
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 25 September 2023
Sec. Pediatric Cardiology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1238847
Undifferentiated mesenchymal tumors from the intimal layer (intimal sarcomas) are rare within the ventricles and exceptional in children. A rare case of an intimal sarcoma located in the right ventricle in a young child is presented with need for urgent surgical resection due to mechanical flow obstruction. Tumor cells showed amplification of MDM2 gene and a homozygous loss of CDKN2A on 9p21. A review of the literature regarding primary cardiac malignancies and intimal sarcoma in children is provided.
Cardiac intimal sarcomas are very rare. In contrast to vascular intimal sarcoma, cardiac involvement is an infrequent finding especially in children. A retrospective study of 100 cardiac sarcomas however, describes intimal sarcoma as the predominant primary sarcoma of the heart (1). These tumors are known to be very aggressive with high metastatic potential and often (micro-)metastatic disease at the time of diagnosis. Prognosis is therefore reserved with full surgical resection as the major outcome parameter for prolonged survival (2–4).
We present a case of a 4-year old child presenting with an intimal sarcoma located in the right ventricle with need for urgent surgical resection. This is a one of the few reports of cardiac intimal sarcoma and the first report of a ventricular localization in a young child.
A 4-year old child was admitted to the pediatric cardiology department with suspicion of right heart failure: ascites, edema and poor circulation. There was a history of progressive fatigue and exertional dyspnea for some days. Medical history was negative apart from food allergy.
The patient presented with edema of the lower limbs with poor circulation, abdominal distention with hepatomegaly. There was no cardiac murmur, heart rate was 53 BPM, blood pressure of 101/73 mmHg and oxygen saturation of 100%.
Abdominal ultrasound confirmed ascites and hepatomegaly. Chest x-ray showed cardiomegaly with presence of bilateral pleural effusion. The cardiac ultrasound revealed a large mass in the right ventricle bulging through tricuspid valve into the right atrium; delineation of the leaflets of the tricuspid valve was impossible due to the mass. (Figure 1) The right atrium was importantly dilated. No regurgitation of the tricuspid or pulmonary valve could be observed. Pericardial and pleural effusion were confirmed.
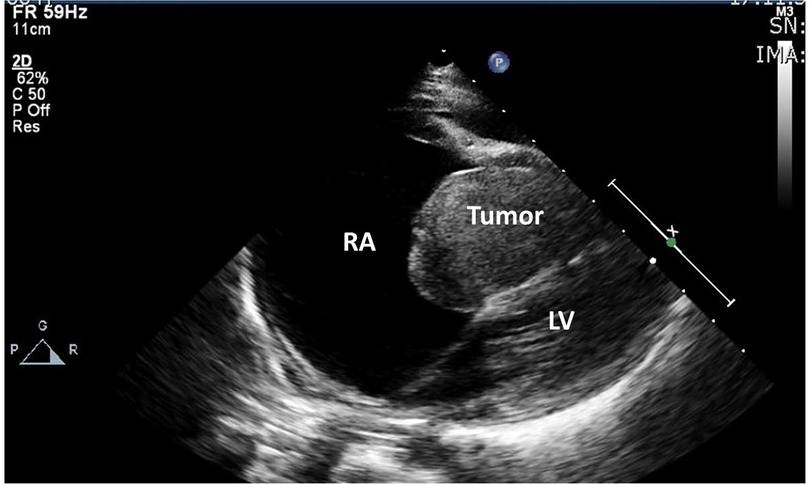
Figure 1. Echocardiographic image of large dilated right atrium with large mass in the right ventricular cavity towards the right atrium; no discernable tricuspid valve.
Urgent surgical resection was needed within 24 h after admission. The tumor was steeled on the anterior papillary muscle of the tricuspid valve (Figure 2A,B). A complete resection could be performed and subsequent a De Vega plasty of the tricuspid valve was established. The post-operative course was uneventful and the patient could be discharged from hospital 4 days after surgery.
