- 1Department of Pediatrics, Eskisehir Osmangazi University Faculty of Medicine, Eskisehir, Türkiye
- 2Klinik für Neonatologie, Darmstädter Kinderkliniken Prinzessin Margaret, Perinatalzentrum Südhessen, Darmstadt, Germany
- 3Pediatric Intensive Care Unit, University Hospital Center “Mother Teresa”, Tirana, Albania
- 4Corporate Medical Affairs, Biotest AG, Dreieich, Germany
- 5Corporate Clinical Research & Development, Biotest AG, Dreieich, Germany
- 6Medical and Scientific Affairs, Biotest UK, Birmingham, United Kingdom
- 7Department of Child Health, Neonatology Division, Dr. Cipto Mangunkusumo National Central General Hospital, Jakarta, Indonesia
Background: Sepsis is a major cause of mortality and morbidity globally, with around one-quarter of all sepsis-related deaths occurring in children under the age of 5. We conducted a meta-analysis and systematic review of the literature to evaluate the clinical effectiveness of an IgM-enriched immunoglobulin preparation in pediatrics patients and neonates with sepsis.
Methods: Systematic searches of PubMed, the Cochrane Library and Embase databases were performed in November 2022, with no date limitations, to identify studies in which IgM-enriched immunoglobulin was used as adjunctive therapy in neonatal and pediatric patients with sepsis.
Results: In total, 15 studies fulfilled the eligibility criteria, 13 neonatal studies and 2 pediatric studies. Pooled estimates from all studies indicated that mortality rates were significantly lower in patients who received treatment with the IgM-enriched immunoglobulin compared with controls (OR 0.41; 95% CI 0.32–0.55). Further analyses in neonatal studies, alone, showed a significant benefit with longer treatment durations (>3 days) vs. the recommended treatment duration (3 days) (OR 0.32; 95% CI 0.22–0.47) vs. (OR 0.61; 95% CI 0.41–0.92). Treatment with IgM-enriched immunoglobulin was associated with a lower mortality risk compared with controls in prospective studies vs. retrospective analyses (OR 0.37; 95% CI 0.27–0.51) vs. (OR 0.73; 95% CI 0.41–1.30).
Conclusions: This systematic review suggests that adjunctive treatment with IgM-enriched immunoglobulin may reduce the risk of mortality in neonatal and pediatric populations. However, large randomized controlled trials are required to further substantiate and evaluate these findings.
1. Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (1, 2). In the pediatric Surviving Sepsis Campaign International Guidelines, which apply to all patients from 37 weeks gestational age at birth to 18 years old, septic shock is defined as severe infection leading to cardiovascular dysfunction (including hypotension, need for treatment with a vasoactive medication, or impaired perfusion). “Sepsis-associated organ dysfunction” is defined as severe infection leading to cardiovascular and/or non-cardiovascular organ dysfunction (3, 4). A consensus definition of neonatal sepsis is still a matter of debate (5).
Approximately one-half of pediatric patients with sepsis have underlying disease such as chronic lung disease, congenital heart disease or neuromuscular disease (6, 7), with sepsis most commonly the result of diarrheal disease or lower respiratory infections (8). However, community-acquired meningococcal infection can cause sepsis even in healthy children.
Neonates, in particular preterm neonates, are at a higher risk of infection as a result of the functional immaturity of their immune system (9–11). Immunoglobulin (Ig) G is transferred from mother to fetus, but the majority isn’t acquired until the last month of pregnancy (10). IgM and IgA do not cross the placenta (12). Consequently, preterm or low birth weight (LBW) neonates are hypogammaglobulinemic (12) in addition to having an immature adaptive immune system and, as a result, are at greater risk of severe infection and mortality (13).
Despite advances in treatment, sepsis is still a significant cause of morbidity and mortality, particularly in neonates and pediatric populations, and even more so in low-and-middle-income countries (9, 13). In 2017, there were an estimated 48.9 million cases of sepsis globally, of which 42% were in children aged under 5 years (8). For those who survive, sepsis is associated with long-term comorbidities (14, 15), including poor neurodevelopmental and growth outcomes in early childhood in preterm and LBW neonates (16, 17) and at least moderate disability in almost one-fifth of older children (15).
Intravenous immunoglobulin (IVIG) preparations, that primarily contain IgG, have been suggested to have benefits as adjunctive therapy in patients with sepsis. However, evaluation of polyclonal IVIG adjunctive therapy in neonates showed no survival benefit in the large multinational International Neonatal Immunotherapy Study (INIS) (18). An alternative immunoglobulin preparation (see methods) enriched with IgM and IgA in addition to IgG, denoted IgM-enriched immunoglobulin for this review, is available for the treatment of sepsis. Said IgM-enriched immunoglobulin is considered to offer greater antibacterial and immunomodulatory activity (19–21), with a higher affinity for the lipopolysaccharides of gram-negative bacteria compared with IgG (19, 22), and the capacity to neutralize bacterial toxins (23) compared with IVIG. In adults, treatment with IgM-enriched immunoglobulin has been associated with improved survival (24–29), however data in neonatal and in particular pediatric populations are limited. Nevertheless, given the potential benefits of IgM-enriched immunoglobulin, in the context of the neonate's compromised immunity, it would be reasonable to believe that IgM-enriched immunoglobulin may have a beneficial effect in neonates.
Systematic meta-analyses have conducted subgroup analyses on adjunctive therapy with IgM-enriched immunoglobulin in neonatal patient populations (29–31). However, the limited studies have precluded robust conclusions on its therapeutic potential and emphasize the need for larger trials. The recent publication of further studies evaluating adjunctive treatment with IgM-enriched immunoglobulin in pediatric and neonatal sepsis, have prompted this systematic review, the aim of which is to provide an updated analysis and review of studies that assess the effectiveness of IgM-enriched immunoglobulin as adjunctive therapy in pediatric and neonatal sepsis.
2. Methods
A systematic review and meta-analysis were performed using the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 guidelines.
2.1. Data sources
A systematic search of PubMed, Cochrane Library, and Embase databases was conducted in November 2022 and was not date limited. The search strategy consisted of ((“IgM-enriched immunoglobulin”) or (“IgM enriched immunoglobulin”) or (“IgM and IgA-enriched immunoglobulin”) or (“IgM/IgA enriched immunoglobulin”) or (IVIGMA) or (IgGAM) or (“IgMA enriched IVIG”) or (“IgMA-enriched IVIG”) or (“polyvalent immunoglobulins”) or (“immunoglobulin M preparation”) or (“immunoglobulin preparation containing IgG, IgM and IgA”) or (pentaglobin or pentaglobulin)) and (sepsis or septic shock). Additional studies were identified by reviewing the reference lists of relevant articles and a manual search of the internet. In addition, results of an unpublished study (32) were included in the meta-analysis.
2.2. Eligibility criteria
Two authors (UW and AB) evaluated the studies independently to determine their eligibility for inclusion in the meta-analysis. Trials were included if they: (1) compared IgM-enriched immunoglobulin, specifically Pentaglobin® Biotest Germany (12% IgM, 12% IgA and 76% IgG) and standard therapy (standard therapy only or placebo and standard therapy); (2) enrolled neonatal or pediatric patients with sepsis; and (3) provided mortality data. The primary outcome was all-cause mortality. Studies on prophylactic use of IgM-enriched immunoglobulin or studies where normal IVIG was used were excluded. Any disagreements between authors were resolved by consensus.
2.3. Data extraction
Two authors (UW and AB) extracted the data independently from each eligible study, including the lead author, year of publication, study design, number of patients, age for pediatric patients and gestational age and birthweight for neonatal patients, dose and duration of treatment, and definition of mortality. The number of patients in the IgM-enriched immunoglobulin treatment and control groups in each study were recorded as well as the number of deceased patients.
2.4. Quality assessment
The methodological quality of the studies included in our analysis was assessed independently by two authors (UW and AB). Randomized controlled studies (RCTs) were assessed using the Cochrane Collaboration's tool for assessing risk of bias (33). Each study was examined for selection bias, performance bias, attrition bias, detection bias and reporting bias, with each criterion graded as either low, high or unclear risk. Observational studies were assessed using the Newcastle–Ottawa Scale (34) which examined selection bias, comparability and outcome. Each study was awarded up to nine stars, with those regarded as high-quality studies receiving ≥6 stars.
