- 1Education Institute, Sheikh Khalifa Medical City (SKMC), Abu Dhabi, United Arab Emirates
- 2Sheikh Khalifa Medical City(SKMC), Purelab-Purehealth, Abu Dhabi, United Arab Emirates
- 3College of Medicine and Health Sciences, United Arab Emirates University (UAEU), Al Ain, United Arab Emirates
- 4Department of Pediatric Neurology, Sheikh Shakhbout Medical City (SSMC), Abu Dhabi, United Arab Emirates
- 5Department of Pediatric Rheumatology, Sheikh Shakhbout Medical City (SSMC), Abu Dhabi, United Arab Emirates
Neonatal lupus erythematosus (NLE) is an autoimmune disease caused by the transplacental passage of anti-Ro/SS-A and anti-La/SS-B. This can be less commonly seen with U1-ribonucleoprotein (U1RNP). Our patient is a 7-day-old male, who first presented with seizures. In addition, during an electroencephalogram, he was found to have an irregular heart rhythm. Looking further into the history, we found that the mother was aware that she had systemic lupus erythematosus (SLE). However, she had not been followed up with a rheumatologist. The workup for NLE found a negative anti-Ro/SS-A and anti-La/SS-B, with a positive U1RNP-70kD. U1RNP-70kD is a diagnostic test for mixed connective tissue disease in adults, but no research has been done on its significance in NLE. Despite having SLE, the infant’s mother did not receive surveillance during her pregnancy, as the current guidelines are tailored for mothers with anti-Ro/SS-A and anti-La/SS-B. As a result, this calls for the extension of these guidelines to include the U1RNP-70kD antibody. In this case, the 70kD subtype of U1RNP was positive, which may have had a role to play in this unusual presentation. However, further research is needed to improve the care of mothers and babies with U1RNP-70kD.
1. Introduction
Neonatal lupus erythematosus (NLE) is an autoimmune disease caused by the transplacental passage of pathological autoantibodies from the mother to the fetus. These antibodies are the anti-Ro/SS-A and anti-La/SS-B and less commonly the anti-U1-ribonucleoprotein (U1RNP) (1). Congenital heart block (CHB) occurs in 2% of mothers with anti-Ro/SS-A and/or anti-La/SS-B. This number increases up to 20% with subsequent pregnancies. The diagnosis for NLE involves the presence of one of the above antibodies in addition to a system involvement. It may present with multi-system involvement, including the skin, cardiac, hematologic, neurologic, and/or hepatobiliary system, usually manifesting as cutaneous lesions, cytopenia, elevated aminotransferases, and rarely heart block (1). Cutaneous manifestations have been associated with positive anti-U1RNP (1). Skin, hepatobiliary, and hematologic symptoms usually resolve within 6 months with the washout of maternal antibodies. On the other hand, the development of complete CHB is irreversible (2), with a mortality rate of 4%–29% (2). The cardiac involvement is not only limited to CHB but can also include structural abnormalities. In addition to CHB, the structural heart abnormalities carry a higher rate of mortality. Thus, detection and early intervention are required to decrease this risk of mortality (2). Here, we detail a case of NLE presenting with seizure and incomplete heart block, with positive anti-U1RNP-70kD and negative anti-Ro/SS-A and anti-La/SS-B.
2. Description of the case
This 7-day-old male was born full term, by a normal vaginal delivery. The mother was gravida 2, para 2. She was found to have group B streptococcus (GBS) and appropriately received antibiotics. She was also known to have hypothyroidism and was on levothyroxine but with an otherwise unremarkable perinatal history. The infant was brought to the emergency department (ED) with a cough and an increased difficulty in breathing. He was found to have respiratory syncytial virus (RSV) and was discharged with supportive management. The next day, he presented again to the ED with decreased activity and decreased oral intake. The mother reported symptoms of choking, coughing up frothy secretions, and experiencing weak extremities for a period of 4–5 min. A physical examination showed a hypoactive infant with normal suckling and Moro reflexes, an open anterior fontanelle, and no skin lesions. He was hemodynamically stable with no fever, weighing 2.6 kg (in the second percentile), with a length of 45 cm (less than second percentile), and a head circumference of 33 cm (in the fourth percentile).
