated Rhinitis Control Assessment Test (61) can be used to evaluate ARC symptom control at each visit to compare with a pretreatment value (Table 7). Digital apps, such as the My Pollen Forecast-Allergies app, allows the patient or caregiver to create a digital diary that tracks improvement over time and may be useful for assessing the efficacy of SLIT-tablet treatment. For children with seasonal allergies, a follow-up visit during peak pollen season may be conducted to assess symptom control when it is most needed.
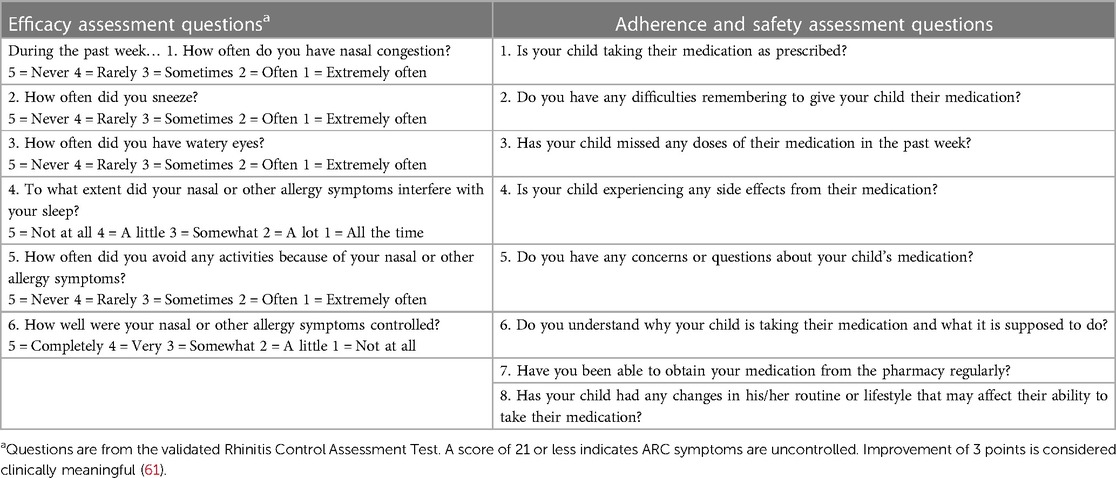
Table 7. Recommended questions to assess efficacy, adherence, and safety of SLIT-tablets at follow-up visits.
As with any medication, adherence to the daily SLIT-tablet treatment is critical for its success. Based on data from a prospective randomized study (62), follow-up visits every 3–6 months are recommended to maximize adherence. During these follow-up visits, the pediatrician can ask the patient or caregiver questions to assess adherence (Table 7). If any issues with adherence are revealed, further conversations should be held to emphasize the importance of adherence and address any barriers or issues that are affecting adherence. The use of digital apps or reminders may be useful for helping patients who have difficulty remembering to take their SLIT-tablet (12). Questions to assess the safety of the SLIT-tablet treatment can also be asked during the follow-up visit (Table 7).
10. Discussion
In North America, pediatricians may be the first (and only) point of care for children with ARC. SLIT-tablets are a convenient at-home, injection-free AIT option that can be prescribed for children with ARC. The proposed decision tree and prescribing checklist provide pediatricians with the tools to prescribe SLIT-tablets to children.
Author contributions
MB and LD provided the clinical practice questions and expertise. HN provided clinical expertise. MO and KR drafted the decision tree and prescribing checklist. All authors critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Medical writing assistance was funded by ALK-Abelló A/S, Hørsholm, Denmark.
Acknowledgments
Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Scott Medical Communications, LLC.
