- 1Faculty of Sports Sciences, Bu-Ali Sina University, Hamedan, Iran
- 2Department of Nursing, School of Nursing and Midwifery, Chronic Disease (Home Care) Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
- 3Department of Pediatric Nursing, School of Nursing and Midwifery, Chronic Disease (Home Care) Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
- 4Department of Pediatrics Endocrinology, School of Medicine, Besat Hospital, Hamadan University of Medical Sciences, Hamadan, Iran
Background: Obesity and central precocious puberty (CPP) are associated with increased anxiety, depression, and anger in girls. The contribution of exercise as an efficacious component in decreasing anxiety, depression, and anger has been increasingly recognized.
Objectives: This study aims to evaluate the effects of combined training on cortisol, anxiety, depression, and anger in overweight and obese girls with CPP.
Methods: The study involved 30 girls aged 7–9 years diagnosed with CPP (undergoing triptorelin treatment) and dealing with obesity. In addition, these girls scored higher than the cut-off line for anxiety, depression, and anger. The participants were divided into two groups, with 15 individuals in each group. The exercise group engaged in 60 min of combined aerobic and resistance training three times per week for a duration of 12 weeks. On the other hand, the control group did not receive any training. Throughout the study, the serum cortisol levels were measured in both groups. Anxiety, anger, and depression questionnaires were also completed at three different stages, namely, baseline, 12 weeks, and 16 weeks (after a 4-week period of detraining).
Results: In the exercise group, there was a significant decrease (P < 0.05) in cortisol serum levels and anxiety, depression, and anger scores. These changes were observed consistently during detraining (P > 0.05). However, in the control group, only the depression score significantly decreased (P < 0.05).
Conclusions: Based on the results, it can be concluded that combined training is a method to improve the mental health of CPP girls.
Clinical Trial Registration: https://en.irct.ir/trial/61990, identifier IRCT20170411033378N10.
1. Introduction
In 2019, the World Health Organization (WHO) reported that approximately 38.2 million children below the age of 5 years were impacted by obesity. In 2016, over 340 million children and adolescents aged 5–19 years were affected by obesity (1). Obesity in children leads to various issues, including the early onset of puberty in girls (2). A retrospective, case–control study examining a large population of children over a 2-year period concluded that overweight and obesity among children, especially in girls, are related to increased odds of central precocious puberty (CPP), and these CPP-predisposing effects are found when overweight/obesity was prolonged for greater than 1 year for girls and 2 years for boys (3). Precocious puberty means the onset of puberty before the age of 8 in girls and 9 in boys (4). Precocious puberty has been linked to short stature, depression, aggression, social withdrawal, and moodiness (5). Also, early pubertal maturation is associated with anxiety symptomatology (6). The relationship between stress and depressive disorders has often been attributed to a dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis (7). Moreover, the HPA axis regulates both the production and secretion of cortisol (8). Abdominal obesity is also associated with long-term elevated cortisol levels, followed by certain mental disorders (9). Therefore, precocious puberty and obesity should be prevented and treated.
The treatment for precocious puberty is gonadotropin-releasing hormone analog (GnRHa) (10). Training is highly effective for weight loss and preventing and treating obesity (11). Girls with a reduced body mass index–standard deviation score (BMI-SDS) experienced a delayed onset of puberty compared with overweight girls who did not have a reduction in BMI-SDS (12). Recent studies have shown that physical activity can positively impact physical and mental health (13). Physical activity promotes improvements in depressed mood (14), anxiety (15), and aggression (16). Physical activity is a long-term lifestyle behavior that should be introduced in some capacity to all able children, adolescents, and adults (17). Using exercise as a stress management technique has been shown to have preventative and treatment effects on stress (18). Researchers have recommended that an individual exercise for 15–30 min at least three times a week in programs of 10 weeks or longer to reduce depression and anxiety (19). In addition, a study revealed that exercise substantially impacted the reduction of cortisol levels (20).
While training offers numerous advantages, individuals may also encounter detraining, resulting in the potential loss of adaptations and performance gained through training (21). The authors’ previous research showed that aerobic training reduces cortisol levels, and detraining eliminates the benefits of training in girls with precocious puberty (22).
While it is widely accepted that training can enhance the quality of life, a lack of sufficient information regarding the impact of combined training (aerobic + resistance) and detraining on the mental wellbeing of girls experiencing precocious puberty remains. If the effects of training and detraining on these girls are comprehended, these children and their families can make informed decisions about whether or not to engage in training programs. Therefore, our study aimed to assess the impact of a combination of training and detraining on depression, aggression, anxiety, and cortisol levels in overweight and obese girls experiencing precocious puberty.
2. Materials and methods
A pediatric endocrinology and metabolism specialist from Hospital Beasat in Hamadan City referred 75 girls with precocious puberty to our department of pediatrics. The selected girls received an intramuscular injection of GnRH analog (triptorelin) at a dose of 80 mg/kg (maximum: 3.75 mg) every 28 days. Only those children who met the inclusion and exclusion criteria of the study were chosen. The hormonal criteria for the diagnosis of CPP include using luteinizing hormone (LH), which is the best biochemical parameter to diagnose CPP. An increase in levels of LH greater than 0.2 IU/L can be considered a pubertal value (23). A provocative test with GnRH agonist with precise cut-offs is challenging to establish. A peak stimulated LH of >∼8 mIU/ml after GnRH and >∼5 IU/L after GnRHa indicates CPP (24).
The inclusion criteria include being overweight or obese based on the tri-ponderal mass index (TMI), falling within the age range of 7–9 years, having been diagnosed with precocious puberty at least 1 year prior, receiving treatment with the triptorelin drug, taking 1 ml of medication every 28 days, and achieving scores above 50 on the Spence Children’s Anxiety Scale, above 14 on the Birleson Depression Self-Rating, and above 95 on the Children’s Inventory of Anger.