2.5. Statistical analyses
All statistical analyses were performed with R Version 4.1.1. Heterogeneity was evaluated using chi–squared tests and the I2 index. If the I2 index was between 50% and 75%, heterogeneity was considered moderate. If the I2 index was >75%, heterogeneity was evaluated as considerable. Potential publication bias was assessed using funnel plots, with asymmetry evaluated using the Egger's test. Independent of heterogeneity a conservative approach was applied by using a Mantel–Haenszel random effects model. A value of 0.5 was added to cells where the mortality count was zero.
A subgroup analysis was performed to evaluate whether any mortality benefit associated with treatment with IgM-enriched immunoglobulin was affected by pediatric vs. neonatal populations. All subsequent subanalyses were performed on studies in neonates only, with further analyses on the effects of: recommended IgM-enriched immunoglobulin treatment dose according to the summary of product characteristics (SmPC) (35) (Pentaglobin® Biotest Germany, 250 mg/kg/day for 3 consecutive days) vs. a greater number of days administered (250 mg/kg/day for >3 consecutive days); patients with proven vs. suspected sepsis; retrospective vs. prospective studies; and studies published before 2000 vs. studies published after 2000 (all studies that were published pre 2000 were conducted and completed before the year 2000 and those published post 2000 were conducted after the year 2000).
3. Results
3.1. Identification of studies
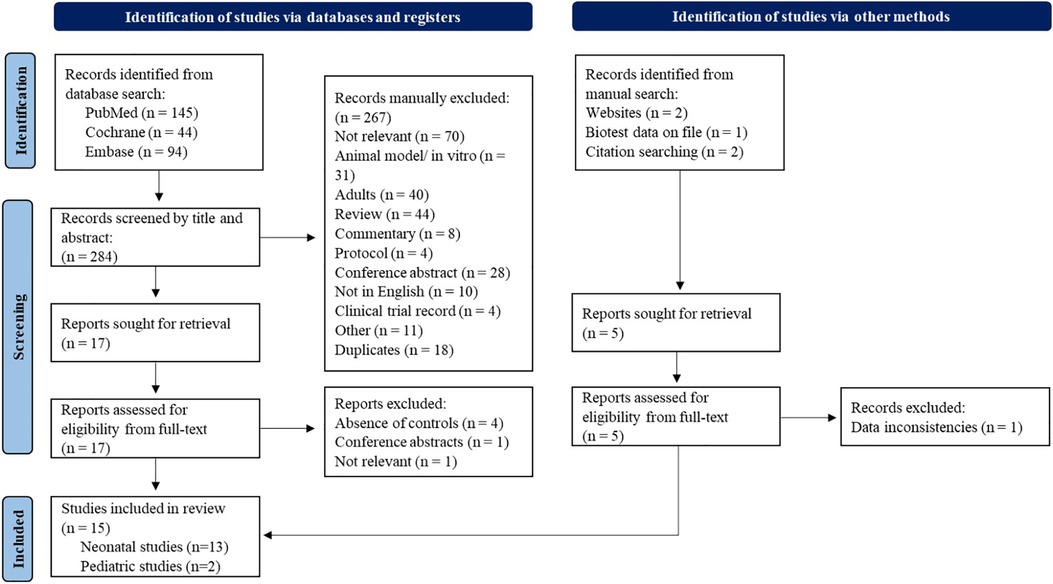
The flow chart of the study selection procedure is shown in Figure 1. The initial search identified 145 studies in PubMed, 44 in the Cochrane Library and 94 from Embase. In addition, four studies were identified by citation searching and manual search. An unpublished report was also identified for inclusion (32). The titles and abstracts of all studies found in the search were screened and 22 articles were evaluated for eligibility from their full-text manuscript. In total, 15 studies (32, 36–49) fulfilled the eligibility criteria (two pediatric and 13 neonatal studies) and were included in the final meta-analysis. These studies included a total of 1,727 patients (1,549 neonates and 178 pediatric patients).
3.2. Neonate study details
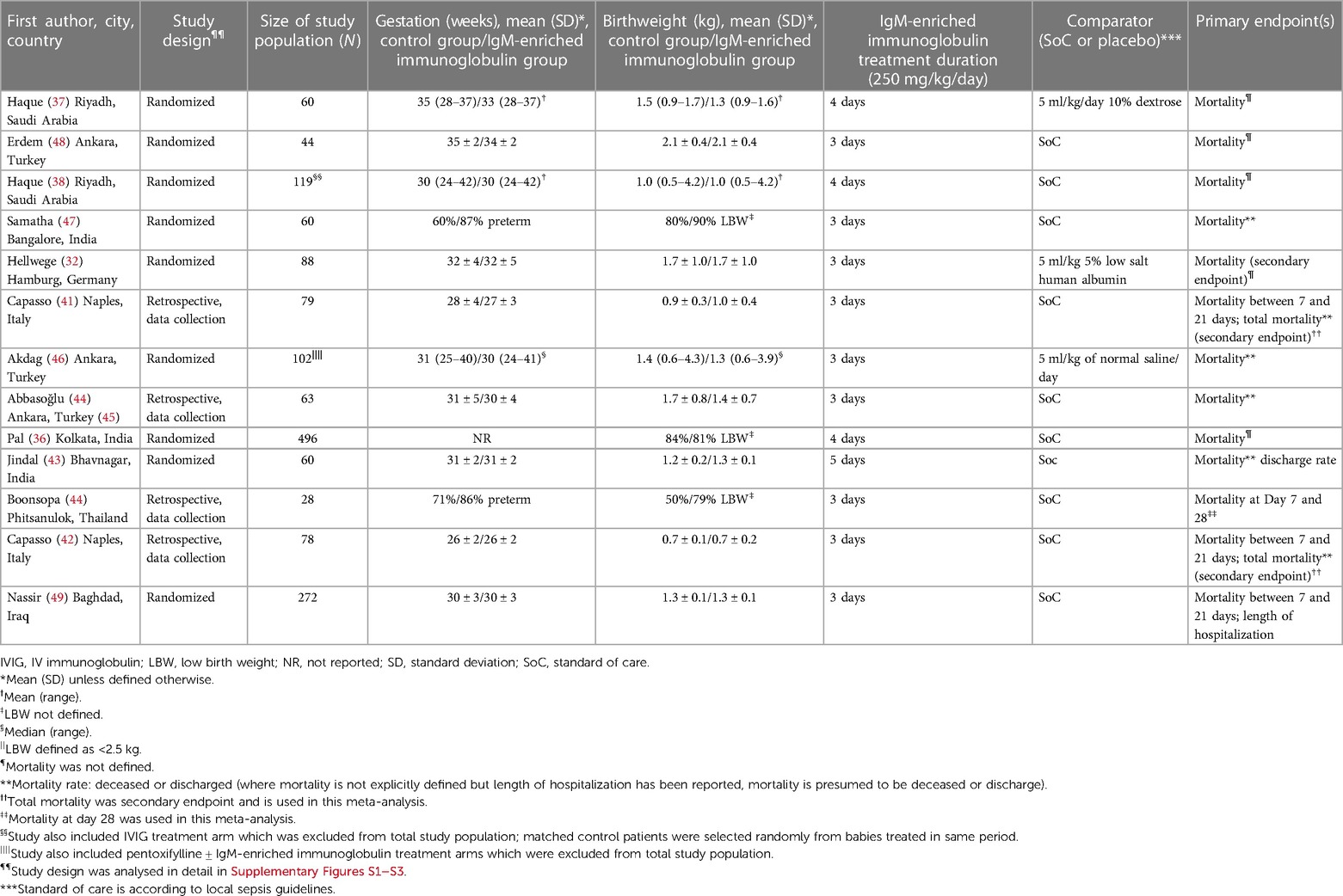
The details of the 13 neonatal studies included in the meta-analysis are shown in Table 1. Of these, nine were controlled studies, eight of which were randomized (32, 36–38, 43, 46, 48, 49) and one was non-randomized (47). The remaining four studies were retrospective, controlled data analyses (41, 42, 44, 45). The studies included varied in size from 28 to 496 patients. The mean gestational age was reported for nine of the neonatal studies and varied from 26 to 35 weeks. Two of the remaining studies defined how many neonates were preterm (<37 weeks) (44, 47), one defined the median gestational age (46) and the final study did not report gestation (36). The mean birthweight was reported for the same nine studies as those with mean gestational age and varied from 0.7–2.1 kg. For the four remaining studies, three studies reported the percent of patients with LBW (defined as <2.5 kg) (36, 44, 47) and one the median birthweight (46). Dose of IgM-enriched immunoglobulin preparation was the same for all neonatal studies (250 mg/kg/day), however, four studies extended the duration of treatment beyond the recommended 3 days, with durations of 4 days (36–38) and 5 days (43). Definitions of mortality varied between the studies included in the analyses: mortality was not defined for five studies (32, 36–38, 48); mortality was defined as deceased or discharged for six studies (41–43, 45–47); and mortality was defined as short-term mortality at 7 and 21 days (49) and 7 and 28 days (44) for the remaining two studies.