Conflict of interest
MB has served as a consultant or speaker for ALK, Sanofi, Regeneron, Merck, Bellus, Hikma, Prollergy, and Lanier Biotherapeutics. LD has served as a consultant or speaker for ALK, Sanofi, Regeneron, Allergy Therapeutics, GSK, AstraZeneca, and Abionic. HN and MO are employees of ALK. KR was an employee of ALK when the work was conducted.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. García-Marcos L, Asher MI, Pearce N, Ellwood E, Bissell K, Chiang CY, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J. (2022) 60:2102866. doi: 10.1183/13993003.02866-2021
2. Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the pediatric allergies in America survey. J Allergy Clin Immunol. (2009) 124:S43–70. doi: 10.1016/j.jaci.2009.05.013
3. Meltzer EO, Farrar JR, Sennett C. Findings from an online survey assessing the burden and management of seasonal allergic rhinoconjunctivitis in US patients. J Allergy Clin Immunol Pract. (2017) 5:779–89.e6. doi: 10.1016/j.jaip.2016.10.010
4. Fereidouni M, Rezapour H, Saharkhiz M, Mahmoudzadeh S, Ayadilord M, Askari M, et al. A study of the association of cognitive abilities and emotional function with allergic disorders in young women. BMC Womens Health. (2021) 21:205. doi: 10.1186/s12905-021-01345-x
5. Papapostolou G, Kiotseridis H, Romberg K, Dahl Å, Bjermer L, Lindgren M, et al. Cognitive dysfunction and quality of life during pollen season in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. (2021) 32:67–76. doi: 10.1111/pai.13328
6. Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. (2007) 120:381–7. doi: 10.1016/j.jaci.2007.03.034
7. Bensnes SS. You sneeze, you lose: the impact of pollen exposure on cognitive performance during high-stakes high school exams. J Health Econ. (2016) 49:1–13. doi: 10.1016/j.jhealeco.2016.05.005
8. Ibáñez MD, Valero AL, Montoro J, Jauregui I, Ferrer M, Dávila I, et al. Analysis of comorbidities and therapeutic approach for allergic rhinitis in a pediatric population in Spain. Pediatr Allergy Immunol. (2013) 24:678–84. doi: 10.1111/pai.12126
9. Westman M, Stjärne P, Asarnoj A, Kull I, van Hage M, Wickman M, et al. Natural course and comorbidities of allergic and nonallergic rhinitis in children. J Allergy Clin Immunol. (2012) 129:403–8. doi: 10.1016/j.jaci.2011.09.036
10. Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep. (2020) 21:2. doi: 10.1007/s11882-020-00977-7
11. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI Guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. (2018) 73:765–98. doi: 10.1111/all.13317
12. Calderon MA, Casale TB, Nelson HS, Bacharier LB, Bansal P, Bernstein DI, et al. Extrapolating evidence-based medicine of AIT into clinical practice in the United States. J Allergy Clin Immunol Pract. (2023) 11:P1100–15. doi: 10.1016/j.jaip.2022.10.033
13. Leatherman B, Skoner DP, Hadley JA, Walstein N, Blaiss MS, Dykewicz MS, et al. The allergies, immunotherapy, and RhinoconjunctivitiS (AIRS) survey: provider practices and beliefs about allergen immunotherapy. Int Forum Allergy Rhinol. (2014) 4:779–88. doi: 10.1002/alr.21349
14. Winders T, DuBuske L, Bukstein DA, Meltzer EO, Wallace D, Rance K. Shifts in allergy practice in a COVID-19 world: implications of pre-COVID-19 national health care provider and patient surveys of treatments for nasal allergies. Allergy Asthma Proc. (2021) 42:301–9. doi: 10.2500/aap.2021.42.210035
15. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. (2011) 127:S1–S55. doi: 10.1016/j.jaci.2010.09.034
16. Skoner DP, Blaiss MS, Dykewicz MS, Smith N, Leatherman B, Bielory L, et al. The allergies, immunotherapy, and RhinoconjunctivitiS (AIRS) survey: patients’ experience with allergen immunotherapy. Allergy Asthma Proc. (2014) 35:219–26. doi: 10.2500/aap.2014.35.3752