The exclusion criteria include having another illness, taking another medicine, having a special diet, being active in another sport, and using narcotics.
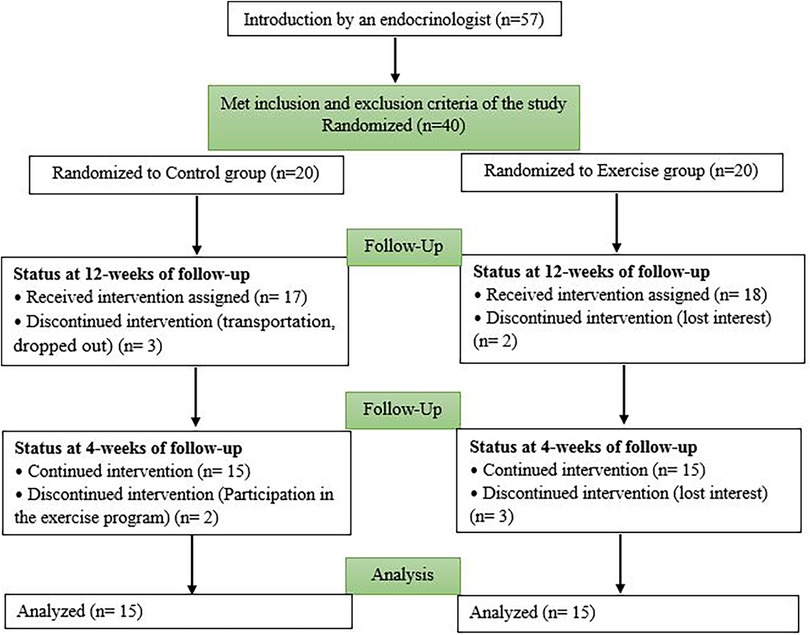
A total of 40 girls who met the inclusion and exclusion criteria were selected. After obtaining informed and written consent from their parents, they were randomly assigned to two separate groups. The exercise group (Ex) participated in a 12-week combined training program (n = 20), and the control group (Con) did not receive any exercise intervention (n = 20). Some were excluded from the study because of lack of interest, transportation, and participation in another sports program (Figure 1). Finally, 30 subjects were analyzed (Figure 1). The Research Ethics Committees of Hamadan University of Medical Sciences approved this research on 5 February 2022, with proprietary ID IR.UMSHA.REC.1400.883. The Iranian Registry of Clinical Trials also approved the study on 27 February 2022 IRCT20170411033378N10.
2.1. Measuring anthropometric indicators and blood samples
All children underwent anthropometric measurements conducted by two experienced physical education teachers. The height of the children was measured using a stadiometer, and their weight was measured using digital weighing scales. Waist circumference (WC, cm) and hip circumference (HC, cm) were measured using an elastic measuring tape, following the WHO recommendations (25). TMI was measured by body weight divided by height cubed. For children aged <16 years, the TMI thresholds to diagnose overweight status were 13.1 ≤ TMI ≤ 14.1 kg/m3, and the TMI thresholds to diagnose obese status were TMI ≥ 14.1 kg/m3 (26). Using a caliper, skinfold thickness was measured from the left side of the body to the nearest 0.1 mm in two places: (1) triceps, halfway between the acromion process and the olecranon process, and (2) subscapular, approximately 20 mm below the tip of the scapula, at an angle of 45° to the lateral side of the body (27). To increase the accuracy, the measurement was repeated three times. To measure muscle mass, the method of Poortmans et al. was used, which has been validated in children and adolescents (28). Peak oxygen uptake (VO2peak) was measured using the 6 min walk test (6MWT) according to the American Thoracic Society rules. The test was performed in a 30 m flat corridor, where contributors were advised to walk as fast as they could without running for 6 min, and they were also encouraged with standard motivational statements (29). This test has already been administered to and validated on children (30). To measure systolic blood pressure (SBP) and diastolic blood pressure (DBP), an automatic barometer was used. Immobile participants were seated during the measurement. To reduce error, the measurements were repeated twice. The watch fastened to the wrist measured the resting heart rate (RHR).
A laboratory specialist collected blood samples between 8:30 and 9:00 a.m. after a fasting period of 12 h, within 2 h of waking up. The samples were taken from the ulnar vein and placed in optocoupler coagulation tubes purchased from Italy. After the blood clotted, the clot was separated by centrifugation at a speed of 1,000–2,000×g for 10 min, resulting in the extraction of the serum. A laboratory kit was used to measure cortisol serum levels (BOSTER, Canada) with a 0.5 ng/ml sensitivity for the enzyme-linked immunosorbent assay (ELISA) method.
2.2. Measuring mental health variables
Children’s anxiety was assessed using the Spence Children’s Anxiety Scale child (SCAS-C) (31). This questionnaire appraises six domains of anxiety, namely, separation anxiety, social phobia, obsessive–compulsive disorder, panic, generalized anxiety, fears, and physical injury. This scale is easy for children and will be completed in 10 min. This questionnaire has 44 items, of which 38 reflect specific signs of anxiety and six relate to positive filler items to reduce negative response bias. Of the 38 anxiety items, six items reflect separation anxiety, six social phobias, six obsessive–compulsive problems, nine panic, six generalized anxiety, and five fears of physical injury. Items are haphazardly allocated within the questionnaire. Anxiety is rated on a 4-point scale from never (0) to always (3). T-scores and cut-offs for the SCAS-C are available on the SCAS website according to age and gender. Using the SCAS-C, for girls aged 8–11 years, a total cut-off score of ≥50 is suggested. There are no suggested cut-offs for children aged 7 years, so the threshold of ≥50 was used for girls of this age. The Persian version of SCAS has acceptable reliability and validity for Iranian children and adolescents (32).