The infant was admitted to the ward for observation, further management, and investigation. Full septic workup was sent, except for the lumbar puncture, which was refused by the parents. An electrocardiogram (ECG) and an electroencephalogram (EEG) were ordered. During the EEG session, the neurology physician noted an irregular heart rhythm on the ECG monitor, which was an evidence of an incomplete heart block (Figure 1). The extended history taken revealed that the mother had systemic lupus erythematosus (SLE), which, despite her being aware of it, was not previously revealed. She did not disclose this earlier as she did not think that it was of relevance because she was in remission, stopped taking her medication, and had not been followed up with a rheumatologist for over 18 months. This history alerted the team to investigate the infant for NLE. The neonate was then put on a Holter monitor and was sent to the pediatric intensive care unit (PICU) for close monitoring.
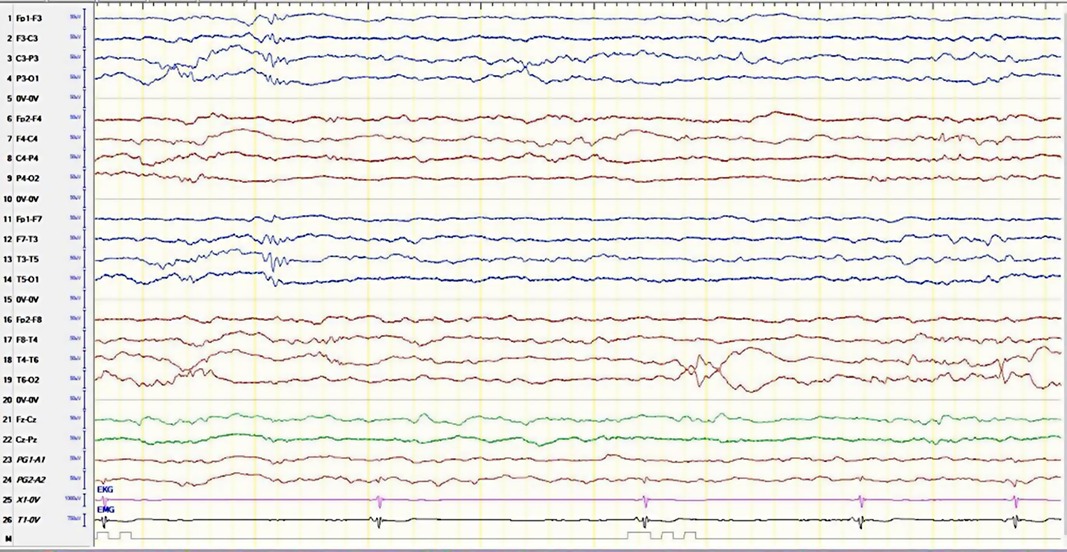
Figure 1. The electroencephalogram of the patient with the bradycardia/irregular heartbeat. Single-lead EKG monitor is in line 25.
The EEG reported an underlying regional disturbance and a low threshold for seizures (the background was continuous with mixed frequencies and short epochs of discontinuity, in addition to frequent bilateral temporal sharp wave activity with no clinical activity). Subsequently, the patient was loaded with an intravenous (IV) levetiracetam at a dose of 30 mg/kg, with a maintenance dose of 15 mg/kg every 12 h. He was also started on empiric antibiotics, cefotaxime, and ampicillin for possible meningitis.
