- Department of Neonatology, The First Affiliated Hospital of Zheng Zhou University, Zhengzhou, China
Objectives: The study aims to investigate whether the time interval between administering antenatal corticosteroids (ACS) and delivery influences the neonatal outcomes in late preterm (LPT) neonates (34 + 0 to 36 + 6 weeks) born to mothers with diabetes.
Study design: This retrospective cohort study included women with any type of diabetes who gave birth between 34 + 0 weeks and 36 + 6 weeks of gestation. Based on the time interval between the first dose of corticosteroid and delivery, the cases were stratified into the following groups: <2, 2–7, and >7 days. Women unexposed to ACS served as the control group. The primary outcomes included the incidence of neonatal hypoglycemia and respiratory distress syndrome/transient tachypnea of the newborn. Multivariate logistic regression was used to assess the relationship between the time interval and neonatal outcomes and adjust for potential confounders.
Results: The study enrolled a total of 636 parturients. Among them, 247 (38.8%) delivered within 2 days after ACS administration, 169 (26.6%) within 2–7 days, and 126 (19.8%) at >7 days. Baseline characteristics such as type of diabetes, methods of glycemic control, preterm premature rupture of membrane, placenta previa, cesarean delivery, indication for delivery, percentage of large for gestational age, birth weight, and HbA1c in the second or third trimester were significantly different among the four groups. The multivariate analysis showed no statistically significant difference in the incidence of primary or secondary neonatal outcomes between the case and control groups.
Conclusions: ACS treatment was not associated with neonatal hypoglycemia and respiratory outcomes in LPT neonates born to diabetic mothers, regardless of the time interval to delivery.
Introduction
In the country, late preterm (LPT: 34 + 0 to 36 + 6 weeks gestation) births comprise approximately 70% of all preterm births (PTB) (1). In addition, this subgroup of infants has a higher risk of suffering from multiple complications than those born at term, such as respiratory distress, leading to respiratory distress syndrome (RDS) and transient tachypnea of the newborn (TTN) (2, 3). In recent years, the prevalence of diabetes mellitus and gestational diabetes mellitus (GDM) has gradually increased in mainland China, and GDM has become a common complication during pregnancy (4). Maternal diabetes [including GDM or diabetes in pregnancy (DIP)] is associated with delayed surfactant synthesis and increased rates of cesarean delivery, which can also lead to increased rates of neonatal RDS and TTN (5–7).
The Antenatal Late Preterm Steroid (ALPS) trial, a large multicenter randomized controlled trial, found that using antenatal corticosteroids (ACS) was significantly associated with reduced complications in the respiratory system of LPT neonates (8). Other studies also confirmed this finding (9–13). Accordingly, many physicians have started routinely using ACS for women at risk of LPT labor (14). However, these studies assessing the efficacy of ACS largely excluded women with diabetes (including GDM and DIP) (10, 11) or included only a small proportion of them (8, 9, 15). ACS may be important in improving respiratory outcomes in infants born to mothers with diabetes. Furthermore, ACS was found to be associated with an increased risk of neonatal hypoglycemia. Hypoglycemia is also a common complication in newborns born to diabetic mothers (7, 16, 17). Therefore, it is necessary to evaluate the efficacy of ACS in women with diabetes who delivered during the LPT period.
Recently, several retrospective studies on the safety and efficacy of ACS treatment in women with diabetes (GDM or DIP) who delivered during the LPT period have obtained inconsistent results (18–20). More importantly, none of those studies specifically assessed the effect of the time interval between corticosteroid administration and delivery on neonatal outcomes, which plays an important role in the clinical effectiveness of ACS. Thus, our study aims to determine if the time interval between ACS administration and delivery influences the outcomes of LPT neonates born to diabetic mothers.