Children’s depression was assessed using the Birleson Depression Self-Rating Scale for Children (DSRS-C). This scale is an 18-item inventory appropriate for children and adolescents aged between 8 and 14 years. Participants must choose one of three options, namely, “most of the time,” “sometimes,” or “never.” The cut-off point of this questionnaire for girls is 14 (33). The reliability and validity of the DSRS-C scale were considered satisfactory (34).
Children’s anger was evaluated using the Children’s Inventory of Anger (ChIA) (35). It has 39 items used to evaluate children aged 6–16 years. This tool measures four subscale scores, namely, frustration, physical aggression, peer relationships, and authority relations. The internal reliability for the total scale is reportedly high (α = 0.95; subscale coefficients ranged from 0.85 to 0.87), and the 1-week test–retest reliability was 0.75 (35).
2.3. Frequency of measurements
All anthropometric indices, blood samples, anxiety, depression, and anger were measured three times from all subjects. Initial measurements were performed 1 day prior to the initiation of the exercise program. The second stage of measurements in the patients in the experimental and control groups was started 48 h after the last exercise session to evaluate the effect of the exercise program on the mentioned indices. The third stage of measurements was performed following the 4-week detraining period. The decision to select a 4-week detraining period was based on the findings that a mere 2-week detraining period is insufficient to diminish the positive impacts of regular exercise fully. However, prolonged detraining may have detrimental consequences (36).
2.4. Intervention
The exercise program was conducted in a gym and supervised by two experienced physical education instructors. The first session was dedicated to measuring anthropometric indicators and familiarizing participants with exercise. The training protocol was performed for 12 weeks, with three sessions per week and 60 min of aerobic + resistance training in each session according to the Lambert protocol (37). Exercise sessions consisted of 30 min of aerobic exercise followed by 20 min of strengthening exercises and 10 min of stretching and cool-down. To promote physical activity in children of different age groups, it is advisable to incorporate unstructured play for young children and engaging activities for older children (38). The aerobic exercise section used fast walking, running, and ball games. Subjects played all the indicated exercises in all sessions at a heart rate corresponding to 55%–65% of individual maximal cardiorespiratory fitness (based on baseline VO2peak measures by 6MWT). In every training session, children were equipped with a heart rate monitor. If their heart rate dropped below 55% or went above 65% of their VO2peak, watch alarms would alert them. The aerobic period was followed by strengthening exercises of the arms, legs, and trunk (two to three series of 10–15 repetitions), with the resistance being provided by the body weight of the child and elastic bands (37). The Con did not receive any interventions, and the researchers asked them not to participate in any exercise activities. After the 3-month intervention, participants were asked not to participate in any exercise programs for 4 weeks.
2.5. Statistical analysis
During the intervention, we utilized the analysis of variance (ANOVA) with repeated measures test to identify significant changes in the difference between groups and within groups. To evaluate the relationship between the variables, we used the Pearson correlation coefficient. All analyses were performed using SPSS 20 software. The findings were declared as mean ± SD. Differences were assigned statistically at P < 0.05.
3. Result
3.1. Effect of the combined training and detraining on anthropometric and clinical parameters
Table 1 presents the individual physiological and anthropometric characteristics of the participants in the three stages (baseline, 12 weeks, and 16 weeks). The results confirm that 12 weeks of combined training decreased the weight, TMI, WC, subscapular skinfold thicknesses (SS), RHR, and SBP and increased the 6MWT, VO2peak, and muscle mass (P < 0.05). Although the detraining period led to increased weight, TMI, WC, and SS, their values did not reach the initial levels before training. 6MWT and VO2peak returned to their initial levels during the detraining period. Muscle mass and SBP did not change during the detraining period.
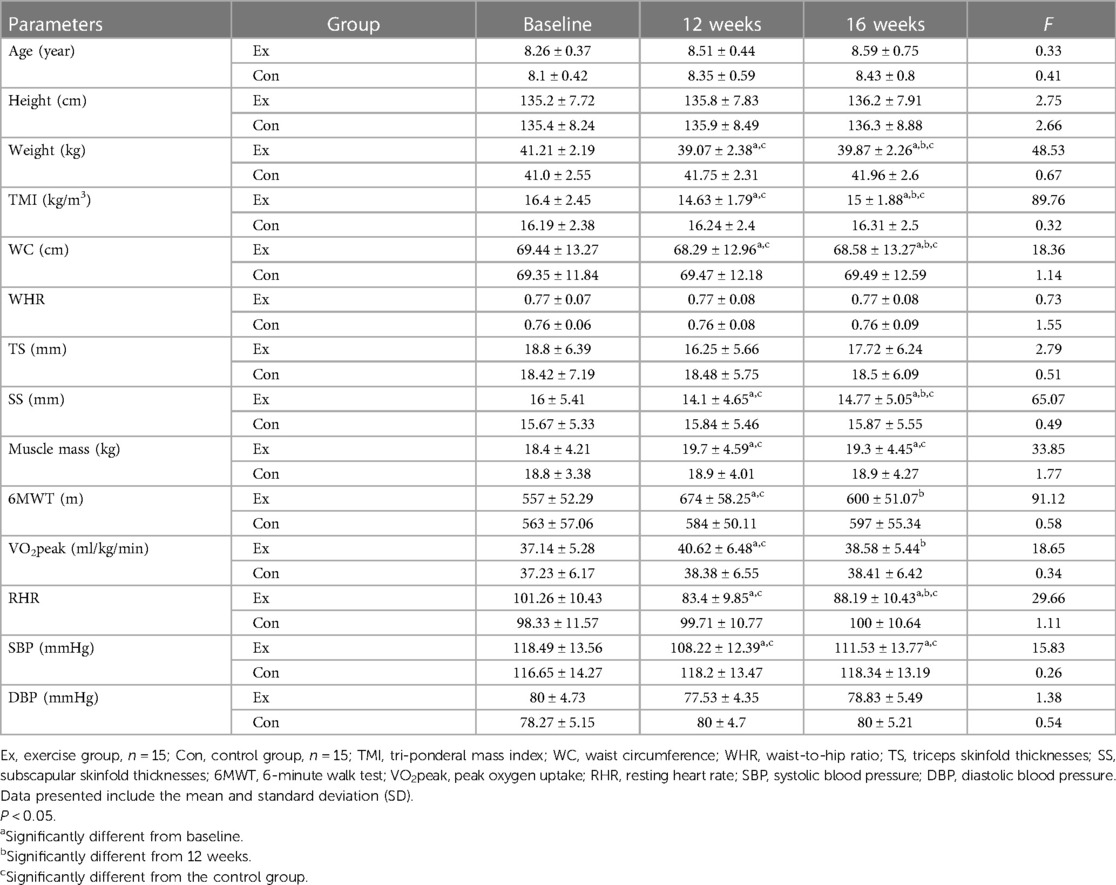
Table 1. Subject’s physiological and anthropometric characteristics in two groups (exercise and control) and three stages (baseline, 12 weeks, and 16 weeks).