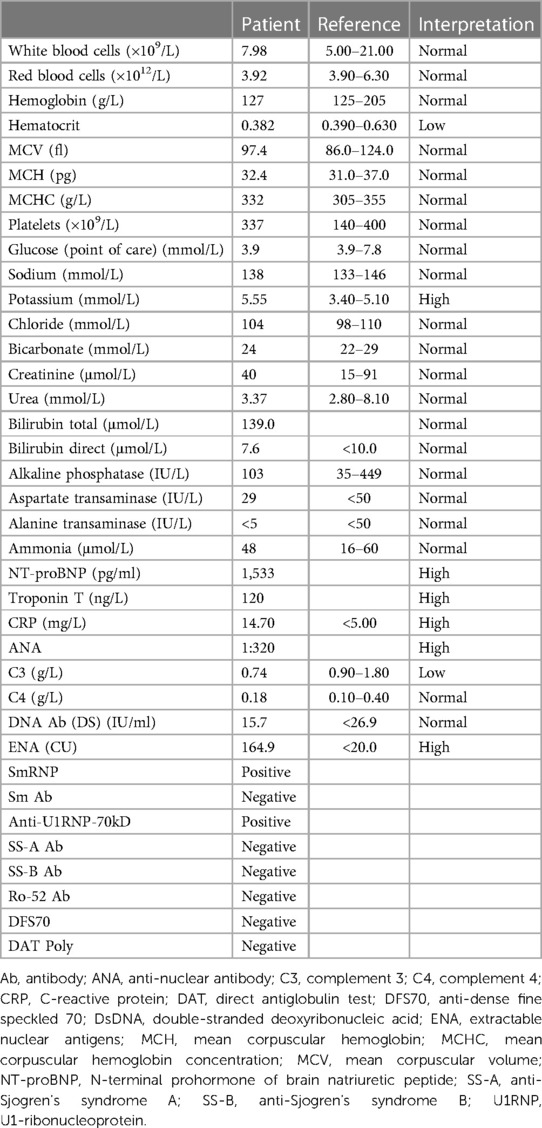
The detailed laboratory studies for the patient are presented in Table 1. In summary, his complete blood count and renal and liver function tests were within a normal range. His ammonia and glucose levels were normal. The anti-nuclear antibody (ANA) by indirect immunofluorescence (IIF) was 1:320 (positive speckled), complement 3 (C3) was low, complement 4 (C4) was normal, the extractable nuclear antigen (ENA) antibody test was positive, anti-Sm/RNP was detected by solid-phase assay, and anti-Sm antibody, anti-Ro/SS-A, and anti-La/SS-B were negative. The other autoantibodies were negative. His cardiac N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and troponin T were high. His blood and urine cultures had no growth of any organism.
As for the imaging, the echocardiogram showed a structurally normal heart with no signs of pulmonary hypertension. The cranial ultrasound showed a slightly increased periventricular echogenicity bilaterally, probably due to mild leukomalacia. No hemorrhage or hydrocephalus was observed. A brain computerized tomography (CT) angiogram was performed to exclude any vascular phenomena as a possible etiology for the seizure in the context of NLE, and it was found to be normal. The Holter monitor showed episodes of sudden sinus slowing, followed with junctional escape beats and periods of marked sinus bradycardia. He remained stable under observation in the PICU without requiring a pacemaker or any additional interventions. He was then discharged and put on oral levetiracetam at a dose of 40 mg twice per day.
Two weeks later, a follow-up in the clinic revealed no recurrence of seizures or development of new symptoms. Repeated laboratory workup showed improved cardiac function tests, with a decrease in ENA, but levels had not yet normalized. The EEG, ECG, and echocardiogram were normal. A month after discharge, a repeat EEG showed a normal result; therefore the levetiracetam was discontinued. With a normal physical exam, a follow-up at 6 months of age revealed that he was appropriately meeting his developmental milestones and the parents refused to repeat the laboratory workup at this visit.
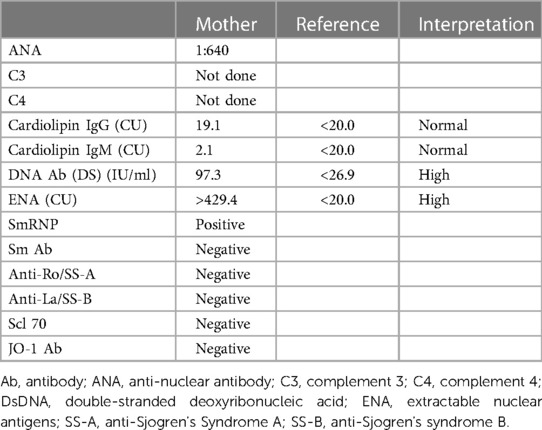
Retrospectively looking at this case, although the mother did not disclose her history of SLE to the pediatric team, she was followed up with an obstetrics medicine physician who was aware of her SLE and immunology status. Since the mother lacked the classical anti-Ro/SS-A and anti-La/SS-B, this did not prompt the obstetrician to do further surveillance or education regarding the possibility of NLE. Table 2 presents the mother's immunology in early pregnancy.