17. Greenhawt M, Oppenheimer J, Nelson M, Nelson H, Lockey R, Lieberman P, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. (2017) 118:276–82.e2. doi: 10.1016/j.anai.2016.12.009
18. Brunton S, Nelson HS, Bernstein DI, Lawton S, Lu S, Nolte H. Sublingual immunotherapy tablets as a disease-modifying add-on treatment option to pharmacotherapy for allergic rhinitis and asthma. Postgrad Med. (2017) 129:1–9. doi: 10.1080/00325481.2017.1308208
19. Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: world allergy organization position paper 2013 update. World Allergy Organ J. (2014) 7:6. doi: 10.1186/1939-4551-7-6
20. Lam K, Pinto JM, Lee SE, Rance K, Nolte H. Delivery options for sublingual immunotherapy for allergic rhinoconjunctivitis: clinical considerations for North America. Rhinology Online. (2022) 5:70–86. doi: 10.4193/RHINOL/22.002
21. Agache I, Ryan D, Rodriguez MR, Yusuf O, Angier E, Jutel M. Allergy management in primary care across European countries – actual status. Allergy. (2013) 68:836–43. doi: 10.1111/all.12150
22. Blaiss MS, Dykewicz MS, Skoner DP, Smith N, Leatherman B, Craig TJ, et al. Diagnosis and treatment of nasal and ocular allergies: the allergies, immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Ann Allergy Asthma Immunol. (2014) 112:322–8.e1. doi: 10.1016/j.anai.2014.02.006
23. Cabrera M, Ryan D, Angier E, Losappio L, Flokstra-de Blok BMJ, Gawlik R, et al. Current allergy educational needs in primary care. Results of the EAACI working group on primary care survey exploring the confidence to manage and the opportunity to refer patients with allergy. Allergy. (2022) 77:378–87. doi: 10.1111/all.15084
24. Cabrera M, Ryan D, Erlewyn-Lajeunesse M, Levin ME, Martinez-Canavate A, Villaizan Perez C, et al. Preliminary pilot study results of the EAACI allergy educational needs in primary care pediatricians task force in managing allergic disorders. Pediatr Allergy Immunol. (2023) 34:e13907. doi: 10.1111/pai.13907
25. Blume SW, Yeomans K, Allen-Ramey F, Smith N, Kim H, Lockey RF, et al. Administration and burden of subcutaneous immunotherapy for allergic rhinitis in U.S. And Canadian clinical practice. J Manag Care Spec Pharm. (2015) 21:982–90. doi: 10.18553/jmcp.2015.21.11.982
26. Ryan D, Gerth van Wijk R, Angier E, Kristiansen M, Zaman H, Sheikh A, et al. Challenges in the implementation of the EAACI AIT guidelines: a situational analysis of current provision of allergen immunotherapy. Allergy. (2018) 73:827–36. doi: 10.1111/all.13264
27. Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. (2011) 127:64–71.e4. doi: 10.1016/j.jaci.2010.11.034
28. Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. (2009) 123:167–73.e7. doi: 10.1016/j.jaci.2008.10.044
29. Maloney J, Bernstein DI, Nelson H, Creticos P, Hebert J, Noonan M, et al. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. (2014) 112:146–53.e2. doi: 10.1016/j.anai.2013.11.018
30. Nolte H, Bernstein DI, Nelson HS, Ellis AK, Kleine-Tebbe J, Lu S. Efficacy and safety of ragweed SLIT-tablet in children with allergic rhinoconjunctivitis in a randomized, placebo-controlled trial. J Allergy Clin Immunol Pract. (2020) 8:2322–31.e5. doi: 10.1016/j.jaip.2020.03.041
31. Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. (2009) 123:160–6.e3. doi: 10.1016/j.jaci.2008.10.009
32. Nolte H, Bernstein DI, Nelson HS, Kleine-Tebbe J, Sussman GL, Seitzberg D, et al. Efficacy of house dust mite SLIT-tablet in North American adolescents and adults in a randomized, placebo-controlled trial. J Allergy Clin Immunol. (2016) 138:1631–8. doi: 10.1016/j.jaci.2016.06.044
33. Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sorensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. (2018) 141:529–38. doi: 10.1016/j.jaci.2017.06.014
34. Devillier P, Wahn U, Zielen S, Heinrich J. Grass pollen sublingual immunotherapy tablets provide long-term relief of grass pollen-associated allergic rhinitis and reduce the risk of asthma: findings from a retrospective, real-world database subanalysis. Expert Rev Clin Immunol. (2017) 13:1199–206. doi: 10.1080/1744666X.2017.1398082
35. Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. (2018) 73:165–77. doi: 10.1111/all.13213
36. Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Macchia L, Di Lorenzo G. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. (2017) 72:691–704. doi: 10.1111/all.13104
37. Nolte H, Calderon M, Bernstein DI, Roberts G, Juhl RG, Hulstrøm V. Anaphylaxis is uncommon in clinical trials of sublingual immunotherapy tablets as identified by a standardized algorithm. J Allergy Clin Immunol. (2023) 151:AB46. doi: 10.1016/j.jaci.2022.12.147
38. Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. (1968) 38:159–88.5302296
39. Bernstein DI, Bardelas JA Jr, Svanholm Fogh B, Kaur A, Li Z, Nolte H. A practical guide to the sublingual immunotherapy tablet adverse event profile: implications for clinical practice. Postgrad Med. (2017) 129:1–8. doi: 10.1080/00325481.2017.1302306
40. Didier A, Bons B. Safety and tolerability of 5-grass pollen tablet sublingual immunotherapy: pooled analysis and clinical review. Exp Opin Drug Safety. (2015) 14:777–88. doi: 10.1517/14740338.2015.1017468
41. Halken S, Roberts G, Valovirta E, Nolte H, Hulstrom V, Blaiss MS. Safety of timothy grass sublingual immunotherapy tablet in children: pooled analyses of clinical trials. J Allergy Clin Immunol Pract. (2020) 8:1387–93.e2. doi: 10.1016/j.jaip.2020.01.008
42. ODACTRA. House dust mite allergen extract tablet for sublingual use. Hørsholm, Denmark: Full Prescribing Information (2023).
43. Ragwitek. Short ragweed pollen allergen extract tablet for sublingual use. Hørsholm, Denmark: Full Prescribing Information (2021).
44. Grastek. Timothy grass pollen allergen extract tablet for sublingual use. Hørsholm, Denmark: Full Prescribing Information (2019).
45. Oralair. Sweet vernal, orchard, perennial rye, timothy, and Kentucky blue grass mixed pollens allergen extract tablet for sublingual use. Antony, France: Full Prescribing Information (2018).
46. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedón JC. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. (2016) 4:1111–22. doi: 10.1016/j.jaip.2016.05.003
47. Yang L, Fu J, Zhou Y. Research progress in atopic march. Front Immunol. (2020) 11:1907. doi: 10.3389/fimmu.2020.01907
48. Dykewicz MS, Wallace DV, Amrol DJ, Baroody FM, Bernstein JA, Craig TJ, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. (2020) 146:721–67. doi: 10.1016/j.jaci.2020.07.007
49. Jutel M, Papadopoulos NG, Gronlund H, Hoffman HJ, Bohle B, Hellings P, et al. Recommendations for the allergy management in the primary care. Allergy. (2014) 69:708–18. doi: 10.1111/all.12382
50. Scadding GK, Smith PK, Blaiss M, Roberts G, Hellings PW, Gevaert P, et al. Allergic rhinitis in childhood and the new EUFOREA algorithm. Front Allergy. (2021) 2:706589. doi: 10.3389/falgy.2021.706589
51. Lee KS, Yum HY, Sheen YH, Park YM, Lee YJ, Choi BS, et al. Comorbidities and phenotypes of rhinitis in Korean children and adolescents: a cross-sectional, multicenter study. Allergy Asthma Immunol Res. (2017) 9:70–8. doi: 10.4168/aair.2017.9.1.70
52. Kim H, Moote W, Waserman S. Allergen immunotherapy pocket guide. Canadian Society of Allergy and Clinical Immunology (2016).