3.2. Effect of the combined training and detraining on cortisol levels
The results indicated that the training intervention decreased the cortisol concentrations, and those concentrations were maintained throughout the detraining period (P < 0.05). In the Con, no significant change was observed throughout the study (Figure 2).
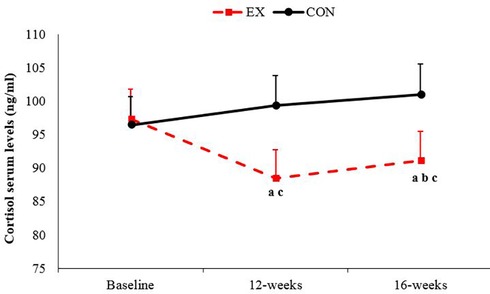
Figure 2. Changes in cortisol serum levels from the baseline to the end of 16 weeks of study in the exercise and control groups: (a) significantly different from baseline in the exercise group; (b) significantly different from 12 weeks; (c) significantly different between the exercise and control groups. P < 0.05. Ex, exercise; Con, control. Data are reported as mean ± standard deviation.
3.3. Effect of the combined training and detraining on mental health indices
The results demonstrated that combined training led to a significant decrease in the overall anxiety score among children. However, in the Con, there were no significant differences in the total anxiety score, except for the panic and generalized anxiety subscales, which showed a notable decrease (Table 2).
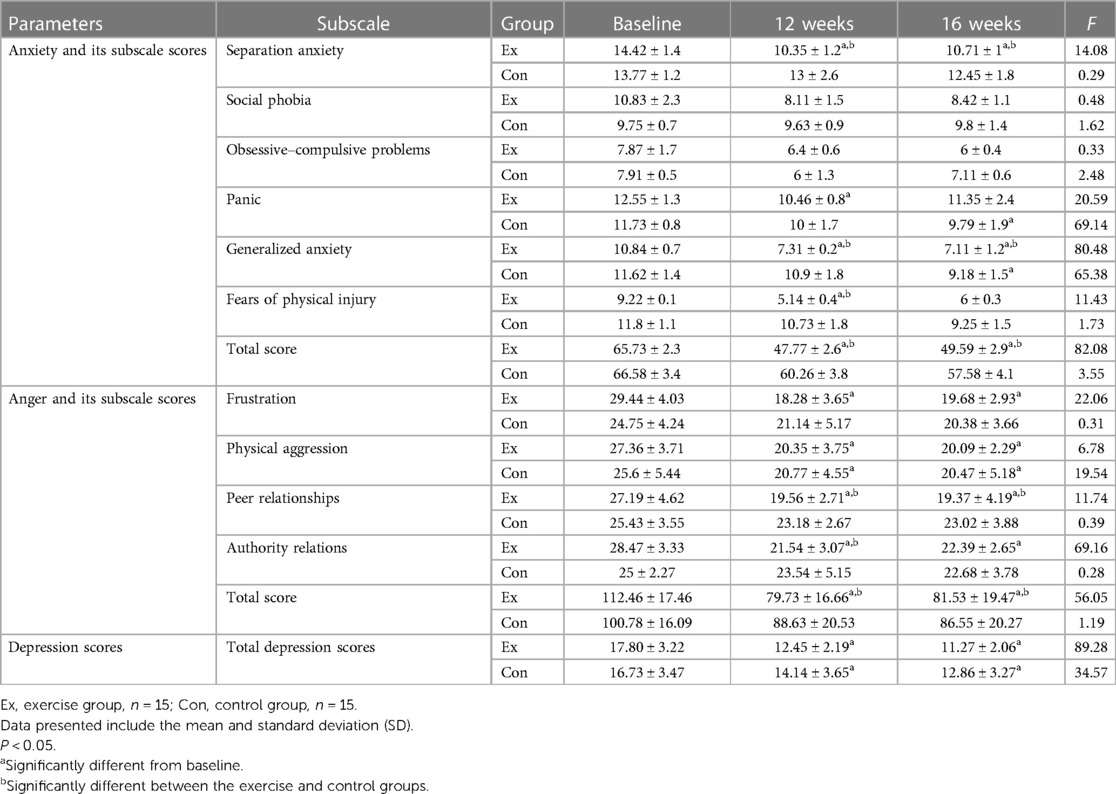
Table 2. Mean and standard deviation of anxiety, anger, and depression scores in two groups (exercise and control) and three stages (baseline, 12 weeks, and 16 weeks).
Furthermore, the results indicated a notable reduction in the depression scores among children who participated in a 12-week exercise program. Likewise, the Con, which received a 12-week treatment involving GnRH agonist, also witnessed a significant decrease in their depression scores (Table 2).
The statistical analysis of the data revealed a significant decrease in the total score of Nelson’s Anger Questionnaire with combined training. Conversely, no significant changes in anger scores were observed in the Con. However, the Con did experience a significant decrease in the physical aggression scores (Table 2).