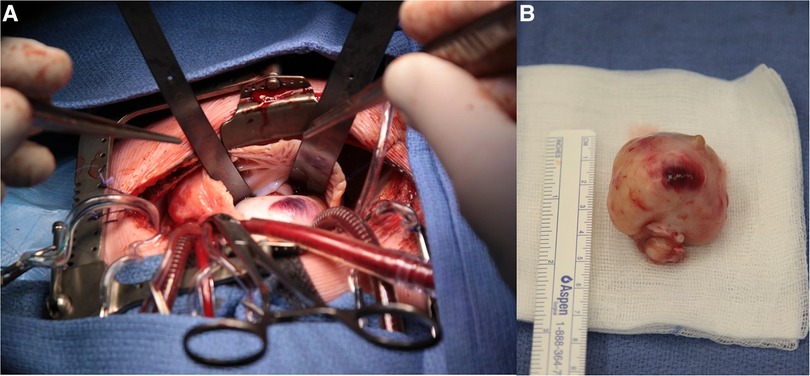
Figure 2. (A) Surgical view on cardiopulmonary bypass: right atrium opened with view on the right atrial appendix and the large mass bulging into the right atrium. (B) macroscopic view of resected tumor.
Macroscopically the lesion was nodular and white with a diameter of 3.7〉× 3.5 × 3.5 cm (Figure 3A). After fixation with 4% buffered formalin, samples were paraffin-embedded and 5-µm-thick sections were stained with haematoxylin and eosin (H&E). Light microscopy revealed a sharply demarcated lesion, not invading the residual myocardial tissue. The lesion consisted of spindle cells arranged in a storiform and herringbone pattern (Figure 3B). Less cellular, more fibrous and sometimes myxoid areas were admixed with cellular and atypical looking zones. Focally there was marked nuclear atypia with bizarre, giant nuclei (Figure 3C). The mitotic count ranged up to 5mitoses/10HPF. This not-specific morphology allows a very broad differential diagnosis. To narrow it down immunohistochemical stains were performed (Table 1). Strong expression of MDM2 and EGFR was seen (Figure 3D). In addition, there was some expression of alfa-SMA neurofilament and EMA. The remaining stains were all negative.
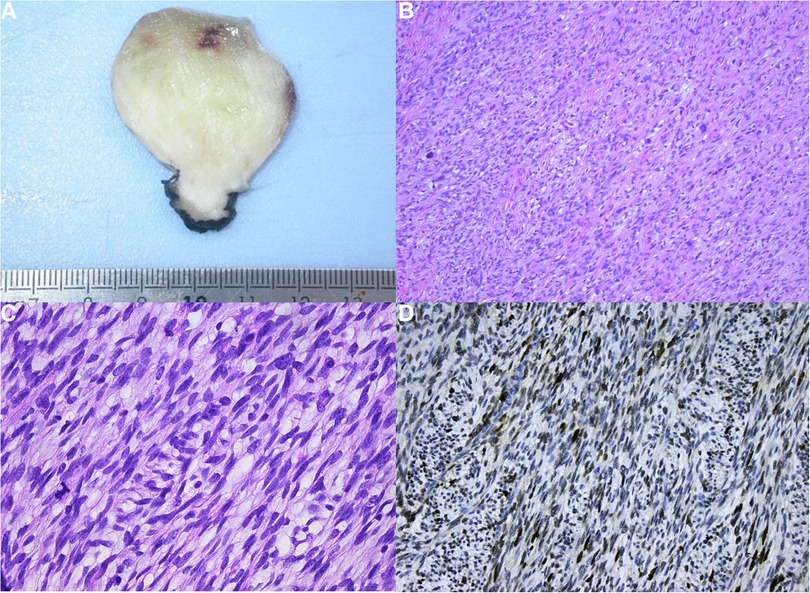
Figure 3. (A) Macroscopic view. (B) Low power view showing the fascicular arrangement of the tumor cells with prominent nuclear atypia. (C) At high power the nuclear atypia is appreciated to a better advantage. (D) Strong nuclear MDM2 expression.
FISH analysis was performed, and showed a gross amplification of MDM2 gene in >50% of tumor cells. There was no amplification of KIT, PDGFRA or EGFR genes. A homozygous loss of CDKN2A on 9p21 was also present. As an intimal sarcoma is malignant, further tumor staging was performed. Blood work-up was unremarkable. Whole-body PET-CT-scan showed multiple hypermetabolic lymph nodes bilaterally in the retromandibular region, in the right supraclavicular and in the anterior part of the mediastinum. Bone scan using 99mTc-MDP as a tracer was unable to show skeletal metastases.