Materials and methods
Data collection
This retrospective cohort included women diagnosed with GDM and DIP (including pre-existing DIP) and who delivered at 34 to 36 + 6 weeks of pregnancy in a tertiary medical center between 1 October 2017 and 31 December 2022. The exclusion criteria included women with multiple pregnancies, fetal chromosomal abnormalities, or major fetal anomalies. According to the time interval between the first dose of ACS and delivery, the cases were divided into the following groups: <2, 2–7, and >7 days. Women unexposed to prenatal steroids served as the control group. The demographic and obstetric variables recorded include the type of diabetes, method of glycemic control, maternal age, gravidity, parity, preterm premature rupture of membrane (PPROM), placenta previa, mode of delivery, indication for delivery, body mass index (BMI), hypertensive disorders, second- or third-trimester glycated hemoglobin (HbA1c), and indication for ACS. Neonatal baseline data included male sex, gestational age (GA) at ACS, GA at delivery, birth weight, small or large for gestational age (LGA), and 1 min Apgar score. The primary outcomes included the incidence of RDS/TTN and neonatal hypoglycemia. The secondary outcomes included the lowest glucose level, neonatal ward admission, admission due to respiratory issue, admission due to hypoglycemia, duration of hospitalization, oxygen requirement (any form of oxygen requirement after birth), continuous positive airway pressure (CPAP) or high flow nasal cannula (HFNC), mechanical ventilation, requirement of resuscitation at birth, and need for surfactant.
Definitions
The diagnosis of GDM or DIP was based on one or more of the following abnormal glucose values: GDM: fasting blood glucose ≥5.1 mmol/L, 1-h post 75 g oral glucose tolerance test (OGTT) ≥ 10.0 mmol/L, and 2-h post 75 g OGTT ≥ 8.5 mmol/L; DIP: fasting glucose ≥7 mmol/L and 2-h glucose ≥11.1 mmol/L, according to the World Health Organization criteria (21). The Fenton 2013 curve was used to calculate the birth weight percentile (22). RDS was characterized as follows: (1) progressive dyspnea accompanied by an expiratory groan, cyanosis, or inspiratory three-concave sign within 6 h after birth and (2) reduced transparency of both lungs, an air bronchogram sign, indistinct septal margins, an indistinct heart, or white lungs on chest x-rays (23). The main chest x-ray findings of TTN were interstitial, alveolar, and interlobular pleural effusion. Hypoglycemia was defined as glucose levels below 2.2 mmol/L within 24 h after birth (24). All newborns with a glucose level ≤2.6 mmol/L subsequently received a protocol including intravenous dextrose therapy and monitoring in our unit. For newborns without hypoglycemic symptoms, blood glucose monitoring should be conducted after the initial feeding (within 1.5 h after birth), and pre-feeding blood glucose should be checked every 3–6 h within 24 h after birth. Newborns with hypoglycemic symptoms require continuous blood glucose monitoring.
ACS exposure was defined as at least one dose of dexamethasone (6 mg) given at any time during pregnancy. A complete course was defined as four intramuscular 6-mg doses of dexamethasone administered 12 h apart. Indications for ACS (25) included preterm labor (regular uterine contractions leading to cervical changes), PPROM, fetal indications (such as placental insufficiency, intrauterine growth restriction, or oligohydramnios), maternal indications (such as GDM or DIP and hypertensive disorders), abnormal vaginal bleeding (such as placenta previa and placental abruption), and asymptomatic changes in the cervix (cervical dilation of >4 cm and/or length of <15 mm). However, prophylactic treatment with dexamethasone was ultimately the decision of the obstetrician.
Statistical analysis
Data analysis was performed using SPSS version 26.0 software. ANOVA test and chi-squared tests were used to compare continuous and categorical variables, respectively. Post hoc analysis was performed using the Bonferroni test to correct for multiple comparisons. The least significant difference test or Tamhane's test was used to compare any two groups according to homogeneity of variance or heterogeneity of variance, respectively. A two-tailed test was used to evaluate the significance of statistical tests at the significance level of 5%. Multivariate logistic regression analysis was performed to assess the relationship between the interval from ACS administration to birth and the neonatal outcomes and to adjust for potential confounders. In the multivariate analysis, the regression model included variables with differences between groups (P < 0.05).