3.4. Correlation analyses
Figure 3 shows the number of parameter changes between baseline and 12 weeks, as well as the significant Pearson correlation between them. A positive correlation existed between changes in weight and changes in TMI (r = 0.311), cortisol (r = 0.104), anxiety (r = 0.265), depression (r = 0.192), and anger (r = 0.209). Also, changes in serum cortisol levels were positively correlated with changes in TMI (r = 0.173) and anxiety (r = 0.164) (P < 0.05).
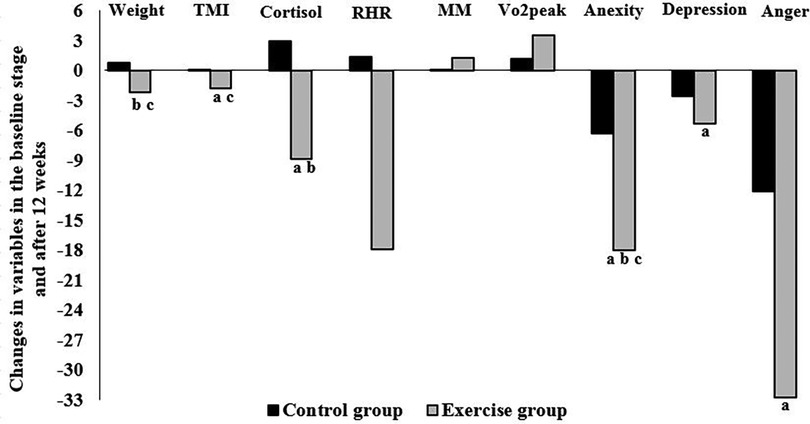
Figure 3. Correlation between the changes in variables in the baseline stage and after 12 weeks in the exercise and control groups: (a) significant correlation with weight, (b) significant correlation with TMI, and (c) significant correlation with cortisol. P < 0.05. TMI, tri-ponderal mass index; RHR, resting heart rate; MM, muscle mass; VO2peak, peak oxygen uptake.
Figure 4 shows the number of parameter changes between 12 weeks and 16 weeks, as well as the significant Pearson correlation between them. As shown in Figure 4, there is a significant positive correlation between weight changes and TMI changes (r = 0.218), and there is a negative correlation between weight changes and VO2peak changes (r = −0.125). Also, there is a significant positive correlation between cortisol changes and anxiety changes (r = 0.14) (P < 0.05).
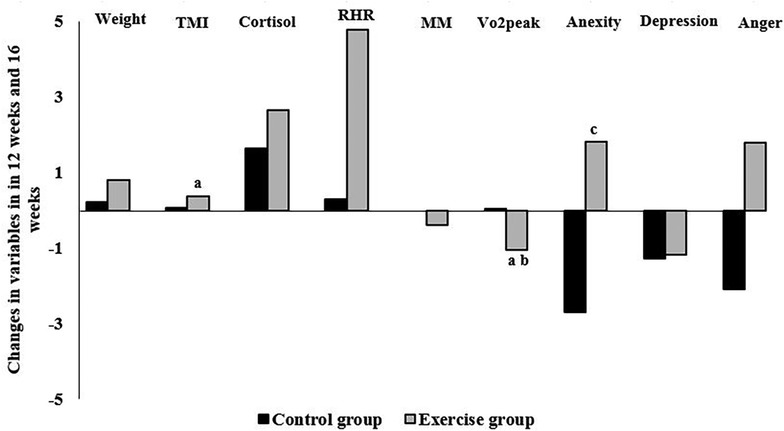
Figure 4. Correlation between the changes in variables in 12 weeks and 16 weeks in the exercise and control groups: (a) significant correlation with weight, (b) significant correlation with TMI, and (c) significant correlation with cortisol. P < 0.05. TMI, tri-ponderal mass index; RHR, resting heart rate; MM, muscle mass; VO2peak, peak oxygen uptake.
4. Discussion
As hypothesized, we found that 12 weeks of combined training led to a decrease in children’s anxiety, depression, and anger scores, as well as a decrease in serum cortisol levels. Meanwhile, in the Con, only depression decreased significantly. Furthermore, the positive effects of the combined training persisted during the 4-week detraining period. Potential mechanisms contributing to the reduction of the puberty onset age with obesity include the presence of aromatase in adipose tissue. Aromatase is an enzyme that can produce estrogens from adrenal androgen (39). Moreover, obesity is associated with insulin-induced reductions of sex hormone-binding globulin (SHBG) (40), which increases the bioavailability of sex steroids including estradiol. However, exercise causes weight loss through several mechanisms. Exercise can increase energy consumption and regulate energy balance, reduce body fat, improve body components, regulate insulin metabolism, and improve lipid metabolism. Obesity can also regulate the expression of genes and contend gene defects to some extent (41). The results of previous studies demonstrate that weight loss, reduced caloric intake, and catabolic state have a very powerful influence on the HPA axis and other endocrine systems (42). As observed in the Ex group during this study, the decrease in weight and TMI could potentially account for the reduction in cortisol levels within this group. Using TMI as a screening tool to identify obesity and overweight status in children and adolescents is worth contemplating (26). Therefore, we focused on TMI. Because of the limitation of BMI in distinguishing adipose mass from muscle, TMI has been proposed as a new indicator in assessing adiposity in children and adolescents better (43).
Another hypothesis to explain our results is to point out that at the level of the central nervous system, neuropeptides and corticosteroid receptors (glucocorticoid and mineralocorticoid receptors) in the brain and anterior pituitary play a major role in regulating circulating cortisol levels. Decreased pituitary sensitivity in endurance-trained athletes is one mechanism to reduce cortisol levels (20). Also, it has been shown in rats that restoring activity in the HPA axis with regular exercise resulted from adaptations in the hypothalamus and adrenal gland that promote a lower corticotropin-releasing hormone production and reduced adrenal sensitivity to adrenocorticotropic hormones, respectively (44).