3.3. Pediatric study details
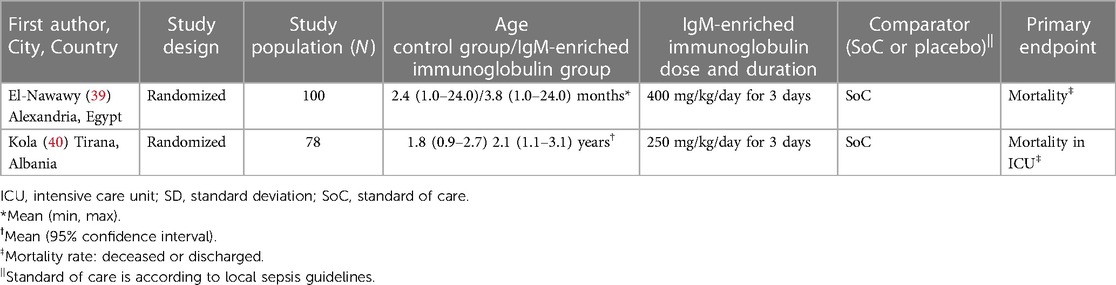
The details of the two pediatric studies included in the meta-analysis are shown in Table 2. Both were RCTs (39, 40). A total of 78 and 100 patients were included in each study, with a mean age from 2.4 months (39) to 2.1 years (40). Dose of IgM-enriched immunoglobulin preparation was different in these studies; the recommended dose (250 mg/kg/day) was used in one study (40) and a higher dose (400 mg/kg/day) for the remaining study (39), with the same duration (3 days) for both. Definitions of mortality were deceased or discharged for one study (39) and mortality in ICU for the other (40).
3.4. Methodological quality of included RCTs
The results of the Cochrane Collaboration's risk of bias assessment of RCTs are reported in Supplementary Figures S1 and S2.
3.4.1. Allocation
The method of random sequence generation was described for four studies (32, 40, 46, 47) but was undefined for a further six studies (36, 37, 39, 43, 48, 49). The blinding of randomization to conceal the allocation sequence was described for three studies (37, 40, 46) however for seven studies there was insufficient information to determine whether allocation had been adequately concealed (32, 36, 39, 43, 47–49).
3.4.2. Blinding
Only two studies reported blinding of participants, personnel and/or outcome (37, 46) with no reports of blinding for the remaining nine studies (32, 36, 38–40, 43, 47–49). However, it should be noted, that it is unlikely that bias due to lack of blinding would have had an impact on mortality outcome (33).
3.4.3. Incomplete outcome data, selective reporting and other bias
Only one study had incomplete outcome data because of missing data following premature termination and dropout, and other bias potentially affecting inferential statistical analysis (32). The remaining ten studies (36–40, 43, 46–49) had no missing mortality data, and no other identifiable potential sources of bias. Conversely, it was not possible to ascertain if there was any selective reporting for these ten studies (36–40, 43, 46–49), but the absence was clear for the study by Hellwege et al. (32).
3.5. Newcastle–Ottawa quality assessment
The result of the quality assessment of the observational studies is reported in Supplementary Table S3. Two studies (41, 42) scored 6 stars and were regarded as high-quality studies and the remaining two studies (44, 45) scored <6 stars.
3.6. Primary outcomes
Pooled estimates from all neonatal and pediatric studies indicated that mortality rates were significantly lower in patients who were treated with IgM-enriched immunoglobulin compared with their respective controls [odds ratio (OR) 0.41; 95% confidence interval (CI) 0.32–0.55] (Figure 2). Statistical homogeneity was met (I2 = 10%).
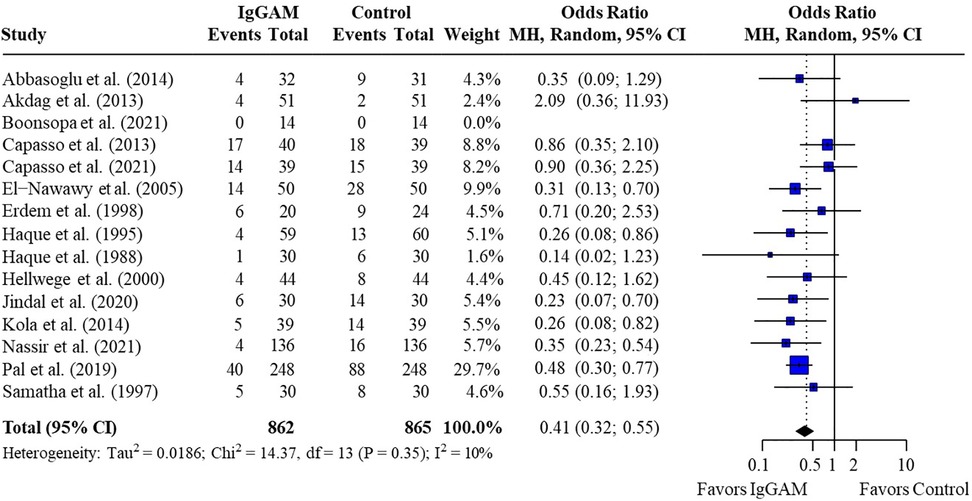
Figure 2. Effect of IgM-enriched immunoglobulin treatment on mortality rates in all patients with sepsis (pediatrics and neonates). CI, confidence interval; IgGAM, IgM-enriched immunoglobulin; MH, Mantel–Haenszel.
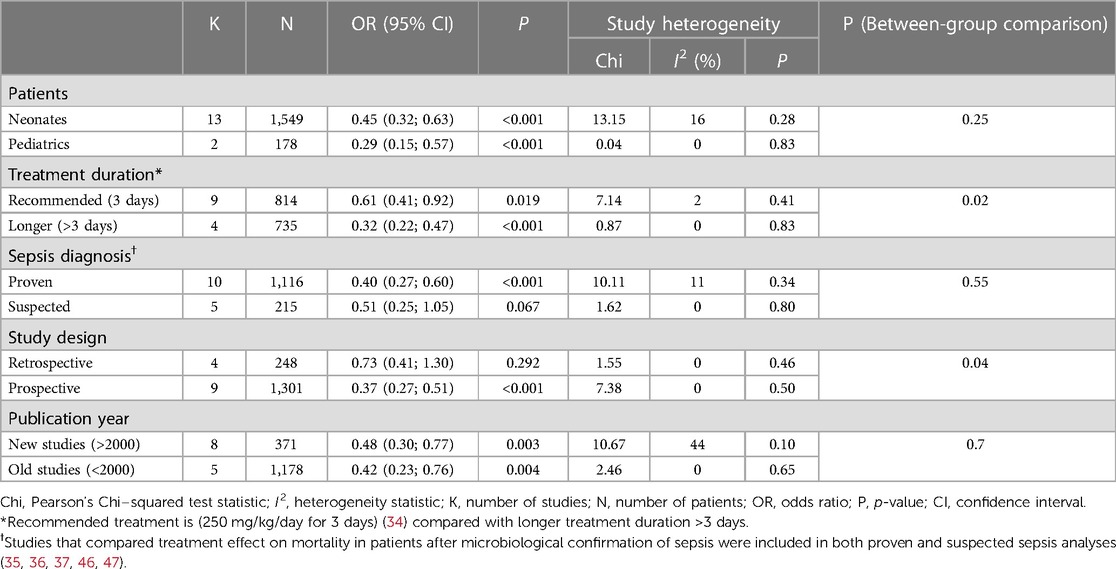
A subgroup analysis was performed to compare mortality benefit in neonatal studies and pediatric studies; mortality rates remained significantly lower in patients treated with IgM-enriched immunoglobulin compared with controls for both neonatal and pediatric populations (OR 0.45; 95% CI 0.32–0.63) vs. (OR 0.29; 95% CI 0.15–0.57), respectively, with no significant differences observed between populations (p = 0.25) (Figure 3 and Table 3). All other analyses were based on neonatal studies only.