Results
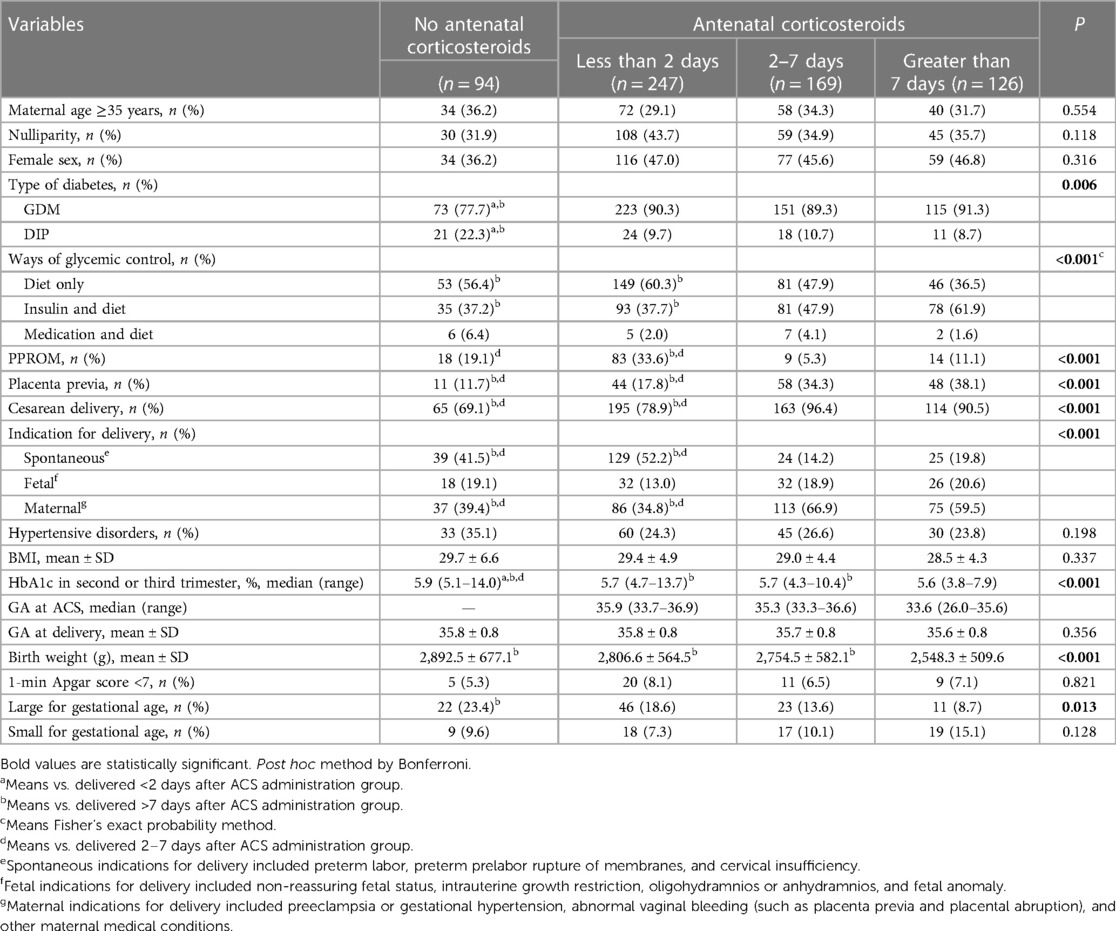
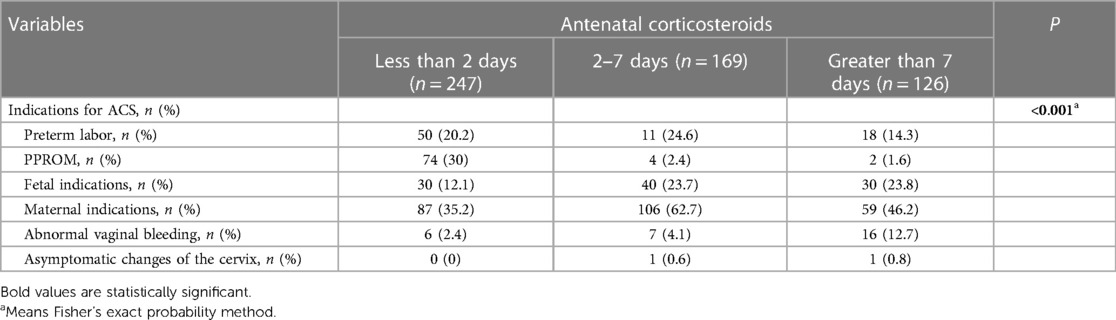
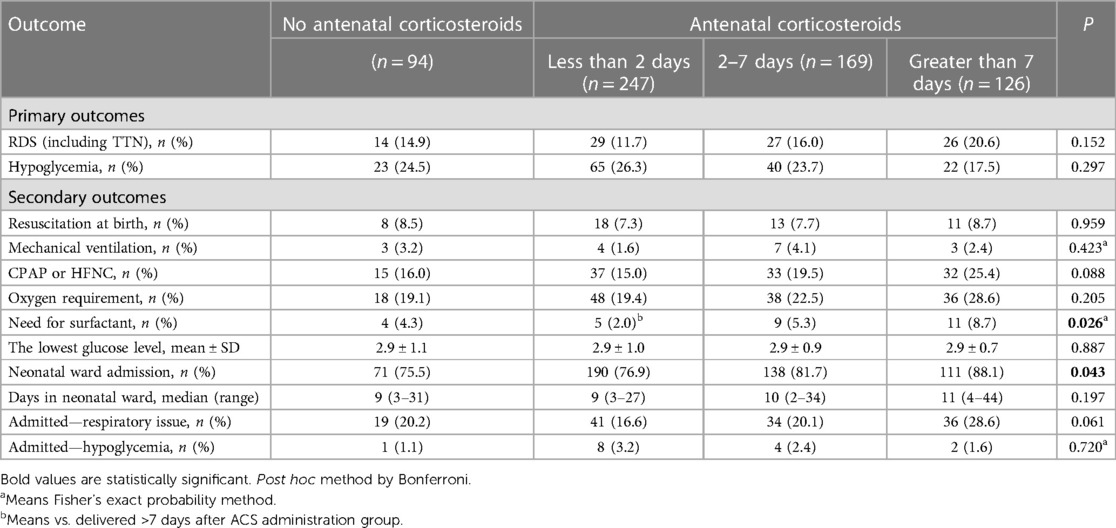
Overall, the study enrolled a total of 636 parturients. Among them, 247 (38.8%) delivered within 2 days after ACS administration, 169 (26.6%) within 2–7 days, and 126 (19.8%) at >7 days. Table 1 shows the baseline characteristics of each group. Baseline characteristics such as type of diabetes, methods of glycemic control, PPROM, placenta previa, cesarean delivery, indication for delivery, percentage of LGA, birth weight, and HbA1c in the second or third trimester were significantly different among the four groups (Table 1). The control group and the <2 days group were more likely to deliver due to spontaneous indications, while the other two groups primarily due to maternal indications. Compared with the other three groups, neonates born after 7 days of ACS treatment had a lower birth weight (2,548.3 ± 509.6, >7 days group, vs. 2,892.5 ± 677.1, control group; 2,806.6 ± 564.5, <2 days group; 2,754.5 ± 582.1, 2–7 days group; P < 0.001). There were no other significant differences in baseline characteristics among the four groups. Women were more likely to receive ACS due to maternal indications (Table 2). Table 3 shows the neonatal outcomes. In the unadjusted estimates, there were no differences in the rates of primary and secondary neonatal outcomes among the four groups, except surfactant use and neonatal ward admission. Post hoc analysis showed that there was a statistical difference in the rate of surfactant use between the <2 days group and the >7 days group (2.0% vs. 8.7%, P = 0.026), but no statistical difference in the hospitalization rate was found between any two groups. We used multivariate logistic regression analysis to control for different baseline characteristics among groups and to evaluate the association between the interval from ACS administration to birth and the neonatal outcomes. Table 4 presents the results. The multivariate analysis revealed no statistically significant difference in the incidence of primary or secondary neonatal outcomes between the case and control groups.