53. Biedermann T, Kuna P, Panzner P, Valovirta E, Andersson M, de Blay F, et al. The SQ tree SLIT-tablet is highly effective and well tolerated: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. (2019) 143:1058–66.e6. doi: 10.1016/j.jaci.2018.12.1001
54. Nolte H, Waserman S, Ellis AK, Biedermann T, Wurtzen PA. Treatment effect of the tree pollen SLIT-tablet on allergic rhinoconjunctivitis during oak pollen season. J Allergy Clin Immunol Pract. (2021) 9:1871–8. doi: 10.1016/j.jaip.2021.01.035
55. Maloney J, Berman G, Gagnon R, Bernstein DI, Nelson HS, Kleine-Tebbe J, et al. Sequential treatment initiation with timothy grass and ragweed sublingual immunotherapy tablets followed by simultaneous treatment is well tolerated. J Allergy Clin Immunol Pract. (2016) 4:301–9.e2. doi: 10.1016/j.jaip.2015.11.004
56. Gotoh M, Okubo K, Yuta A, Ogawa Y, Nagakura H, Ueyama S, et al. Safety profile and immunological response of dual sublingual immunotherapy with house dust mite tablet and Japanese cedar pollen tablet. Allergol Int. (2020) 69:104–10. doi: 10.1016/j.alit.2019.07.007
57. Creticos PS, Bernstein DI, Casale TB, Lockey RF, Maloney J, Nolte H. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. (2016) 4:1194–204. doi: 10.1016/j.jaip.2016.05.014
58. Kim H, Bernstein DI, Smith IM, Nolte H. Adverse event profile of SQ house dust mite sublingual immunotherapy tablet after longer treatment interruption. J Allergy Clin Immunol. (2023) 151:AB48. doi: 10.1016/j.jaci.2022.12.154
59. Ogulur I, Pat Y, Ardicli O, Barletta E, Cevhertas L, Fernandez-Santamaria R, et al. Advances and highlights in biomarkers of allergic diseases. Allergy. (2021) 76:3659–86. doi: 10.1111/all.15089
60. Bernstein D, Durham S, Biedermann T, Nolte H. Onset of clinical effect with sublingual immunotherapy tablets for allergic rhinoconjunctivitis. J Allergy Clin Immunol. (2023) 151:AB125. doi: 10.1016/j.jaci.2022.12.394
61. Meltzer EO, Schatz M, Nathan R, Garris C, Stanford RH, Kosinski M. Reliability, validity, and responsiveness of the rhinitis control assessment test in patients with rhinitis. J Allergy Clin Immunol. (2013) 131:379–86. doi: 10.1016/j.jaci.2012.10.022
Keywords: allergic rhinitis, allergen immunotherapy, children, checklist, decision tree, management, sublingual
Citation: Blaiss M, DuBuske L, Nolte H, Opstrup M and Rance K (2023) A practical guide to prescribing sublingual immunotherapy tablets in North America for pediatric allergic rhinoconjunctivitis: an injection-free allergy immunotherapy option. Front. Pediatr. 11:1244146. doi: 10.3389/fped.2023.1244146
Received: 21 June 2023; Accepted: 12 September 2023;
Published: 4 October 2023.
Edited by:
Marzia Duse, Sapienza University of Rome, ItalyReviewed by:
Antigoni Mavroudi, Aristotle University of Thessaloniki, GreeceHideyuki Kawauchi, Shimane University, Japan
Riccardo Castagnoli, National Institute of Allergy and Infectious Diseases (NIH), United States
© 2023 Blaiss, DuBuske, Nolte, Opstrup and Rance. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hendrik Nolte SGVuZHJpay5ub2x0ZUBhbGsubmV0
 Michael Blaiss1
Michael Blaiss1 Hendrik Nolte
Hendrik Nolte Karen Rance
Karen Rance