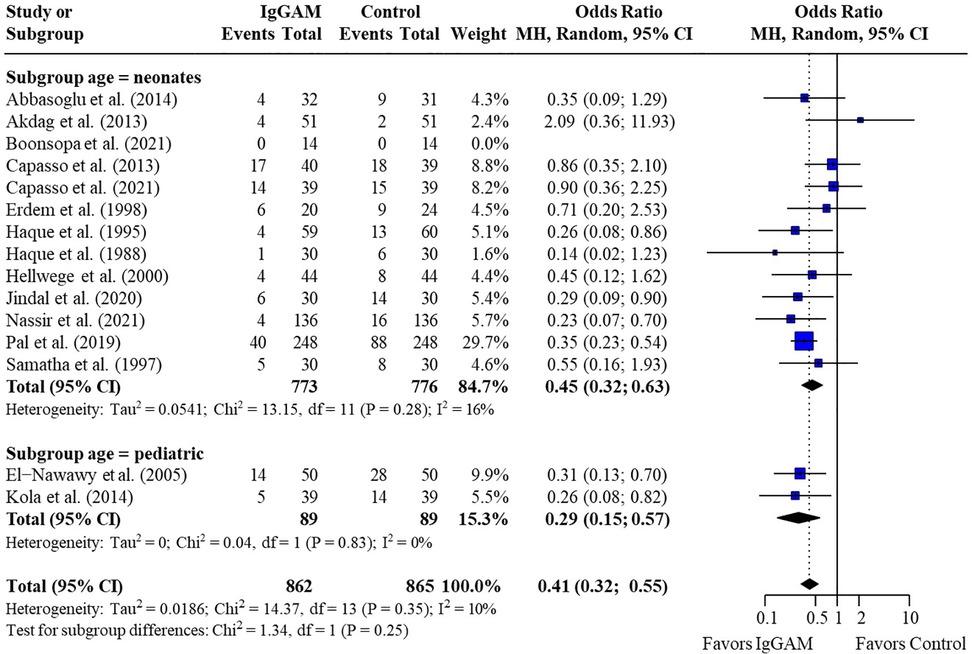
Figure 3. Subgroup analyses to compare the effect of IgM-enriched immunoglobulin treatment on mortality rates in neonatal and pediatric sepsis. CI, confidence interval; IgGAM, IgM-enriched immunoglobulin; MH, Mantel–Haenszel.
Subsequent subgroup analyses of neonatal studies observed that IgM-enriched immunoglobulin significantly reduced the mortality risk (p = 0.02) compared with controls according to the duration of treatment. Compared with controls, mortality risk was more markedly reduced with prolonged IgM-enriched immunoglobulin treatment (250 mg/kg/day for >3 days) (OR 0.32; 95% CI 0.22–0.47) vs. the recommended dose (250 mg/kg/day for 3 days) (OR 0.61; 95% CI 0.41–0.92) (Figure 4 and Table 3). A significant difference (p = 0.04) was also observed for the study design subgroup analysis, with a greater reduction in mortality risk compared with controls observed with IgM-enriched immunoglobulin treatment in prospective studies vs. the retrospective analyses (OR 0.37; 95% CI 0.27–0.51) vs. (OR 0.73; 95% CI 0.41–1.30) (Figure 5 and Table 3). There were no differences in comparative mortality risk for subgroup analyses for sepsis diagnosis (Figure 6 and Table 3) or publication year (Figure 7 and Table 3). Although treatment with IgM-enriched immunoglobulin was associated with a significantly lower risk of mortality compared with controls for patients with proven sepsis (p < 0.001), significance was not reached in the analysis of patients with suspected sepsis (p = 0.067).
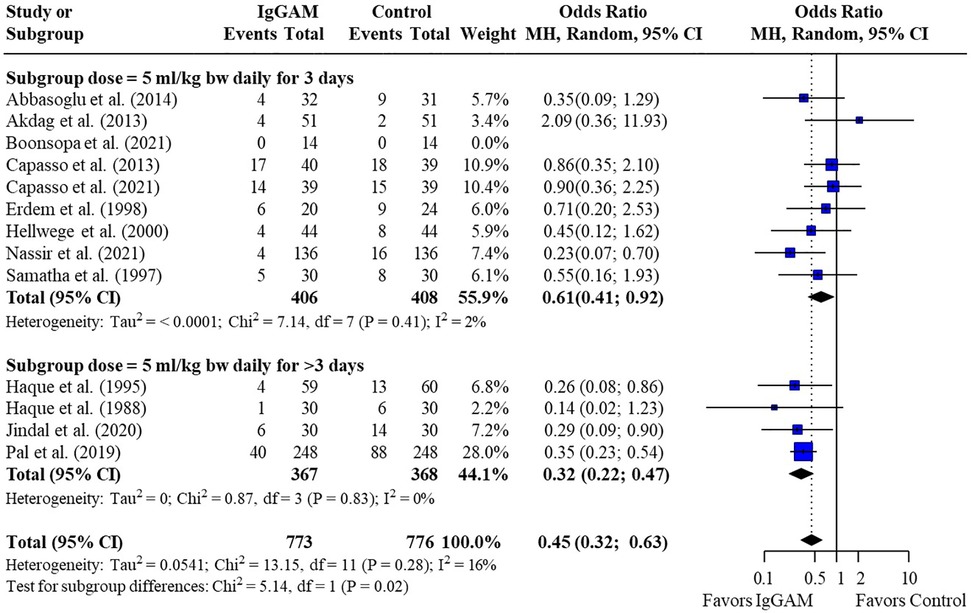
Figure 4. Subgroup analyses to compare the effect of a longer treatment duration (>3 days) of IgM-enriched immunoglobulin compared with the recommended regimen (3 days) on mortality rates in neonatal sepsis. BW, body weight; CI, confidence interval; IgGAM, IgM-enriched immunoglobulin; MH, Mantel–Haenszel.
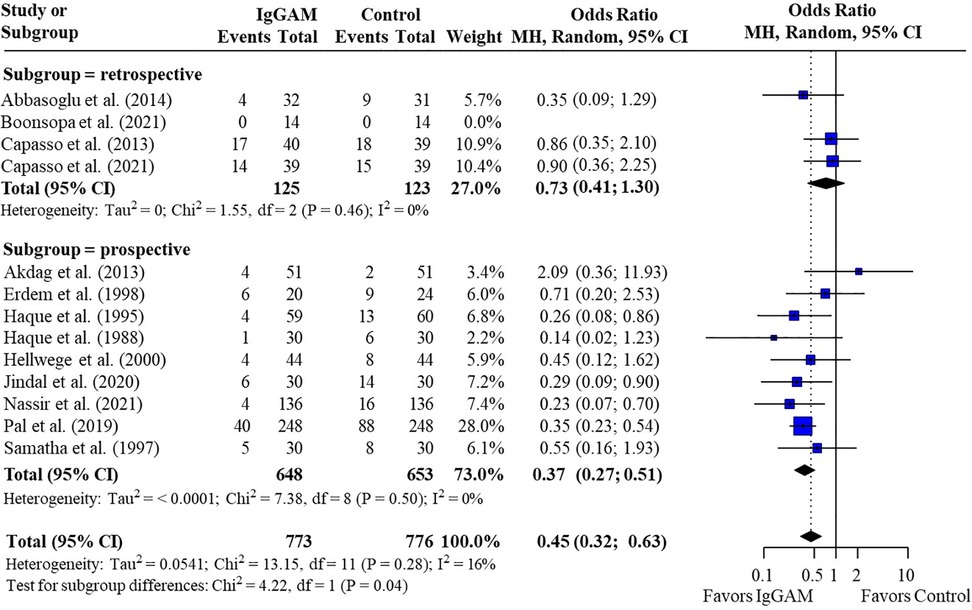
Figure 5. Subgroup analyses to compare the effect of IgM-enriched immunoglobulin treatment in retrospective vs. prospective studies on mortality rates in neonatal sepsis. CI, confidence interval; IgGAM, IgM-enriched immunoglobulin; MH, Mantel–Haenszel.