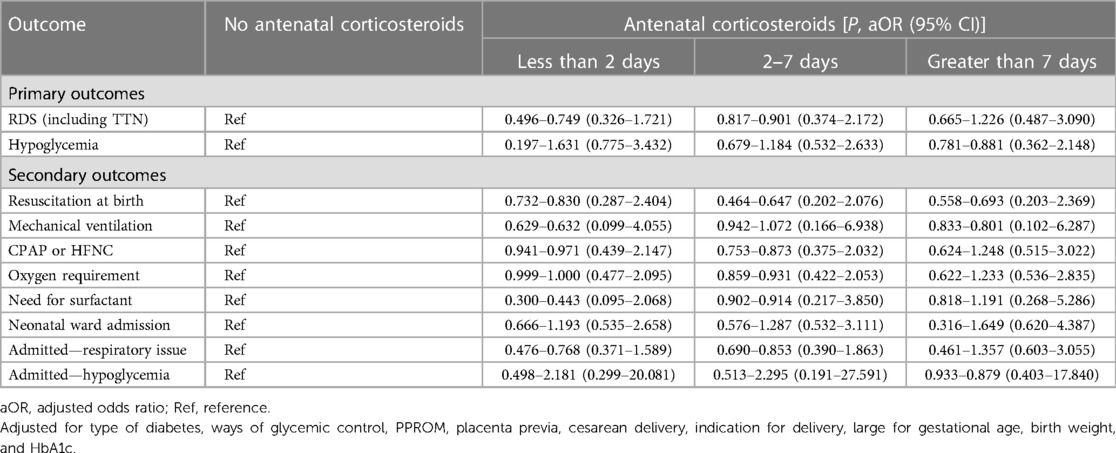
Table 4. Multivariable analysis of the association between the antenatal corticosteroid administration to birth interval and adverse neonatal outcomes.
Discussion
In our study, ACS treatment did not reduce the incidence of respiratory system diseases in LPT neonates born to diabetic mothers. In addition, it did not increase the incidence of neonatal hypoglycemia, regardless of the time interval to delivery.
The ALPS trial found that administering ACS significantly reduced the incidence of neonatal respiratory complications in LPT infants (8). However, the trial included only a small sample of women with GDM (306/2,831), and it excluded women with GDM requiring insulin treatment or those with pregestational diabetes. Dude et al. (18) and Krispin et al. (19) conducted retrospective cohort studies of diabetic mothers. One study included women with all types of diabetes, and ACS treatment was administered exclusively during the LPT period (18). The other study included 161 mothers with GDM who delivered during the LPT period and 2,101 mothers with GDM who delivered at term. The ACS group included women who received ACS treatment between 24 + 0 and 33 + 6 weeks of gestation (19). In contrast to these studies, our study included women with any type of diabetes and those treated with ACS at any time during pregnancy as a case group. Despite the differences, their findings are consistent with ours in that ACS treatment was not associated with reducing respiratory morbidity in LPT infants born to diabetic mothers. Furthermore, some studies found that ACS treatment does not improve neonatal respiratory outcomes even in mothers with diabetes who delivered between 23 + 0 and 33 + 6 weeks or underwent early-term scheduled cesarean section (ETSCS) (26–28). ACS treatment can result in transient maternal hyperglycemia and subsequent fetal hyperinsulinemia, which leads to delayed surfactant synthesis (29–31). In addition, Refuerzo et al. found that hyperglycemia following ACS treatment is more severe in diabetic pregnant women, with glucose levels increasing nearly 50% higher than that in non-diabetic pregnant women (32). Therefore, neonates born to diabetic women may not receive the same benefits from ACS, compared with those born to mothers without diabetes.
Studies have found that ACS may increase the incidence of neonatal hypoglycemia by inducing transient maternal hyperglycemia, leading to fetal reactive hyperinsulinemia and fetal adrenal suppression (33–35). In addition, previous experimental studies demonstrated that ACS exposure may induce the production of fetal hepatic enzymes involved in regulating glucose metabolism (36, 37). Thus, ACS exposure may further increase the risk of hypoglycemia in infants born to diabetic mothers. However, the effect of ACS on the blood glucose levels of neonates has been inconsistent across different studies. The ALPS trial, which included only a small sample of women with GDM (306/2,831), also showed that ACS could significantly increase the incidence of neonatal hypoglycemia [relative risk: 1.6; 95% confidence interval (CI): 1.37–1.87; P < 0.001] (8). The study of Dude et al. found a higher incidence of hypoglycemia in newborns born to mothers with diabetes treated with LPT corticosteroids (adjusted odds ratio 2.96, 95% CI: 1.29–6.82) (18). Two retrospective cohort studies by Gupta et al. (27) and Li et al. (28) on women with all types of diabetes recently found that ACS treatment before ETSCS was related to more neonatal hypoglycemia. Moreover, Li et al. found that this association only existed in newborns born within 2 days after ACS administration. However, our study found no association between ACS administration at any time during pregnancy and neonatal hypoglycemia in the population of pregnant women with diabetes. The study by Krispin et al. (19) and another study (38), which included 54 mothers with pregestational diabetes who delivered during the LPT period, also did not find a correlation between the two. Although none of the studies took into account the timing of administration, maternal hyperglycemia following corticosteroid treatment may be transient. The influences of ACS on maternal and subsequent fetal glucose homeostasis may disappear or decrease with the extension of the time interval between ACS administration and delivery (39). In the future, larger prospective studies are required to assess the effects of ACS exposure in this particular group.