Adjuvant chemotherapy with doxorubicin (60 mg/m2 day1) and ifosfamide (3 g/m2 day1, 2 and 3) was proposed, but parents refused postoperative chemotherapy. Cardiac magnetic resonance imaging did not show any macroscopic neoplasm 6 months after surgical resection.
Follow-up was performed initially every 3 months the first year and yearly afterwards. To date 8 years after surgical resection echocardiography does show an unchanged small fibrotic zone at the base of the papillary muscle of the tricuspid valve, without tumor recurrence. There is a residual low grade of tricuspid valve regurgitation (1–2/4) and no stenosis.
Primary cardiac sarcomas are rare but represent the majority of primary malignant cardiac tumors. There are few series describing sarcomas of the heart, and the majority of publications are case reports (2, 5–10). Ramlawi et al. reports a single-institution experience on 95 cases over 25-year period and Isambert et al. on 124 cases in 33-year period of the French Sarcoma Group with a mean age of 43 and 47.1 years respectively (11, 12). Recently a large multi-institutional cohort of 747 patients with primary cardiac malignancies is reported from the US National Cancer Database (13). Among the reported sarcomas, angiosarcomas appear to be the most prominent type (32.3–40.4%) (5, 11–14). The most common sites for the sarcomas are the right (38.8–39%) and left atrium (33–37.2%), and far less frequent the right ventricle (5%–5.8%) (11, 12). Neuville et al. reports in a retrospective clinicopathologic and molecular analysis of one hundred primary sarcomas, intimal sarcoma as the predominant tumor type with occurrence rate of 42%, followed by angiosarcoma in only 26 of 100 cases (1). All but one angiosarcoma originated from the right heart, whereas 83% of intimal sarcomas were from the left heart. Most mentioned case series and case reports are findings in adult population, occurring mostly in fourth and fifth decade of life (7, 8, 15, 16). In children, the estimated incidence of all cardiac tumors, benign or malignant, is 0.0017%–0.027%, and primary malignancy is approximately 10% (13, 17). Primary cardiac malignancies have been studied in the SEER experience (4). A total of 25 pediatric patients (<20 years of age) were identified with primary cardiac tumor in a period between 1973 and 2008. Age-adjusted incidence is 0.00686 per 100,000 US population. Most common histology type was soft tissue sarcoma (40%), which was not further specified, followed by non-Hodgkin lymphoma and teratoma with the incidence of each of them being 12%.
Intimal sarcoma is a malignant mesenchymal tumor which is usually a poorly differentiated sarcoma composed of atypical spindle cells and/or pleomorphic cells with the possibility of myxoid areas or epithelioid morphology. Tumor cells generally exhibit immunoreactivity for vimentin and variable expression for smooth muscle actin but are usually negative for desmin and endothelial markers (18). In the study by Neuville et al. all cases showed overexpression and amplification of MDM2. More than two thirds were also positive for CDK4 and HMGA2, which has been confirmed in earlier reports (1, 18).
Primary cardiac sarcomas are highly aggressive tumors which rapidly infiltrate all the layers of the heart and metastasize rapidly, especially when right-sided (11). At the time of diagnosis up to 80% of patients have metastatic disease (19). Mean survival rates are poor and range from 3 months to 20 months (11, 12, 15, 20). The overall 5-year survival in the US National Cancer Database was 11.5% (13). Therapy is primarily surgical, with achievement of negative resection margins significantly prolonging patient survival (5, 11, 12, 15). Unfortunately complete tumor resection is only possible in less than 50% of patients. Adjuvant chemo- and/or radiation therapy remains controversial (13). Some groups suggest that these adjuvant therapies have no added value, while others suggest better survival rates in patients who had received chemotherapy and radiation therapy (5, 11, 12, 20).
The presented case is exceptionally rare for age of presentation as well as for tumor location for this type of tumor. According to the literature this is the first report of an intimal sarcoma within the ventricle in a young child. Previously a case of pulmonary artery intimal sarcoma has been reported in an infant and in the left atrium in another child (21, 22). Soft tissue sarcoma has been reported as the predominant tumor type in primary malignant pediatric cardiac tumors, though no further tumor type specification was mentioned. The location of the tumor in the presented case is rare as 83% of intimal sarcomas arise from the left heart (1, 4). On the other hand, intimal sarcoma is more often reported as a tumor of the large vessels, especially pulmonary artery and thus in some way arising from the right heart. Given the disproportion between reported cases of cardiac intimal sarcoma and the retrospective finding of intimal sarcoma as the predominant primary cardiac sarcoma in adults, it might be that intimal sarcoma of the heart in children is more prevalent than is assumed, however still extremely rare (1, 23). In cases of (pediatric) sarcoma of the heart it is important to search for amplification by FISH at least for MDM2 (22, 24). In our case morphology and immune histochemistry results suggested the presence of intimal sarcoma. FISH analysis with amplification of MDM2, as well as loss of CDKN2A, supported the diagnosis.