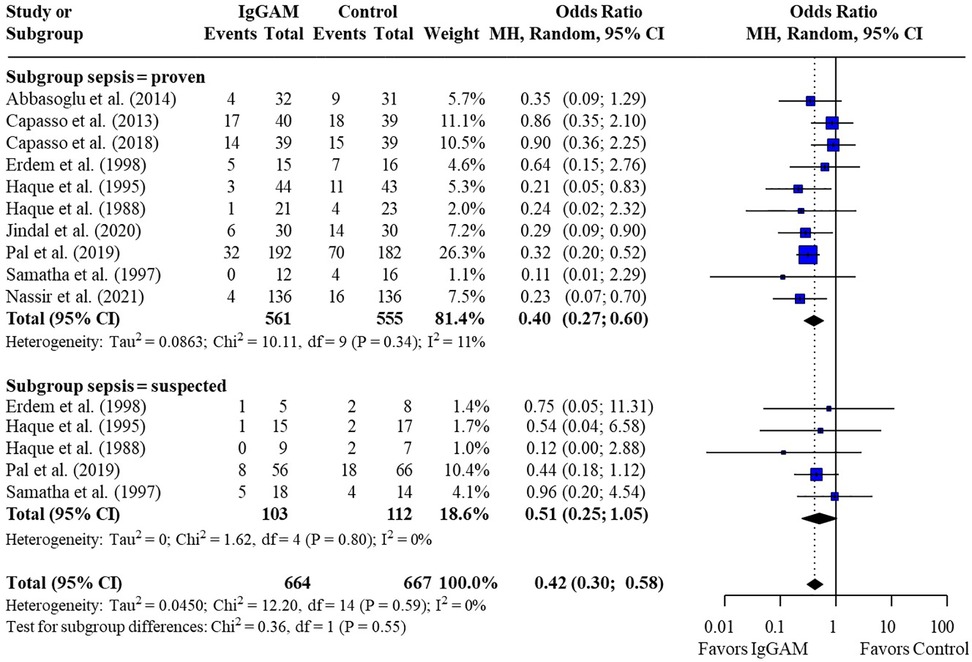
Figure 6. Subgroup analyses to compare the effect of IgM-enriched immunoglobulin treatment on mortality rates in neonates with proven or suspected sepsis. Studies which compared treatment effect on mortality in patients after microbiological confirmation of sepsis were included in both proven and suspected sepsis analyses (35, 36, 37, 46, 47). CI, confidence interval; IgGAM, IgM-enriched immunoglobulin; MH, Mantel–Haenszel.
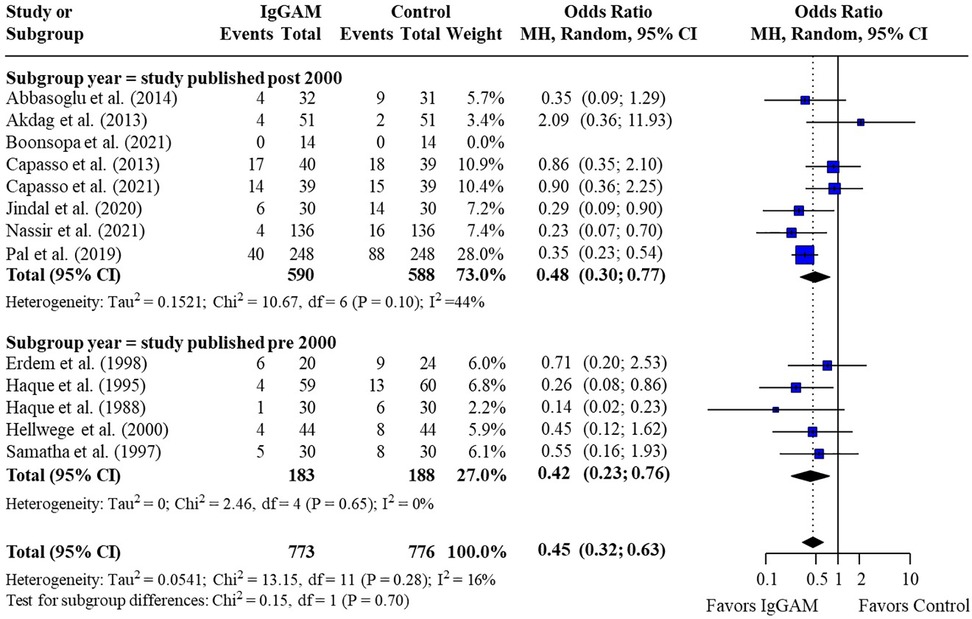
Figure 7. Subgroup analyses to compare the effect of IgM-enriched immunoglobulin treatment in older (pre 2000)* vs. newer (post 2000) studies on mortality rates in neonatal sepsis. *All studies pre and post 2000 were enrolled and published within these cut-off dates. CI, confidence interval; IgGAM, IgM-enriched immunoglobulin; MH, Mantel–Haenszel.
3.7. Publication bias
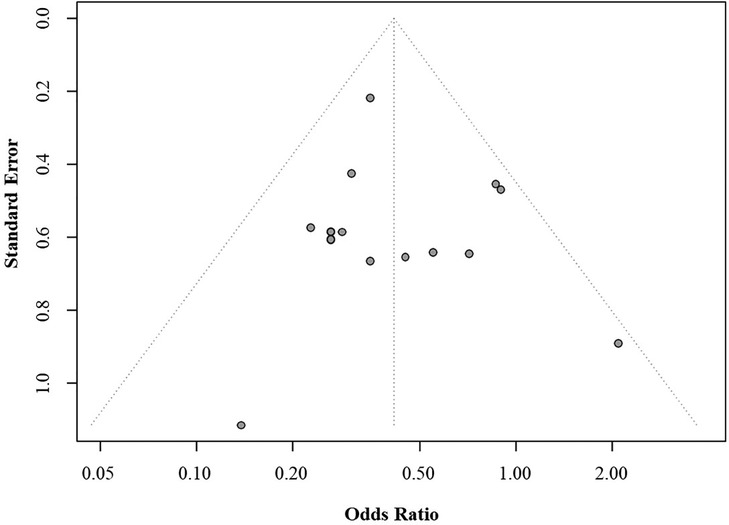
Assessment of publication bias showed that no bias was evident in the analyses, as indicated by the presence of all results within the funnel (Egger's test p > 0.05) (Figure 8).
4. Discussion
This meta-analysis evaluating IgM-enriched immunoglobulin as adjunctive therapy in neonatal and pediatric sepsis, included 13 studies in neonates (n = 1,549) and two studies in pediatric patients (n = 178). Overall, treatment with IgM-enriched immunoglobulin significantly reduced risk of mortality compared with controls in both populations.
The pathology and pathophysiology of sepsis differs between neonatal and pediatric patients. Neonates, especially preterm neonates, are highly susceptible to infection due to their immature immune system, which can lead to an inadequate or dysregulated immune response to pathogens (50, 51). In contrast, almost one-half of all pediatric patients who develop sepsis have a comorbid condition that increases their susceptibility to infection (52). The cause of infection may also be different; preterm neonates may be more at risk of infection from iatrogenic sources or acquire vertically transmitted early-onset sepsis (50), whilst pediatric patients may be more likely to develop sepsis from a hospital-acquired infection such as methicillin-resistant Staphylococcus aureus if they have a chronic illness, or community-acquired meningococcal infection if they are otherwise healthy (3). Accordingly therapeutic considerations will differ (3), and it is not inevitable that IgM-enriched immunoglobulin will have an equal benefit in both populations. In the pediatric analysis, the observed significant benefit following adjunctive treatment with IgM-enriched immunoglobulin was based on just two studies, highlighting the need for further larger studies to validate these results.