Another point to discuss is that multi-facet exercises incorporating both aerobic and resistance training produce varied responses to cortisol production. The increase in VO2peak and muscle mass in the intervention group confirmed an expected effect of the aerobic and resistance training performed during the intervention period, respectively. It has already been reported that high cardiorespiratory fitness levels are associated with a lower diurnal cortisol output (45). In this research, VO2peak increased and had a negative relationship with cortisol, which could be a possible mechanism of cortisol reduction. On the other hand, cortisol is a catabolic hormone that stimulates degradation and inhibits the synthesis of muscle proteins (46). However, in our study, muscle mass increased due to combined training.
A recent meta-analysis has confirmed that physical activity provides significant benefits in reducing symptoms of depression, anxiety, and distress among different adult populations. These populations include the general public, individuals with mental health disorders, and those suffering from chronic diseases (47). The findings prove that exercise can have multiple beneficial effects on the physical and mental health of people with a wide range of mental disorders (48). The mechanisms behind the possible stress-buffering effects of exercise are still unclear. An early meta-analysis proved that aerobically fit subjects had a reduced psychosocial stress response (e.g., heart rate and blood pressure) compared with unfit individuals (15). It was previously argued that the benefits associated with training might be due to neurotransmitter, neuromodulator, and psychological mechanisms (49), oxidative stress, pro-inflammatory cytokines, and anti-inflammatory cytokines (7). In this regard, our previous research also confirmed that combined training and aerobic training increased adiponectin and decreased resistin and the signs of puberty (50, 51). Training may also increase body temperature, blood circulation in the brain, and impact on the HPA axis and physiological reactivity to stress (19).
Exercise has been found to have an antidepressant effect in both human and animal studies. This is achieved by boosting mitochondrial activity in brain neurons, promoting the release of monoamine neurotransmitters, increasing the levels of neurotropic factors, preventing the excessive production of inflammatory factors, and controlling the expression of microRNAs (14).
Serotonin has been linked to exercise and anger expression. In human adults, aggression has been shown to correlate negatively with the serotonergic activity of the central nervous system (52). In rats, exercise has been shown to buffer stress by altering serotonin and norepinephrine brain systems (53), so this may account for the reduced anger scores observed in the exercise condition. However, in the current study, serotonin was not evaluated.
A significant decrease in depression scales in Con is noted in our study, while anxiety and anger scores did not change during the 16-week treatment with GnRH agonist. In the same field, Yu et al. stated that a longer follow-up duration may lead to a stronger improvement in more patients with clinical psychological issues from a larger pool (54).
5. Conclusion
Overall, it can be concluded that overweight and obese children with CPP who participated in the training program experienced a decrease in cortisol, anxiety, depression, and anger compared with the Con. For overweight girls with CPP, engaging in physical activity alongside GnRH treatment not only improves physiological aspects like weight, heart rate, and cardiorespiratory fitness but also enhances psychological wellbeing. However, treatment with GnRH alone does not provide the same benefit levels. In addition, the effects of training on psychological and physiological characteristics were not completely eliminated during the 4-week detraining period, except for VO2peak. Exercise interventions should be integrated into the regular care of girls with CPP to promote physical and mental health.
6. Limitations
Some limitations of our study merit consideration. The sample size was small. The impact of diet, chemicals, and industry was not controlled, and the changes in the dietary or physical habits of the participants were not monitored in this study. Another limitation of the study was the inadequacy of the measurement of cortisol alone as a stress marker.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved. This research was approved by the Research Ethics Committees of Hamadan University of Medical Sciences on 5 February 2022, with proprietary ID IR.UMSHA.REC.1400.883. The study was also approved by the Iranian Registry of Clinical Trials, IRCT20170411033378N10, on 27 February 2022. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent for the publication of any potentially identifiable images or data included in this article was obtained from the individual(s) and minor(s)’ legal guardian/next of kin.
Author contributions
AH, ESa, and FC presented the idea for research, designed the research studies, supervised, provided guidance, and wrote the manuscript. ESh conducted the research, monitored the implementation of the exercise protocol, acquired data, analyzed data, and wrote the manuscript. ZR diagnosed and introduced children with precocious puberty. All authors contributed to the article and approved the submitted version.
Funding
The work was conducted as a research project and was supported by Hamadan University of Medical Sciences (grant number 140103242065).
Acknowledgments
The authors would like to thank the participants and their families. The authors also appreciate the financial support of Hamadan University of Medical Sciences (No.140103242065).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Obesity and Overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. (Accessed May 2, 2023).
2. Zhai L, Liu J, Zhao J, Liu J, Bia Y, Jia L, et al. Association of obesity with onset of puberty and sex hormones in Chinese girls: a 4-year longitudinal study. PLoS One. (2015) 10(8):e0134656. doi: 10.1371/journal.pone.0134656
3. Liu G, Guo J, Zhang X, Lu Y, Miao J, Xue H. Obesity is a risk factor for central precocious puberty: a case–control study. BMC Pediatr. (2021) 21(1):509. doi: 10.1186/s12887-021-02936-1
4. Berberoğlu M. Precocious puberty and normal variant puberty: definition, etiology, diagnosis and current management. J Clin Res Pediatr Endocrinol. (2009) 1(4):164–74. doi: 10.4274/jcrpe.v1i4.3
5. Synovitz L, Chopak-Foss J. Precocious puberty: pathology, related risks, and support strategies. Open J Prev Med. (2013) 3:504–9. doi: 10.4236/ojpm.2013.39068
6. Blumenthal H, Leen-Feldner EW, Babson KA, Gahr JL, Trainor CD, Frala JL. Elevated social anxiety among early maturing girls. Dev Psychol. (2011) 47(4):1133–40. doi: 10.1037/a0024008
7. Gerber M, Imboden C, Beck J, Brand S, Colledge F, Eckert A, et al. Effects of aerobic exercise on cortisol stress reactivity in response to the Trier social stress test in inpatients with major depressive disorders: a randomized controlled trial. J Clin Med. (2020) 9(5):1419. doi: 10.3390/jcm9051419
8. Thau L, Gandhi J, Sharma S. Physiology, cortisol. In: Statpearls. Treasure Island, FL: StatPearls Publishing (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK538239/. (Accessed April 23, 2023).