In the case reported here complete resection of tumor could be obtained. Further tumor staging was negative apart from presence of multiple hypermetabolic lymph nodes on PET-CT scan. The lymphadenopathy could happen due to multiple reasons, as inflammation, a recent infection or more likely due to congestion caused by the sarcoma blocking the flow in the right ventricle and causing the effusions in the pericardium and the pleura, as well as causing the ascites. In the diagnostic work-up of intimal sarcomas CT and MRI are optimal for assessing tissue characteristics, infiltration and metastases (25). Given the high probability of occult dissemination at the time of diagnosis in this type of malignancy adjuvant chemotherapy was proposed, which was refused by the parents. Close follow-up is performed and with disease-free survival up to currently 8 years after surgical resection.
Intimal sarcomas, undifferentiated mesenchymal tumors arising from the intimal layer of the ventricle are an extremely rare entity in children. The molecular diagnosis is confirmed by a MDM2 amplification. Although the prognosis of primary cardiac sarcomas is overall poor, the presented case did show a good survival up to 8 years after surgical resection of the tumor.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The ethical approval has been granted for the specific case report by the ethical committee of UZ Leuven, Herestraat 49, Leuven Belgium. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because the written informed consent was required. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SV: analysis and interpretation of the data, writing and critical revising the manuscript and final approval of the manuscript RS: conception and design, interpretation of the data, critical revising the manuscript and final approval of the manuscript MD-R: analysis of the data, critical revising the manuscript and final approval of the manuscript. VL: critical revising the manuscript and final approval of the manuscript. BM: critical revising the manuscript and final approval of the manuscript. BC: conception and design, analysis and interpretation of the data, critical revising the manuscript and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Neuville A, Collin F, Bruneval P, Parrens M, Thivolet F, Gomez-Brouchet A, et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am J Surg Pathol. (2014) 38(4):461–9. doi: 10.1097/PAS.0000000000000184
2. Mayer F, Aebert H, Rudert M, Königsrainer A, Horger M, Kanz L, et al. Primary malignant sarcomas of the heart and great vessels in adult patients — a single-center experience. Oncologist. (2007) 12(9):1134–42. doi: 10.1634/theoncologist.12-9-1134
3. Thomas-de-Montpréville V, Nottin R, Dulmet E, Serraf A. Heart tumors in children and adults: clinicopathological study of 59 patients from a surgical center. Cardiovasc Pathol. (2007) 16(1):22–8. doi: 10.1016/J.CARPATH.2006.05.008
4. Davis JS, Allan BJ, Perez EA, Neville HL, Sola JE. Primary pediatric cardiac malignancies: the SEER experience. Pediatr Surg Int. (2013) 29(5):425–9. doi: 10.1007/s00383-013-3261-4
5. Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. (1992) 69(2):387–95. doi: 10.1002/1097-0142(19920115)69:2%3C387::AID-CNCR2820690219%3E3.0.CO;2-N
6. Ropp AM, Burke AP, Kligerman SJ, Leb JS, Frazier AA. Intimal sarcoma of the great vessels. Radiographics. (2021) 41(2):361–79. doi: 10.1148/rg.2021200184
7. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. (1999) 34(4):295–304. doi: 10.1046/J.1365-2559.1999.00636.X
8. Kim CH, Dancer JY, Coffey D, Zhai QJ, Reardon M, Ayala AG, et al. Clinicopathologic study of 24 patients with primary cardiac sarcomas: a 10-year single institution experience. Hum Pathol. (2008) 39(6):933–8. doi: 10.1016/j.humpath.2007.12.018
9. Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. (2008) 112(11):2440–6. doi: 10.1002/cncr.23459
10. Kumar N, Agarwal S, Ahuja A, Das P, Airon B, Ray R. Spectrum of cardiac tumors excluding myxoma: experience of a tertiary center with review of the literature. Pathol Res Pract. (2011) 207(12):769–74. doi: 10.1016/j.prp.2011.09.014
11. Ramlawi B, Leja MJ, Saleh WKA, Al Jabbari O, Benjamin R, Ravi V, et al. Surgical treatment of primary cardiac sarcomas: review of a single-institution experience. Ann Thorac Surg. (2016). 101(2):698–702. doi: 10.1016/j.athoracsur.2015.07.087
12. Isambert N, Ray-Coquard I, Italiano A, Rios M, Kerbrat P, Gauthier M, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. (2014) 50(1):128–36. doi: 10.1016/j.ejca.2013.09.012
13. Sultan I, Bianco V, Habertheuer A, Kilic A, Gleason TG, Aranda-Michel E, et al. Long-term outcomes of primary cardiac malignancies multi-institutional results from the national cancer database. J Am Coll Cardiol. (2020) 75(18):2338–47. doi: 10.1016/j.jacc.2020.03.041
14. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol). (2007) 19(10):748–56. doi: 10.1016/J.CLON.2007.06.009
15. Li Z, Hsieh T, Salehi A. Recurrent cardiac intimal (spindle cell) sarcoma of the left atrium. J Cardiothorac Vasc Anesth. (2013) 27(1):103–7. doi: 10.1053/j.jvca.2011.07.027
16. Modi A, Lipnevicius A, Moorjani N, Haw M. Prolonged survival with left atrial spindle cell sarcoma. Interact Cardiovasc Thorac Surg. (2009) 8(6):703–4. doi: 10.1510/icvts.2009.203562
17. Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. (2007) 2:11. doi: 10.1186/1750-1172-2-11
18. Bode-Lesniewska B, Zhao J, Speel EJM, Biraima AM, Turina M, Komminoth P, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. (2001) 438(1):57–65. doi: 10.1007/s004280000313
19. Silverman NA. Primary cardiac tumors. Ann Surg. (1980) 191(2):127–38. doi: 10.1097/00000658-198002000-00001
20. Ibrahim A, Luk A, Singhal P, Wan B, Zavodni A, Cusimano RJ, et al. Primary intimal (spindle cell) sarcoma of the heart: a case report and review of the literature. Case Rep Med. (2013) 2013:461815. doi: 10.1155/2013/461815
21. Chappell T, Creech CB, Parra D, Strauss A, Scholl F, Whitney G. Presentation of pulmonary artery intimal sarcoma in an infant with a history of neonatal valvular pulmonic stenosis. Ann Thorac Surg. (2008) 85(3):1092–4. doi: 10.1016/J.ATHORACSUR.2007.08.072
22. Rahmouni K, Al Abri Q, Janelle M, Nguyen VH, Sabapathy C, Bernier PL. Intimal cardiac sarcoma in an adolescent presenting with sudden cardiogenic shock. Pediatr Blood Cancer. (2021) 68(9):e29083. doi: 10.1002/pbc.29083
23. Yi ES. Tumors of the pulmonary vasculature. Cardiol Clin. (2004) 22(3):431–40. doi: 10.1016/j.ccl.2004.05.001
24. Koelsche C, Benhamida JK, Kommoss FKF, Stichel D, Jones DTW, Pfister SM, et al. Intimal sarcomas and undifferentiated cardiac sarcomas carry mutually exclusive MDM2, MDM4, and CDK6 amplifications and share a common DNA methylation signature. Mod Pathol (2021) 34(12):2122–9. doi: 10.1038/s41379-021-00874-y
Keywords: intimal sarcoma, cardiac, child, MDM2, right ventricle (RV)
Citation: Verbeek S, Sciot R, Debiec-Rychter M, Labarque V, Meyns B and Cools B (2023) Case report: Cardiac intimal sarcoma in a young child. Front. Pediatr. 11:1238847. doi: 10.3389/fped.2023.1238847
Received: 12 June 2023; Accepted: 11 September 2023;
Published: 25 September 2023.
Edited by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeReviewed by:
Abdallah Al-Mohammad, Sheffield Teaching Hospitals NHS Foundation Trust, United Kingdom© 2023 Verbeek, Sciot, Debiec-Rychter, Labarque, Meyns and Cools. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bjorn Cools Ympvcm4uY29vbHNAdXpsZXV2ZW4uYmU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.