Due to the fundamental differences in the neonatal and pediatric immune systems and the small number of pediatric studies, subgroup analyses were limited to studies conducted in neonates. Overall, IgM-enriched immunoglobulin treatment reduced the mortality risk in neonates by 55% compared with controls. Of the 13 neonatal studies included in the analysis, only two studies observed no effect of IgM-enriched immunoglobulin treatment on mortality risk (44, 46). Boonsopa et al. reported no deaths for either control or the IgM-enriched immunoglobulin treatment group (44), but noted improvements in respiratory rates, mean arterial pressure and serum pH with IgM-enriched immunoglobulin treatment, suggesting a clinical benefit. Akdag et al, reported similar mortality rates in neonates treated with IgM-enriched immunoglobulin compared with controls (46). However the authors also analyzed pro-inflammatory biomarkers including cluster of differentiation 64 (CD64) which has high sensitivity and specificity in children as a diagnostic marker of infection and sepsis (53). They observed a significant reduction in CD64 levels with IgM-enriched immunoglobulin treatment compared with controls and concluded that IgM-enriched immunoglobulin may support a reduction of infection.
Interestingly, the comparative risk of mortality between IgM-enriched immunoglobulin-treated and control patients was influenced by the design of the study, with a significant reduction (p = 0.04) in risk of mortality observed with prospective vs. retrospective studies. In our analysis, the secondary endpoints from both Capasso et al. retrospective studies (41, 42) were selected to be more analogous with studies where mortality was evaluated at discharge (43, 45–47). However, despite no reduction in total mortality with IgM-enriched immunoglobulin therapy (41, 42), Capasso et al. still observed a >20% reduction in short-term mortality in both analyses. RCTs are considered to have a lower risk of bias and confounding variables when compared with retrospective analyses. The significant effect observed in the prospective studies in this analysis adds credence to the overall beneficial effect of adjunctive therapy with IgM-enriched immunoglobulin in neonates with sepsis.
Adjunctive therapy with IgM-enriched immunoglobulin in patients with proven sepsis saw a greater reduction in risk of mortality when compared with patients with suspected sepsis. In the Ohlsson Cochrane Database systematic review (30), the authors commented that they had excluded comparisons where sepsis was subsequently proven, as a diagnosis of sepsis is often not confirmed at the time of treatment initiation. Whilst this is an important perspective, three more recent studies included in this analysis waited for confirmation of sepsis by either positive blood or cerebrospinal fluid culture before initiating adjunctive therapy with IgM-enriched immunoglobulin (36, 43, 49), and still saw a significant mortality benefit compared with controls. Nevertheless, early administration of IgM-enriched immunoglobulin has been shown to be an important consideration and early treatment has been associated with a reduced risk of in-ICU mortality in adult patients with septic shock (54).
The INIS study, an RCT with more than 3,000 neonates, evaluated the administration of normal human low-dose IVIG (500 mg/kg; two doses, 48 h apart) in neonatal sepsis and concluded that there was no mortality benefit with standard IVIG adjunctive therapy (18). It was established that for treatment of neonatal sepsis, benefits of immunoglobulin are dependent on the type of immunoglobulin solution and on dose (18). Previous systematic reviews have performed subgroup analysis on IgM-enriched immunoglobulin therapy for neonatal sepsis (29–31). Whilst Kreymann et al. (31) observed a 50% reduction in risk of mortality compared with controls, Alejandria et al. (29) concluded that the evidence was not sufficient to draw robust conclusions and Ohlsson et al. (30) concluded that IgM-enriched immunoglobulin therapy was unable to reduce the risk of mortality. Since these meta-analyses, a further six studies on IgM-enriched immunoglobulin adjunctive therapy in neonatal sepsis have been published (36, 42–44, 46, 49). In total, these studies included 1,036 patients, of which three were prospective studies (36, 43, 49) and three retrospective analyses (42, 44, 46).
The retrospective PIGMENT study, which included 254 pediatric patients with a median age of 13 months with either sepsis, septic shock or multi-organ failure, compared 3-day and 5-day treatment with IgM-enriched immunoglobulin adjunctive therapy (250 mg/kg/day) (55). Although the absence of a control group precluded its inclusion in the current analysis, the study reported a significant reduction in mortality rate with longer treatment durations. In our study, a longer treatment duration was also associated with significantly reduced mortality compared with the SmPC-recommended 3-day regimen (35).
More contemporary studies are often more rigorous and consider advances in standard of care for sepsis and advances in antibiotic therapies. Interestingly, no difference was observed between studies conducted pre- and post- 2000 which may reflect the increases in antibiotic resistance rates now seen in hospitals.
The relationship between immunoglobulin levels at diagnosis of sepsis or septic shock and the outcome is controversial. Studies which evaluate the impact of IgG levels on mortality risk in adult patients with sepsis have reported conflicting outcomes (56–62), and similar disparities have been reported on the association of IgA levels and mortality risk (54, 57, 61). Whilst the data for IgM appear to be marginally more prognostic, with several studies in adults reporting a significant association between decreased IgM levels and reduced survival, at sepsis onset (63) and over time (54, 57, 64), two studies reported no association between IgM levels and survival (56, 61). Notably, Bermejo et al. evaluated the synergy between the three immunoglobulin isotypes and observed that the greatest risk of mortality was associated with the combined deficiency of IgA, IgG and IgM (57).
In view of these observations, adjunctive IgM-enriched immunoglobulin preparations with increased concentrations of IgM and IgA (12% each) may be more effective than standard human IVIG preparations. Berlot et al. evaluated treatment with IgM-enriched immunoglobulin in adult patients with sepsis and observed significant increases in levels of IgM and IgA (but not IgG) over time in survivors compared with non-survivors (65). In addition, Haque et al. also observed significant increases in serum IgM and IgA levels (but not IgG) in neonates treated with IgM-enriched immunoglobulin compared with controls (37). This suggests that IgM-enriched immunoglobulin adjunctive therapy may have the potential to supplement a patient's humoral immunity to within normal parameters.
IgM mediates a range of immune defenses; it is a potent activator of the complement system and is required for the maximal induction of the IgG antibody response (66). IgM also rapidly removes self-antigens, such as apoptotic cell debris, preventing the stimulation of further inflammatory responses (66). As a result, IgM has higher opsonization activity and activation of complement compared with IgG, and accordingly IgM-enriched immunoglobulin have been shown to have higher antimicrobial activity than immunoglobulin preparations containing IgG alone (19, 20, 67, 68). IgA is the second most prevalent antibody and induces either anti-pathogenic or immunomodulatory effects, especially on neutrophils. Therefore, in addition to IgM, the IgA component of the IgM-enriched immunoglobulin preparation could mediate beneficial effects compared with standard IVIG (69).
This systematic review has several limitations. Firstly study characteristics were varied with different IgM-enriched immunoglobulin treatment durations, different control arms and/or study designs and importantly an inconsistent definition of mortality. Additionally for neonates there were differences in undefined early- vs. late-onset sepsis, gestational age and birthweight, all of which will have a significant impact on the neonate's susceptibility to sepsis. Due to the limited number of studies available and the disparities in study reporting, the effect of these parameters on mortality benefit cannot be evaluated in this analysis. The complex nature of sepsis itself makes it very difficult to ascertain the cause of death in neonates and pediatric patients; causes of mortality in sepsis are multifactorial and treatment is one of many factors that may influence mortality risk in these patients. Finally, given the 34-year timespan over which the studies included in the analyses were conducted, there will have been many advances in intensive care medicine. This however, has been tempered by new challenges associated with increases in antimicrobial-resistance (70).
5. Conclusions
This meta-analysis and systematic review have shown that IgM-enriched immunoglobulin adjunctive therapy may reduce the risk of mortality in neonatal and pediatric patients with sepsis when compared with controls. Whilst there are limitations with the studies included in this analysis, this systematic review reveals a clear mortality benefit when prospective studies only were evaluated, highlighting the need for additional large multicentered RCTs to substantiate and further evaluate the observations of our analysis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AB, AS, ED, EK, GF, MA, PG, RR, UW contributed to conception and design of the study. AS performed the statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
Medical writing support was funded by Biotest AG. The opinions expressed in this manuscript reflect those of the authors.
Acknowledgments
We would like to thank Corina Heinz for her helpful review and Anna Atkinson from Elements Communications Ltd for her medical writing assistance.