9. Van der Valk ES, Savas M, van Rossum EFC. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. (2018) 7(2):193–203. doi: 10.1007/s13679-018-0306-y
10. Anık A, Çatlı G, Abacı A, Böber E. Effect of gonadotropin-releasing hormoneagonist therapy on body mass index and growth in girls with idiopathiccentral precocious puberty. Indian J Endocrinol Metab. (2015) 19(2):267–71. doi: 10.4103/2230-8210.131770
11. Stone T, DiPietro L, Stachenfeld NS. Exercise treatment of obesity. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth, MA: MDText.com, Inc (2000). p. 1–21. Available at: https://www.ncbi.nlm.nih.gov/books/NBK278961/. (Accessed March 27, 2023).
12. Reinehr T, Bosse C, Lass N, Rothermel J, Knop C, Roth CL. Effect of weight loss on puberty onset in overweight children. J Pediatr. (2017) 184:143–50.e1. doi: 10.1016/j.jpeds.2017.01.066
13. Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. (2005) 18(2):189–93. doi: 10.1097/00001504-200503000-00013
14. Xie Y, Wu Z, Sun L, Zhou L, Wang G, Xiao L, et al. The effects and mechanisms of exercise on the treatment of depression. Front Psychiatry. (2021) 5(12):705559. doi: 10.3389/fpsyt.2021.705559
15. Crews DJ, Landers DM. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Med Sci Sports Exerc. (1987) 19(5 Suppl):S114–20.3316910
16. Zhu Y, Li J, Zhang M, Li C, Lau EY, Tao S. Physical activity participation and physical aggression in children and adolescents: a systematic review and meta-analysis. Psychol Sport Exerc. (2022) 63:102288. doi: 10.1016/j.psychsport.2022.102288
17. Elliott LD, Wilson OWA, Holland KE, Bopp M. Using exercise as a stress management technique during the COVID-19 pandemic: the differences between men and women in college. Int J Exerc Sci. (2021) 14:1234–46.35096245
18. Jackson EM. Stress relief: the role of exercise in stress management. ACSMS Health Fit J. (2013) 17(3):14–19. doi: 10.1249/FIT.0b013e31828cb1c9
19. Guszkowska M. Effects of exercise on anxiety, depression and mood. Psychiatr Pol. (2004) 38(4):611–20.15518309
20. Duclos M, Tabarin A. Exercise and the hypothalamo–pituitary–adrenal axis. Front Horm Res. (2016) 47:12–26. doi: 10.1159/000445149
21. Rosa CS, Giannaki CD, Krase A, Mplekou M, Grigoriou SS, Stefanidis I, et al. Effects of 12 months of detraining on health-related quality of life in patients receiving hemodialysis therapy. Int Urol Nephrol. (2020) 52:1771–8. doi: 10.1007/s11255-020-02560-5
22. Heidarianpour A, Shokri E, Baghian T, Shokri B. Benefits of aerobic training in girls with precocious puberty: involvement of CRP and cortisol. J Pediatr Endocrinol Metab. (2019) 32(9):1005–11. doi: 10.1515/jpem-2018-0484
23. Harrington J, Palmert MR, Hamilton J. Use of local data to enhance uptake of published recommendations: an example from the diagnostic evaluation of precocious puberty. Arch Dis Child. (2014) 99:15–20. doi: 10.1136/archdischild-2013-304414
24. Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. (2013) 98:2198–207. doi: 10.1210/jc.2013-1024
25. WHO Expert Committee on Physical Status: the Use and Interpretation of Anthropometry (1993: Geneva, Switzerland) and World Health Organization. Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. World Health Organization (1995). Available at: https://apps.who.int/iris/handle/10665/37003. (Accessed February 4, 2023).
26. Wang X, Ma J, Huang S, Dong B, Dong Y, Yang Z, et al. Use of tri-ponderal mass Index in predicting late adolescent overweight and obesity in children aged 7–18. Front Nutr. (2022) 9:785863. doi: 10.3389/fnut.2022.785863
27. Addo OY, Himes JH. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. (2010) 91:635–42. doi: 10.3945/ajcn.2009.28385
28. Poortmans JR, Boisseau N, Moraine JJ, Moreno-Reyes R, Goldman S. Estimation of total-body skeletal muscle mass in children and adolescents. Med Sci Sports Exercise. (2005) 37(2):316–22. doi: 10.1249/01.mss.0000152804.93039.ce
29. ATS committee on proficiency standards for clinical pulmonary function laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102
30. Morinder G, Mattsson E, Sollander C, Marcus C, Larsson UE. Six-minute walk test in obese children andadolescents: reproducibility and validity. Physiother Res Int. (2009) 14:91–104. doi: 10.1002/pri.428
31. Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. (1998) 36(5):545–66. doi: 10.1016/S0005-7967(98)00034-5
32. Hoseini S, Sadeghi M, Qaderi Bagajan K, Soleimani ZA, Jafari M, Zolfaghari S. Evaluation of the psychometric properties of the Persian version of Spence Children’s Anxiety Scale in Iranian adolescents. Clin Child Psychol Psychiatry. (2022) 28(4):1565–79. doi: 10.1177/13591045221112467
33. Birelson PA. Self-rating scale for depressive disorder in childhood. [M.Phil. thesis]. Scotland: University of Edinburgh (1978).