3. Discussion
In this case, the interpretation of the immunology workup is important. Please note that this panel may appear differently in different labs. To summarize the immunology workup, ENA was high with negative anti-Ro/SS-A and anti-La/SS-B and positive Sm/RNP with negative anti-smith (anti-Sm) antibody. Sm/RNP is also known as U1-small nuclear RNP (U1-SnRNP) complex (Figure 2) (3). It is an ENA and is a complex protein consisting of both anti-Sm and anti-RNP. Both have their own subtypes. The protein subtypes of anti-RNP are 70kD, A, and C. U1RNP A and C are seen more in SLE patients, while U1RNP-70kD is specific for mixed connective tissue disease (MCTD) (4). In our lab, the anti-Sm is ordered as a separate entity, and it is negative. Hence, this concludes that the anti-RNP is positive. The U1RNP subtypes are not routinely tested as they pose no clinical significance or implication. We ordered it for our patients for future reference and for further studies. We have found that the U1RNP-70kD subtype is positive. No available data are reported on U1RNP subtypes in the literature in the context of NLE to compare this with.
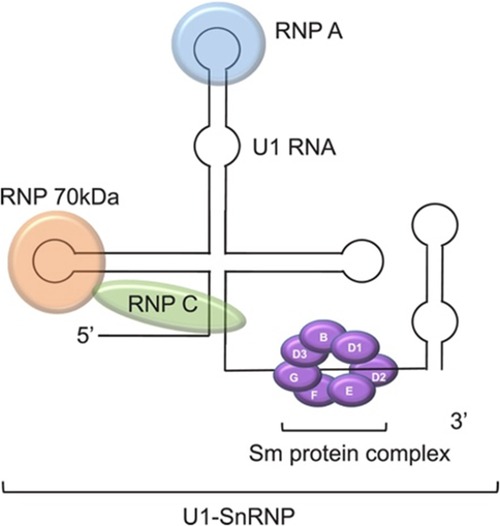
Figure 2. U1-SnRNP complex. Photograph obtained from an open-access article distributed under the Creative Commons Attribution License (3).
Limited data on the clinical significance and pathogenicity of U1RNP subtypes in NLE were found; however some evidence from adult studies was outlined. Specifically looking at the central nervous system (CNS) involvement, the cerebrospinal fluid (CSF) of SLE and MCTD had been examined and found to have elevated indices of anti-U1RNP-70kD (5). Another study investigating the U1RNP in MCTD found that CNS involvement is higher in MCTD patients who have symptoms associated with SLE (6). The long-term follow-up of patients with MCTD found that 17% developed CNS involvement (7). Different subtypes of U1RNP may be associated with different clinical manifestations; therefore, we hypothesize that neonates of SLE mothers with U1RNP A/C present with cutaneous lesions, while neonates of SLE mothers with U1RNP-70Kd present with CHB and/or CNS involvement.