In addition, in our study, the admission rate for hypoglycemia was much lower than the incidence of neonatal hypoglycemia. In China, apart from issues such as respiratory problems, jaundice, and infections, many neonates are hospitalized due to the lack of confidence of their parents in taking care of premature infants. As a result, many cases of neonatal hypoglycemia were detected during hospitalization, which is also one of the reasons for the high rates of hospitalization in our study. Therefore, regardless of hospitalization, routine blood glucose monitoring should be conducted for LPT infants born to mothers with diabetes.
Strength and limitations
Our study has several strengths. To our knowledge, this is the first study to assess the association between different time intervals from ACS administration to delivery and neonatal outcomes in LPT infants born to diabetic mothers. Another strength of our study is the sample size, which is the largest among reports focusing on this particular population. Our study also has several limitations. As a retrospective cohort study, some confounding factors that might have influenced the results were not reported, such as blood glucose levels in the third trimester of pregnancy. However, we collected HbA1c levels in the third trimester as a measure. In addition, because our hospital is the largest tertiary medical center in Henan province, we have many high-risk parturients and a high rate of cesarean deliveries in the study population. Lastly, due to the high rate of ACS exposure in our hospital, there was a difference in sample size between our case groups and the control group, which may have influenced the results. Therefore, a larger randomized controlled trial is needed to confirm these findings in the future.
Conclusions
In conclusion, our findings suggest that ACS treatment is not associated with neonatal hypoglycemia and respiratory outcomes of LPT neonates born to mothers with any type of diabetes, regardless of the time interval to delivery. Further studies are required to evaluate the effect of ACS treatment in this specific population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2023-KY-0537). The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation was not required from the participants or their legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XL contributed to the study conception and design. XL, QH, and YD performed the data collection and analysis. XL wrote the first draft of the manuscript. JZ and XC edited and revised the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Syst. (2015) 64(1):1–65. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf
2. Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. (2013) 132(4):741–51. doi: 10.1542/peds.2013-1131
3. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. (2013) 14 (1):29–36; quiz 36–7. doi: 10.1016/j.prrv.2012.02.002
4. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. (2019) 10(1):154–62. doi: 10.1111/jdi.12854
5. Vignoles P, Gire C, Mancini J, Bretelle F, Boubli L, Janky E, et al. Gestational diabetes: a strong independent risk factor for severe neonatal respiratory failure after 34 weeks. Arch Gynecol Obstet. (2011) 284(5):1099–104. doi: 10.1007/s00404-010-1810-9
6. Becquet O, El Khabbaz F, Alberti C, Mohamed D, Blachier A, Biran V, et al. Insulin treatment of maternal diabetes mellitus and respiratory outcome in late-preterm and term singletons. BMJ Open. (2015) 5(6):e008192. doi: 10.1136/bmjopen-2015-008192