34. Birelson P. The validity of depressive disorder in childhood and the development of a self-rating scale: a research report. J Psychol Psyc. (1981) 22:73–88. doi: 10.1111/j.1469-7610.1981.tb00533.x
35. Nelson WM, Finch AJ. Children’s inventory of anger. Los Angeles, CA: Western Psychological Services (2000).
36. Agarwal D, Dange RB, Vila J, Otamendi AJ, Francis J. Detraining differentially preserved beneficial effects of exercise on hypertension: effects on blood pressure, cardiac function, brain inflammatory cytokines and oxidative stress. PLoS One. (2012) 7:e52569. doi: 10.1371/journal.pone.0052569
37. Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. (2009) 54:2396–406. doi: 10.1016/j.jacc.2009.08.030
38. Yi DY, Kim SC, Lee JH, Lee EH, Kim JY, Kim YJ, et al. Clinical practice guideline for the diagnosis and treatment of pediatric obesity: recommendations from the Committee on Pediatric Obesity of the Korean Society of Pediatric Gastroenterology Hepatology and Nutrition. Korean J Pediatr. (2019) 62(1):3–21. doi: 10.3345/kjp.2018.07360
39. Dunger DB, Ahmed ML, Ong KK. Effects of obesity on growth and puberty. Best Pract Res Clin Endocrinol Metab. (2005) 19:375–90. doi: 10.1016/j.beem.2005.04.005
40. Nokoff N, Thurston J, Hilkin A, Pyle L, Zeitler PS, Nadeau KJ, et al. Sex differences in effects of obesity on reproductive hormones and glucose metabolism in early puberty. J Clin Endocrinol Metab. (2019) 104:4390–7. doi: 10.1210/jc.2018-02747
41. Shen TY, Li JH, Huang L, Tang W. Biological mechanism of losing weight by exercise. J Clin Rehabil Tiss Eng Res. (2007) 11(17):3415–8.
42. Fichter MM, Pirke KM. Effect of experimental and pathological weight loss upon the hypothalamo–pituitary–adrenal axis. Psychoneuroendocrinology. (1986) 11(3):295–305. doi: 10.1016/0306-4530(86)90015-6
43. Sun J, Yang R, Zhao M, Bovet P, Xi B. Tri-ponderal mass index as a screening tool for identifying body fat and cardiovascular risk factors in children and adolescents: a systematic review. Front Endocrinol (Lausanne). (2021) 12:694681. doi: 10.3389/fendo.2021.694681
44. Campbell JE, Király MA, Atkinson DJ, D’souza AM, Vranic M, Riddell MC. Regular exercise prevents the development of hyperglucocorticoidemia via adaptations in the brain and adrenal glands in male Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. (2010) 299(1):R168–76. doi: 10.1152/ajpregu.00155.2010
45. Lucertini F, Ponzio E, Di Palma M, Galati C, Federici A, Barbadoro P, et al. High cardiorespiratory fitness is negatively associated with daily cortisol output in healthy aging men. PLoS One. (2015) 10(11):e0141970. doi: 10.1371/journal.pone.0141970
46. Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. (2003) 58(10):M911–6. doi: 10.1093/gerona/58.10.M911
47. Singh B, Olds T, Curtis R, Dumuid D, Virgara R, Watson A, et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med. (2023) 0:1–10. doi: 10.1136/bjsports-2022-106195
48. Schuch FB, Vancampfort D. Physical activity, exercise, and mental disorders: it is time to move on. Trends Psychiatry Psychother. (2021) 43(3):177–84. doi: 10.47626/2237-6089-2021-0237
49. DeBoer LB, Powers MB, Utschig AC, Otto MW, Smits JA. Exploring exercise as an avenue for the treatment of anxiety disorders. Expert Rev Neurother. (2012) 12(8):1011–22. doi: 10.1586/ern.12.73
50. Shokri E, Heidarianpour A, Razavi Z. Positive effect of combined exercise on adipokines levels and pubertal signs in overweight and obese girls with central precocious puberty. Lipids Health Dis. (2021) 20(1):152. doi: 10.1186/s12944-021-01588-5
51. Shokri B, Heidarianpour A, Shokri E. Effect of exercise and detraining on signs of puberty and selected inflammatory markers in girls with precocious puberty. Med Sci Sports Exerc. (2023) 55(7):1133–42. doi: 10.1249/MSS.0000000000003138
52. Coccaro EF. Central serotonin and impulsive aggression. Br J Psychiatry Suppl. (1989) 8:52–62. doi: 10.1192/S0007125000291769
53. Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. (2003) 23(7):2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003
Keywords: combined training, cortisol, mental health, obesity, central precocious puberty
Citation: Heidarianpour A, Shokri E, Sadeghian E, Cheraghi F and Razavi Z (2023) Combined training in addition to cortisol reduction can improve the mental health of girls with precocious puberty and obesity. Front. Pediatr. 11:1241744. doi: 10.3389/fped.2023.1241744
Received: 17 June 2023; Accepted: 17 October 2023;
Published: 10 November 2023.
Edited by:
Pierluigi Marzuillo, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Gianvincenzo Zuccotti, University of Milan, ItalyPaul B. Kaplowitz, Children’s National Hospital, United States
© 2023 Heidarianpour, Shokri, Sadeghian, Cheraghi and Razavi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Efat Sadeghian c2FkZWdoaWFuX2VAdW1zaGEuYWMuaXI=
†ORCID Ali Heidarianpour orcid.org/0000-0001-8152-1489 Elnaz Shokri orcid.org/0000-0002-8249-7107 Efat Sadeghian orcid.org/0000-0001-6075-3811 Fatemeh Cheraghi orcid.org/0000-0001-5571-7250 Zahra Razavi orcid.org/0000-0001-5318-9087
 Ali Heidarianpour
Ali Heidarianpour Elnaz Shokri1,†
Elnaz Shokri1,†