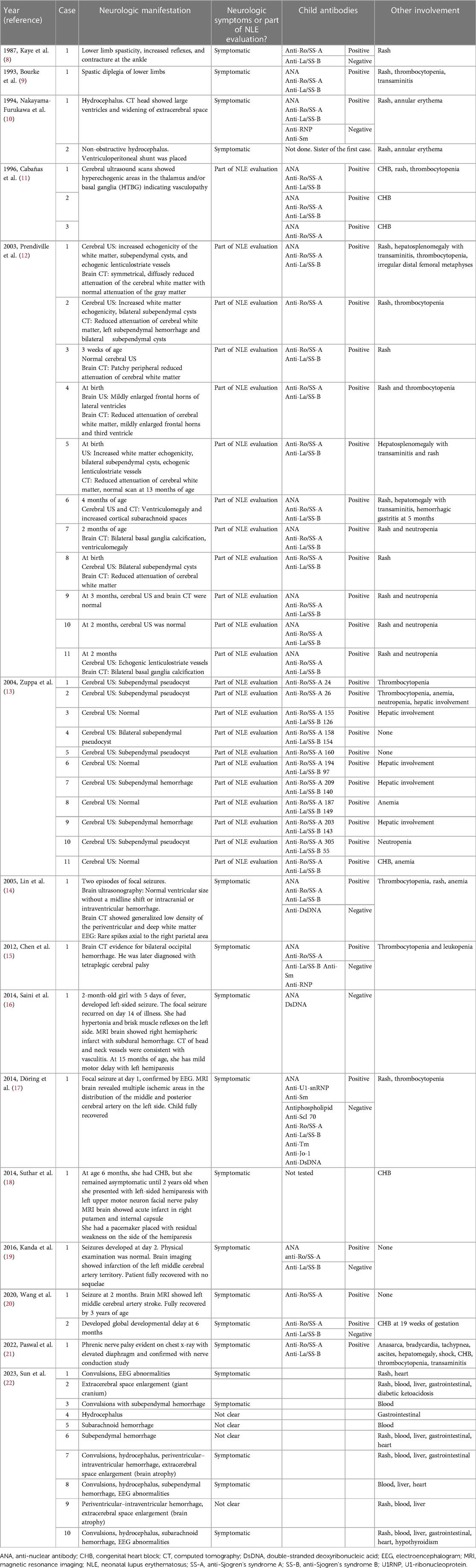
NLE rarely presents with neurologic manifestations. Few patients described in the literature that presented with neurologic symptoms, with the majority of cases finding CNS abnormalities in the imaging as a part of the NLE evaluation, were reported. Subependymal cysts, increased echogenicity, and hydrocephalus were the most widely seen radiologic changes. Table 3 summarizes the NLE patients in the literature with CNS manifestations (8–22). Psychiatric research and publications not in English are not included in this table. Development of seizure was reported in 10 patients (14, 16, 17, 19, 20, 22), eight of which had an underlying cerebral infarct or hemorrhage. The antibody profile is summarized in Table 3. One of the patients without a stroke is the most similar to our patient (14), but unfortunately, the U1RNP profile was not reported. The other symptomatic patients varied in presentation having myelopathy (8), spastic paraparesis (9), tetraplegia (15), facial nerve palsy with hemiparesis (18), phrenic nerve palsy (21), and hydrocephalus (10, 23). The majority of these neurologic symptoms resolved with no long-term sequelae.
CHB is mostly seen with anti-Ro/SS-A and/or anti-La/SS-B. Only recently, CHB is being reported with anti-U1RNP with negative anti-Ro/SS-A and anti-La/SS-B (24). Similar to our case, a total of three patients who reported of having CHB with positive U1RNP (protein subtype not mentioned) and negative anti-Ro/SS-A and anti-La/SS-B but without any neurologic symptoms (24–26) were reported.
The current NLE recommendations for mothers with rheumatic diseases are targeted only for those with anti-Ro/SS-A and/or anti-La/SS-B antibodies. One of the recommendations is to use hydroxychloroquine during pregnancy (27). The other recommendation is early detection of CHB in the most vulnerable period of gestation. This is performed by measuring the mechanical PR interval with a weekly or bi-weekly Doppler fetal echocardiogram during 16–28 weeks of gestation (28).
It is important to rule out other possible causes or insults to diagnose NLE. In our case, GBS meningitis cannot be fully ruled out in the presence of a positive GBS result in the mother. In this admission, the mother received one dose of IV antibiotics, and the baby received a course of antibiotics. Our limitation here was the missing lumbar puncture. On the other hand, GBS meningitis was unlikely as it cannot explain the presence of heart block, and the patient remained well, afebrile with negative blood and urine cultures.