Conflict of interest
ED performs contract work for the Eskisehir Osmangazi University funded by GSK, Sanofi Pasteur and Pfizer, outside of this publication and serves as a consultant and speaker for Biotest. GF has served as a speaker with support from Biotest. AB, AS, PG and UW are employees of Biotest AG.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1239014/full#supplementary-material
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. (2021) 49(11):e1063–143. doi: 10.1097/CCM.0000000000005337
3. Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. (2020) 21(2):e52–106. doi: 10.1097/PCC.0000000000002198
4. Peshimam N, Nadel S. Sepsis in children: state-of-the-art treatment. Ther Adv Infect Dis. (2021) 8:20499361211055332. doi: 10.1177/20499361211055332
5. Hayes R, Hartnett J, Semova G, Murray C, Murphy K, Carroll L, et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr Res. (2023) 93(5):1141–8. doi: 10.1038/s41390-021-01749-36
6. Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. (2005) 6(3 suppl):S3–5. doi: 10.1097/01.PCC.0000161289.22464.C3
7. Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. (2003) 167(5):695–701. doi: 10.1164/rccm.200207-682OC
8. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. (2020) 395(10219):200–11. doi: 10.1016/S0140-6736(19)32989-7
9. Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. (2014) 68(Suppl 1):S24–32. doi: 10.1016/j.jinf.2013.09.011
10. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. (2012) 2012:985646. doi: 10.1155/2012/985646
11. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. (2014) 27(1):21–47. doi: 10.1128/CMR.00031-13
12. Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. (1986) 20(9):899–904. doi: 10.1203/00006450-198609000-00019
13. Gan MY, Lee WL, Yap BJ, Seethor STT, Greenberg RG, Pek JH, et al. Contemporary trends in global mortality of sepsis among young infants less than 90 days: a systematic review and meta-analysis. Front Pediatr. (2022) 10:890767. doi: 10.3389/fped.2022.890767
14. Global report on the epidemiology and burden of sepsis. Available at: https://www.who.int/publications-detail-redirect/9789240010789 (Accessed May, 2023).
15. Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. (2015) 191(10):1147–57. doi: 10.1164/rccm.201412-2323OC
16. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. (2004) 292(19):2357–65. doi: 10.1001/jama.292.19.2357
17. Cai S, Thompson DK, Anderson PJ, Yang JYM. Short- and long-term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: a systematic review and meta-analysis. Children (Basel). (2019) 6(12):131. doi: 10.3390/children6120131
18. Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, Haque K, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. (2011) 365(13):1201–11. doi: 10.1056/NEJMoa1100441
19. Trautmann M, Held TK, Susa M, Karajan MA, Wulf A, Cross AS, et al. Bacterial lipopolysaccharide (LPS)-specific antibodies in commercial human immunoglobulin preparations: superior antibody content of an IgM-enriched product. Clin Exp Immunol. (1998) 111(1):81–90. doi: 10.1046/j.1365-2249.1998.00445.x
20. Nierhaus A, Berlot G, Kindgen-Milles D, Müller E, Girardis M. Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis. Ann Intensive Care. (2020) 10(1):132. doi: 10.1186/s13613-020-00740-1
21. Rossmann FS, Kropec A, Laverde D, Saaverda FR, Wobser D, Huebner J. In vitro and in vivo activity of hyperimmune globulin preparations against multiresistant nosocomial pathogens. Infection. (2015) 43(2):169–75. doi: 10.1007/s15010-014-0706-1
22. Barratt-Due A, Sokolov A, Gustavsen A, Hellerud BC, Egge K, Pischke SE, et al. Polyvalent immunoglobulin significantly attenuated the formation of IL-1β in Escherichia coli-induced sepsis in pigs. Immunobiology. (2013) 218(5):683–9. doi: 10.1016/j.imbio.2012.08.268
23. Norrby-Teglund A, Ihendyane N, Kansal R, Basma H, Kotb M, Andersson J, et al. Relative neutralizing activity in polyspecific IgM, IgA, and IgG preparations against group A streptococcal superantigens. Clin Infect Dis. (2000) 31(5):1175–82. doi: 10.1086/317423
24. Giamarellos-Bourboulis EJ, Tziolos N, Routsi C. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin Microbiol Infect. (2016) 22(6):499–506. doi: 10.1016/j.cmi.2016.01.021
25. Rodríguez A, Rello J, Neira J, Maskin B, Ceraso D, Vasta L, et al. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock. (2005) 23(4):298–304. doi: 10.1097/01.shk.0000157302.69125.f8
26. Schedel I, Dreikhausen U, Nentwig B, Höckenschnieder M, Rauthmann D, Balikcioglu S, et al. Treatment of gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med. (1991) 19(9):1104–13. doi: 10.1097/00003246-199109000-00003
27. Martinez JI, Sánchez HF, Velandia JA, Urbina Z, Florián MC, Martínez MA, et al. Treatment with IgM-enriched immunoglobulin in sepsis: a matched case-control analysis. J Crit Care. (2021) 64:120–4. doi: 10.1016/j.jcrc.2021.03.015
28. Cui J, Wei X, Lv H, Li Y, Li P, Chen Z, et al. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: a meta-analysis with trial sequential analysis. Ann Intensive Care. (2019) 9(1):27. doi: 10.1186/s13613-019-0501-3
29. Alejandria MM, Lansang MAD, Dans LF, Mantaring JB 3rd. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. (2013) 2013(9):CD001090. doi: 10.1002/14651858.CD001090.pub2
30. Ohlsson A, Lacy J. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst Rev. (2020) 2020(1):CD001239. doi: 10.1002/14651858.CD001239.pub3
31. Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. (2007) 35(12):2677–85.18074464
32. Hellwege HH, Seitz RC. Efficacy of Pentaglobin—an IgM enriched intravenous human immunoglobulin—in the treatment of neonatal sepsis. (2000). Biotest data on file.
33. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. (2011). (The Cochrane Collaboration; vol. Version 5.1.0). Available at: https://handbook-5-1.cochrane.org/ (Accessed May, 2023).
34. The Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May, 2023).
36. Pal S, Banerjee S, Roy B, Chattopadhyay A, Mazumder T, Bandyopadhyay S, et al. A study to see the efficacy of IGM enriched IVIG in reducing mortality in neonatal sepsis. Sch Bull. (2019) 5(7):370–3. doi: 10.21276/sb.2019.5.7.9
37. Haque KN, Zaidi MH, Bahakim H. IgM-enriched intravenous immunoglobulin therapy in neonatal sepsis. Am J Dis Child. (1988) 142(12):1293–6. doi: 10.1001/archpedi.1988.02150120047038
38. Haque KN, Remo C, Bahakim H. Comparison of two types of intravenous immunoglobulins in the treatment of neonatal sepsis. Clin Exp Immunol. (1995) 101(2):328–33. doi: 10.1111/j.1365-2249.1995.tb08359.x
39. El-Nawawy A, El-Kinany H, El-Sayed MH, Boshra N. Intravenous polyclonal immunoglobulin administration to sepsis syndrome patients: a prospective study in a pediatric intensive care unit. J Trop Pediatr. (2005) 51(5):271–8. doi: 10.1093/tropej/fmi011
40. Kola E, Çelaj E, Bakalli I, Lluka R, Kuli-Lito G, Sallabanda S. Efficacy of an IgM preparation in the treatment of patients with sepsis: a double-blind randomized clinical trial in a pediatric intensive care unit. South East Eur J Public Health. (2015) 1(1). doi: 10.4119/seejph-1770
41. Capasso L, Borrelli AC, Parrella C, Lama S, Ferrara T, Coppola C, et al. Are IgM-enriched immunoglobulins an effective adjuvant in septic VLBW infants? Ital J Pediatr. (2013) 39:63. doi: 10.1186/1824-7288-39-63
42. Capasso L, Borrelli AC, Pirozzi MR, Bucci L, Albachiara R, Ferrara T, et al. IgM and IgA enriched polyclonal immunoglobulins reduce short term mortality in extremely low birth weight infants with sepsis: a retrospective cohort study. Minerva Pediatr (Torino). (2021) 73(1):3–7. doi: 10.23736/S2724-5276.18.04850-8
43. Jindal SV, Gohil JR, Nikhileshwar A. Efficacy of IgM-rich immunoglobin for treating bacterial sepsis in very-low-birth-weight preterm neonates. Perinatology. (2020) 20(4):114–9.