7. Abell SK, Boyle JA, de Courten B, Knight M, Ranasinha S, Regan J, et al. Contemporary type 1 diabetes pregnancy outcomes: impact of obesity and glycaemic control. Med J Aust. (2016) 205(4):162–7. doi: 10.5694/mja16.00443
8. Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. (2016) 374(14):1311–20. doi: 10.1056/NEJMoa1516783
9. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. Br Med J. (2016) 355:i5044. doi: 10.1136/bmj.i5044
10. Ustun N, Hocaoglu M, Turgut A, Arslanoglu S, Ovali F. Does antenatal corticosteroid therapy improve neonatal outcomes in late preterm birth? J Matern Fetal Neonatal Med. (2022) 35(25):9105–11. doi: 10.1080/14767058.2021.2015576
11. Mirzamoradi M, Hasani Nejhad F, Jamali R, Heidar Z, Bakhtiyari M. Evaluation of the effect of antenatal betamethasone on neonatal respiratory morbidities in late preterm deliveries (34–37 weeks). J Matern Fetal Neonatal Med. (2020) 33(15):2533–40. doi: 10.1080/14767058.2018.1554051
12. Janssen O, Ratner V, Lin J, Fox N, Green R. Respiratory and glycemic control outcomes of late preterm infants after antenatal corticosteroid exposure. J Perinatol. (2021) 41(11):2607–13. doi: 10.1038/s41372-021-01162-y
13. Ventolini G, Neiger R, Mathews L, Adragna N, Belcastro M. Incidence of respiratory disorders in neonates born between 34 and 36 weeks of gestation following exposure to antenatal corticosteroids between 24 and 34 weeks of gestation. Am J Perinatol. (2008) 25(2):079–83. doi: 10.1055/s-2007-1022470
14. Clapp MA, Melamed A, Freret TS, James KE, Gyamfi-Bannerman C, Kaimal AJ. US incidence of late-preterm steroid use and associated neonatal respiratory morbidity after publication of the antenatal late preterm steroids trial, 2015–2017. JAMA Netw Open. (2022) 5(5):e2212702. doi: 10.1001/jamanetworkopen.2022.12702
15. Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecol Obstet Investig. (2010) 70(2):95–9. doi: 10.1159/000295898
16. Abell SK, Boyle JA, de Courten B, Soldatos G, Wallace EM, Zoungas S, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. (2017) 57(3):308–14. doi: 10.1111/ajo.12521
17. Shand AW, Bell JC, McElduff A, Morris J, Roberts CL. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998–2002. Diabet Med. (2008) 25(6):708–15. doi: 10.1111/j.1464-5491.2008.02431.x
18. Dude AM, Yee LM, Henricks A, Eucalitto P, Badreldin N. Neonatal hypoglycemia after antenatal late preterm steroids in individuals with diabetes. J Perinatol. (2021) 41(12):2749–53. doi: 10.1038/s41372-021-01267-4
19. Krispin E, Hochberg A, Chen R, Wiznitzer A, Hadar E, Borovich A. Neonatal outcome in gestational-diabetic mothers treated with antenatal corticosteroids delivering at the late preterm and term. Arch Gynecol Obstet. (2018) 298(4):689–95. doi: 10.1007/s00404-018-4848-8
20. Ali H, Salama H, Robertson N, Olukade T, Al-Obaidly S, Al-Qubaisi M, et al. Antenatal corticosteroids and short-term neonatal outcomes in term and near-term infants of diabetic mothers. Analysis of the Qatar PEARL-peristat registry. J Perinat Med. (2021) 49(3):377–82. doi: 10.1515/jpm-2020-0249
21. World Health Organization. WHO recommendation on the diagnosis of gestational diabetes in pregnancy. Geneva: WHO (2018).
22. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. (2013) 13(1):1–13. doi: 10.1186/1471-2431-13-59