In summary, our patient had negative anti-Ro/SS-A and anti-La/SS-B and positive anti-U1RNP-70kD, presented with a seizure episode, and was found to have an incomplete heart block. The mother with SLE did not receive surveillance during pregnancy as the current guidelines are tailored for mothers with anti-Ro/SS-A and anti-La/SS-B. It is important to consider testing for U1RNP-70kD in newborns presenting with seizure and maternal SLE. In our case, the 70kD subtype of U1RNP is positive which may have a role in this unusual presentation. Further research looking at U1RNP and its subtypes in the context of NLE is needed to improve the care of mothers and babies with this antibody.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MA wrote the first draft of the manuscript. All authors contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the baby and his family for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. (2012) 2012:301274. doi: 10.1155/2012/301274
2. Capone C, Buyon JP, Friedman DM, Frishman WH. Cardiac manifestations of neonatal lupus: a review of autoantibody-associated congenital heart block and its impact in an adult population. Cardiol Rev. (2012) 20(2):72–6. doi: 10.1097/CRD.0b013e31823c808b
3. van Beers JJBC, Schreurs MWJ. Anti-Sm antibodies in the classification criteria of systemic lupus erythematosus. J Transl Autoimmun. (2022) 5:100155. doi: 10.1016/j.jtauto.2022.100155
4. Poole BD, Schneider RI, Guthridge JM, Velte CA, Reichlin M, Harley JB, et al. Early targets of nuclear RNP humoral autoimmunity in human systemic lupus erythematosus. Arthritis Rheum. (2009) 60(3):848–59. doi: 10.1002/art.24306
5. Sato T, Fujii T, Yokoyama T, Fujita Y, Imura Y, Yukawa N, et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum. (2010) 62(12):3730–40. doi: 10.1002/art.27700
6. Nawata M, Matsushita M, Matsudaira RAN, Yamada H, Kaneda K, Asano M, et al. Clinical significance of anti-U1 RNP antibodies recognizing the conformation structure on U1 RNA/70-kd protein complex in patients with mixed connective tissue disease. Juntendo Med J. (2011) 57:477–87. doi: 10.14789/pjmj.57.477
7. Burdt MA, Hoffman RW, Deutscher SL, Wang GS, Johnson JC, Sharp GC. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum. (1999) 42(5):899–909. doi: 10.1002/1529-0131(199905)42:5%3C899::AID-ANR8%3E3.0.CO;2-L
8. Kaye EM, Butler IJ, Conley S. Myelopathy in neonatal and infantile lupus erythematosus. J Neurol Neurosurg Psychiatry. (1987) 50(7):923–6. doi: 10.1136/jnnp.50.7.923
9. Bourke JF, Burns DA. Neonatal lupus erythematosus with persistent telangiectasia and spastic paraparesis. Clin Exp Dermatol. (1993) 18(3):271–3. doi: 10.1111/j.1365-2230.1993.tb02186.x
10. Nakayama-Furukawa F, Takigawa M, Iwatsuki K, Sato N, Sato H. Hydrocephalus in two female siblings with neonatal lupus erythematosus. Arch Dermatol. (1994) 130(9):1210–2. doi: 10.1001/archderm.1994.01690090144029
11. Cabañas F, Pellicer A, Valverde E, Morales C, Quero J. Central nervous system vasculopathy in neonatal lupus erythematosus. Pediatr Neurol. (1996) 15(2):124–6. doi: 10.1016/0887-8994(96)00159-2
12. Prendiville JS, Cabral DA, Poskitt KJ, Au S, Sargent MA. Central nervous system involvement in neonatal lupus erythematosus. Pediatr Dermatol. (2003) 20(1):60–7. doi: 10.1046/j.1525-1470.2003.03014.x
13. Zuppa AA, Gallini F, De Luca D, Luciano R, Frezza S, de Turris PL, et al. Cerebral ultrasound findings in neonatal lupus syndrome. Biol Neonate. (2004) 86(4):230–4. doi: 10.1159/000079820
14. Lin SC, Shyur SD, Li Hsin H, Wu JY, Ma YC. Focal seizures as an unusual presentation of neonatal lupus erythematosus. Asian Pac J Allergy Immunol. (2005) 23(1):61–4.15997876