44. Boonsopa C, Poonnarattanakul W, Srijuntongsiri S. Comparison of adjunctive treatment with IgM- enriched IVIG and antibiotics alone in treatment of neonatal sepsis. Siriraj Med J. (2021) 73(2):84–91. doi: 10.33192/Smj.2021.12
45. Abbasoğlu A, Ecevit A, Tuğcu AU, Yapakçı E, Tekindal MA, Tarcan A, et al. The influence of IgM-enriched immunoglobulin therapy on neonatal mortality and hematological variables in newborn infants with blood culture-proven sepsis. Turk J Pediatr. (2014) 56(3):267–71.
46. Akdag A, Dilmen U, Haque K, Dilli D, Erdeve O, Goekmen T. Role of pentoxifylline and/or IgM-enriched intravenous immunoglobulin in the management of neonatal sepsis. Am J Perinatol. (2014) 31(10):905–12. doi: 10.1055/s-0033-1363771
47. Samatha S, Jalalu MP, Hegde RK, Vishwanath D, Maiya PP. Role of IgM enriched intravenous immunoglobulin as an adjuvant to antibiotics in neonatal sepsis. Karnataka Pediatr J. (1997) 11(3):1–6.
48. Erdem G, Yurdakök M, Tekinalp G, Ersoy F. The use of IgM-enriched intravenous immunoglobulin for the treatment of neonatal sepsis in preterm infants. Turk J Pediatr. (1993) 35(4):277–81.8160279
49. Nassir KF, Al-Saddi YI, Abbas HM, Al Khames Aga QA, Al Khames Aga LA, Oudah AA. Pentaglobin (immunoglobulin M-enriched immunoglobulin) as adjuvant therapy for premature and very low-birth-weight neonates with sepsis. Indian J Pharmacol. (2021) 53(5):364–70. doi: 10.4103/ijp.ijp_881_20
50. Giannoni E, Schlapbach LJ. Editorial: sepsis in neonates and children. Front Pediatr. (2020) 8:621663. doi: 10.3389/fped.2020.621663
51. Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol. (2017) 17(8):495–507. doi: 10.1038/nri.2017.54
52. Mathias B, Mira JC, Larson SD. Pediatric sepsis. Curr Opin Pediatr. (2016) 28(3):380–7. doi: 10.1097/MOP.0000000000000337
53. Hoffmann JJML. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med. (2009) 47(8):903–16. doi: 10.1515/CCLM.2009.224
54. Berlot G, Vassallo MC, Busetto N, Nieto Yabar M, Istrati T, Baronio S, et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann Intensive Care. (2018) 8(1):122. doi: 10.1186/s13613-018-0466-7
55. Abdullayev E, Kilic O, Bozan G, Kiral E, Iseri Nepesov M, Dinleyici EC. Clinical, laboratory features and prognosis of children receiving IgM-enriched immunoglobulin (3 days vs. 5 days) as adjuvant treatment for serious infectious disease in pediatric intensive care unit: a retrospective single-center experience (PIGMENT study). Hum Vaccines Immunother. (2020) 16(8):1997–2002. doi: 10.1080/21645515.2019.1711298
56. Taccone FS, Stordeur P, De Backer D, Creteur J, Vincent JL. Gamma-globulin levels in patients with community-acquired septic shock. Shock. (2009) 32(4):379–85. doi: 10.1097/SHK.0b013e3181a2c0b2
57. Bermejo-Martín JF, Rodriguez-Fernandez A, Herrán-Monge R, Andaluz-Ojeda D, Muriel-Bombín A, Merino P, et al. Immunoglobulins IgG1, IgM and IgA: a synergistic team influencing survival in sepsis. J Intern Med. (2014) 276(4):404–12. doi: 10.1111/joim.12265
58. Akatsuka M, Tatsumi H, Sonoda T, Masuda Y. Low immunoglobulin G level is associated with poor outcomes in patients with sepsis and septic shock. J Microbiol Immunol Infect. (2021) 54(4):728–32. doi: 10.1016/j.jmii.2020.08.013
59. Myrianthefs PM, Boutzouka E, Baltopoulos GJ. Gamma-globulin levels in patients with community-acquired septic shock. Shock. (2010) 33(5):556–7. doi: 10.1097/01.shk.0000370606.30525.21
60. Dietz S, Lautenschläger C, Müller-Werdan U, Pilz G, Fraunberger P, Päsler M, et al. Serum IgG levels and mortality in patients with severe sepsis and septic shock : the SBITS data. Med Klin Intensivmed Notfallmedizin. (2017) 112(5):462–70. doi: 10.1007/s00063-016-0220-6
61. Alagna L, Meessen JMTA, Bellani G, Albiero D, Caironi P, Principale I, et al. Higher levels of IgA and IgG at sepsis onset are associated with higher mortality: results from the Albumin Italian Outcome Sepsis (ALBIOS) trial. Ann Intensive Care. (2021) 11(1):161. doi: 10.1186/s13613-021-00952-z
62. Shankar-Hari M, Fear D, Lavender P, Mare T, Beale R, Swanson C, et al. Activation-associated accelerated apoptosis of memory B cells in critically ill patients with sepsis. Crit Care Med. (2017) 45(5):875–82. doi: 10.1097/CCM.0000000000002380
63. Krautz C, Maier SL, Brunner M, Langheinrich M, Giamarellos-Bourboulis EJ, Gogos C, et al. Reduced circulating B cells and plasma IgM levels are associated with decreased survival in sepsis—a meta-analysis. J Crit Care. (2018) 45:71–5. doi: 10.1016/j.jcrc.2018.01.013
64. Giamarellos-Bourboulis EJ, Apostolidou E, Lada M, Perdios I, Gatselis NK, Tsangaris I, et al. Kinetics of circulating immunoglobulin M in sepsis: relationship with final outcome. Crit Care. (2013) 17(5):R247. doi: 10.1186/cc13073
65. Berlot G, Scamperle A, Istrati T, Dattola R, Longo I, Chillemi A, et al. Kinetics of immunoglobulins in septic shock patients treated with an IgM- and IgA-enriched intravenous preparation: an observational study. Front Med (Lausanne). (2021) 8:605113. doi: 10.3389/fmed.2021.605113
66. Blandino R, Baumgarth N. Secreted IgM: new tricks for an old molecule. J Leukoc Biol. (2019) 106(5):1021–34. doi: 10.1002/JLB.3RI0519-161R
67. Capasso L, Borrelli A, Cerullo J, Pisanti R, Figliuolo C, Izzo F, et al. Role of immunoglobulins in neonatal sepsis. Transl Med UniSa. (2014) 11:28–33.25674546
68. Stehr SN, Knels L, Weissflog C, Schober J, Haufe D, Lupp A, et al. Effects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock. (2008) 29(2):167–72. doi: 10.1097/shk.0b013e318067df15
69. Bohländer F. A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases. Front Immunol. (2023) 14:1127339. doi: 10.3389/fimmu.2023.1127339
Keywords: IgM-enriched immunoglobulin, neonate, pediatric, sepsis, mortality, infection, meta-analysis
Citation: Dinleyici EC, Frey G, Kola E, Wippermann U, Bauhofer A, Staus A, Griffiths P, Azharry M and Rohsiswatmo R (2023) Clinical efficacy of IgM-enriched immunoglobulin as adjunctive therapy in neonatal and pediatric sepsis: a systematic review and meta-analysis. Front. Pediatr. 11:1239014. doi: 10.3389/fped.2023.1239014
Received: 12 June 2023; Accepted: 31 July 2023;
Published: 11 August 2023.
Edited by:
Stefano Busani, University Hospital of Modena, ItalyReviewed by:
Gokhan Ceylan, University of Health Sciences, TürkiyeThomas Vincent Brogan, Seattle Children's Hospital, United States
© 2023 Dinleyici, Frey, Kola, Wippermann, Bauhofer, Staus, Griffiths, Azharry and Rohsiswatmo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ener Cagri Dinleyici dGltYm9vdGh0ckB5YWhvby5jb20=
 Ener Cagri Dinleyici
Ener Cagri Dinleyici Georg Frey2
Georg Frey2 Ermira Kola
Ermira Kola Ulrike Wippermann
Ulrike Wippermann Peter Griffiths
Peter Griffiths Rinawati Rohsiswatmo
Rinawati Rohsiswatmo