23. Shao XM, Ye HM, Qiu XS. Practice of neonatology. 4th ed. Beijing: People’s Medical Publishing House (China) (2011).
24. Persson M, Shah PS, Rusconi F, Reichman B, Modi N, Kusuda S, et al. Association of maternal diabetes with neonatal outcomes of very preterm and very low-birth-weight infants: an international cohort study. JAMA Pediatr. (2018) 172(9):867–75. doi: 10.1001/jamapediatrics.2018.1811
25. Committee opinion no. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. (2017) 130(2):e102–9. doi: 10.1097/AOG.0000000000002237
26. Battarbee AN, Sandoval G, Grobman WA, Bailit JL, Reddy UM, Wapner RJ, et al. Antenatal corticosteroids and preterm neonatal morbidity and mortality among women with and without diabetes in pregnancy. Am J Perinatol. (2022) 39(1):67–74. doi: 10.1055/s-0040-1714391
27. Gupta K, Rajagopal R, King F, Simmons D. Complications of antenatal corticosteroids in infants born by early term scheduled cesarean section. Diabetes Care. (2020) 43(4):906–8. doi: 10.2337/dc19-2126
28. Li J, Zhang J, Hao Q, Du Y, Lu J, Chen H, et al. Time interval from early-term antenatal corticosteroids administration to delivery and the impact on neonatal outcomes. Front Pediatr. (2022) 10:836220. doi: 10.3389/fped.2022.836220
29. Sifianou P, Thanou V, Karga H. Metabolic and hormonal effects of antenatal betamethasone after 35 weeks of gestation. J Pediatr Pharmacol Ther. (2015) 20(2):138–43. doi: 10.5863/1551-6776-20.2.138
30. Gewolb IH, O’Brien J. Surfactant secretion by type II pneumocytes is inhibited by high glucose concentrations. Exp Lung Res. (1997) 23(3):245–55. doi: 10.3109/01902149709087370
31. Gewolb IH. Effect of high glucose on fetal lung maturation at different times in gestation. Exp Lung Res. (1996) 22(2):201–11. doi: 10.3109/01902149609050847
32. Refuerzo JS, Garg A, Rech B, Ramin SM, Vidaeff A, Blackwell SC. Continuous glucose monitoring in diabetic women following antenatal corticosteroid therapy: a pilot study. Am J Perinatol. (2012) 29(5):335–8. doi: 10.1055/s-0031-1295642
33. Weiss SJ, Niemann S. Effects of antenatal corticosteroids on cortisol and heart rate reactivity of preterm infants. Biol Res Nurs. (2015) 17(5):487–94. doi: 10.1177/1099800414564860
34. Abrantes MA, Valencia AM, Bany-Mohammed F, Aranda JV, Beharry KD. Combined antenatal and postnatal steroid effects on fetal and postnatal growth, and neurological outcomes in neonatal rats. Am J Transl Res. (2019) 11(3):1697–710. PMID: 30972194; PMCID: PMC6456541
35. Kalra S, Kalra B, Gupta Y. Glycemic management after antenatal corticosteroid therapy. N Am J Med Sci. (2014) 6(2):71–6. doi: 10.4103/1947-2714.127744
36. di Pasquo E, Saccone G, Angeli L, Dall’Asta A, Borghi E, Fieni S, et al. Determinants of neonatal hypoglycemia after antenatal administration of corticosteroids (ACS) for lung maturation: data from two referral centers and review of the literature. Early Hum Dev. (2020) 143:104984. doi: 10.1016/j.earlhumdev.2020.104984
37. Ni L, Pan Y, Tang C, Xiong W, Wu X, Zou C. Antenatal exposure to betamethasone induces placental 11beta-hydroxysteroid dehydrogenase type 2 expression and the adult metabolic disorders in mice. PLoS One. (2018) 13(9):e0203802. doi: 10.1371/journal.pone.0203802
38. Cassimatis IR, Battarbee AN, Allshouse AA, Badreldin N, Vranes CM, Oler AM, et al. Neonatal outcomes associated with late preterm betamethasone administration in women with pregestational diabetes. Pediatr Neonatol. (2020) 61(6):645–6. doi: 10.1016/j.pedneo.2020.07.002
Keywords: antenatal corticosteroids, gestational diabetes mellitus, late preterm, hypoglycemia, respiratory system diseases
Citation: Li X, Zhang J, Hao Q, Du Y and Cheng X (2023) The effect of time interval between antenatal corticosteroid administration and delivery on outcomes in late preterm neonates born to mothers with diabetes: a retrospective cohort study. Front. Pediatr. 11:1239977. doi: 10.3389/fped.2023.1239977
Received: 14 June 2023; Accepted: 14 August 2023;
Published: 25 August 2023.
Edited by:
Gil Klinger, Schneider Children’s Medical Center, IsraelReviewed by:
Rachana Singh, Tufts University, United StatesKok Lim Kua, Indiana University Bloomington, United States
David Haas, Indiana University Bloomington, United States
© 2023 Li, Zhang, Hao, Du and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuyong Cheng Y2hlbmd4eTE4OEAxNjMuY29t
 Xiaoyu Li
Xiaoyu Li Jing Zhang
Jing Zhang Qingfei Hao
Qingfei Hao Xiuyong Cheng
Xiuyong Cheng