15. Chen CC, Huang JL, Hsu JF, Chung CY, Lin KL. Neonatal lupus complicated by hemorrhagic stroke. Lupus. (2012) 21(14):1582–5. doi: 10.1177/0961203312463981
16. Saini AG, Sankhyan N, Bhattad S, Vyas S, Saikia B, Singhi P. CNS vasculitis and stroke in neonatal lupus erythematosus: a case report and review of literature. Eur J Paediatr Neurol. (2014) 18(3):444–8. doi: 10.1016/j.ejpn.2014.01.007
17. Döring M, Rohrer KM, Tsiflikas I, Buchenau W, Wilke M, Handgretinger R, et al. A newborn with grouped facial skin lesions and subsequent seizures. BMC Pediatr. (2014) 14:126. doi: 10.1186/1471-2431-14-126
18. Suthar R, Sahu JK, Rohit M, Khandelwal NK, Singhi P. Stroke in a case of neonatal lupus: an uncommon complication. J Child Neurol. (2014) 29(11):Np157–60. doi: 10.1177/0883073813513497
19. Kanda K, Sato A, Abe D, Nishijima S, Ishigami T. The unique coexistence of anti-SS-A/Ro antibodies in a neonate with symptomatic ischemic stroke. Pediatr Neurol. (2016) 62:47–50. doi: 10.1016/j.pediatrneurol.2016.06.005
20. Wang YA, Sibbald C, Moon AT. Retrospective, single-center case series of neonatal lupus. Pediatr Dermatol. (2020) 37(3):484–9. doi: 10.1111/pde.14132
21. Paswal CM, Kaur N, Baranwal AK, Jindal A, Saini AG, Bhatia A. Intravenous immunoglobulin for bilateral phrenic nerve palsy due to neonatal lupus erythematosus. Indian J Pediatr. (2022) 89(7):734. doi: 10.1007/s12098-022-04217-w
22. Sun W, Ding L, Li M, Fu C, Yang Z, Zhu X. Neurological and endocrinological involvement in neonatal lupus erythematosus: a retrospective study at a tertiary hospital in Eastern China. Clin Rheumatol. (2023) 42(9):2461–8. doi: 10.1007/s10067-023-06622-8
23. Chen CC, Lin KL, Chen CL, Wong AM-K, Huang JL. Central nervous system manifestations of neonatal lupus: a systematic review. Lupus. (2013) 22(14):1484–8. doi: 10.1177/0961203313509294
24. Izmirly PM, Halushka MK, Rosenberg AZ, Whelton S, Rais-Bahrami K, Nath DS, et al. Clinical and pathologic implications of extending the spectrum of maternal autoantibodies reactive with ribonucleoproteins associated with cutaneous and now cardiac neonatal lupus from SSA/Ro and SSB/La to U1RNP. Autoimmun Rev. (2017) 16(9):980–3. doi: 10.1016/j.autrev.2017.07.013
25. Radin M, Schreiber K, Cuadrado MJ, Cecchi I, Andreoli L, Franceschini F, et al. Pregnancy outcomes in mixed connective tissue disease: a multicentre study. Rheumatology. (2019) 58(11):2000–8. doi: 10.1093/rheumatology/kez141
26. Acherman RJ, Friedman DM, Buyon JP, Schwartz J, Castillo WJ, Rollins RC, et al. Doppler fetal mechanical PR interval prolongation with positive maternal anti-RNP but negative SSA/Ro and SSB/La auto-antibodies. Prenat Diagn. (2010) 30(8):797–9. doi: 10.1002/pd.2544
27. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody–associated cardiac manifestations of neonatal lupus. Circulation. (2012) 126(1):76–82. doi: 10.1161/CIRCULATIONAHA.111.089268
Keywords: neonatal lupus, systemic lupus erythematosus, heart block, seizure, case report
Citation: Alfalasi M, ElGhazali G, Fathalla W and Khawaja K (2023) Anti-U1RNP-70kD-positive case of neonatal lupus presenting with seizure and incomplete heart block: a case report and literature review. Front. Pediatr. 11:1239327. doi: 10.3389/fped.2023.1239327
Received: 13 June 2023; Accepted: 4 August 2023;
Published: 23 August 2023.
Edited by:
Lovro Lamot, University of Zagreb, CroatiaReviewed by:
Mikhail Kostik, Saint Petersburg State Pediatric Medical University, RussiaAndrea Trombetta, IRCCS Local Health Authority of Reggio Emilia, Italy
© 2023 Alfalasi, ElGhazali, Fathalla and Khawaja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khulood Khawaja a2h1bG9vZEBkb2N0b3JzLm9yZy51aw==
 Maryam Alfalasi
Maryam Alfalasi Gehad ElGhazali
Gehad ElGhazali Waseem Fathalla
Waseem Fathalla Khulood Khawaja5*
Khulood Khawaja5*