- 1Department of Pediatrics, Zagreb Children’s Hospital, School of Medicine, University of Zagreb, Zagreb, Croatia
- 2School of Medicine, Josip Juraj Strossmayer University of Osijek, Osijek, Croatia
- 3Department of Platelet and Leukocyte Diagnosis and Hemostasis, Croatian Institute of Transfusion Medicine, Zagreb, Croatia
- 4Department of Anesthesiology, Resuscitation and Intensive Care Medicine, Zagreb Children’s Hospital, Zagreb, Croatia
- 5Department of Pediatric Surgery, Zagreb Children’s Hospital, Zagreb, Croatia
Introduction: Transfusion-related acute lung injury is a rare but potentially fatal complication, which may appear during or post-transfusion of blood products. Patients with macrophage activation syndrome, a serious life-threatening complication associated with systemic juvenile idiopathic arthritis, often require transfusion or administration of blood products for correction of cytopenia, coagulopathy and hypofibrinogenemia.
Case report: A 6-year-old girl with a past medical history of systemic juvenile idiopathic arthritis had the first relapse of the disease during which she developed macrophage activation syndrome. During this life-threatening complication, she received a second dose of whole blood derived filtered and irradiated platelets from a single male donor due to profound thrombocytopenia. Approximately one hour post-infusion, the patient developed progressive dyspnea, hypoxemia and bilateral pulmonary edema. She was promptly intubated and placed on mechanical ventilation for 40 h. Clinical, laboratory and radiological findings, as well as the success of supportive ventilation therapy were highly suggestive of transfusion-related acute lung injury, a life-threatening complication that occurs within six hours of blood component transfusion. Blood immunology showed no presence of anti-human neutrophil antigen and anti-leukocyte antigen class I and class II antibodies in the donor's or patient's plasma.
Conclusion: To the best of our knowledge, we report the first case of a child with systemic juvenile idiopathic arthritis complicated with macrophage activation syndrome who developed type II transfusion-related acute lung injury following platelet transfusion. It is important to consider transfusion-related acute lung injury in transfusion settings in these children and apply critical and restrictive approach for platelet transfusion.
1 Introduction
Systemic juvenile idiopathic arthritis (sJIA) is a rare type of juvenile idiopathic arthritis (JIA) characterized by high fever and extra-articular findings, triggered by presumed dysregulation of the innate immune system (1–3). In contrast to other types of JIA, sJIA has a disproportionally higher rate of complications, especially macrophage activation syndrome (MAS) that requires early recognition and prompt therapy (4–7). MAS can result in progressive multi-organ failure and eventually a fatal outcome in 4.1%–15.2% of affected children, making timely diagnosis and prompt initiation of appropriate treatment imperative (5). Based on the severity of macrophage activation syndrome that often leads to profound anemia, thrombocytopenia, hypofibrinogenemia and coagulation disorder triggered by hyperinflammation, some children will require concentrated red blood cells, platelet pools, intravenous fibrinogen and fresh frozen plasma. However, the potential risk of adverse events, including transfusion-related acute lung injury (TRALI) following transfusion of blood or blood products in these patients has not been investigated in detail to date.
TRALI is defined by the presence of respiratory insufficiency and hypoxemia that develop during or within six hours of transfusion of blood or blood products, and imaging will reveal bilateral fluffy infiltrates consistent with pulmonary edema. Although TRALI is considered a rare complication, it is the leading cause of transfusion related-mortality (8–10). Based on a recent consensus redefinition of TRALI, the terminology of TRALI type I [without acute respiratory distress syndrome (ARDS) risk factor] and TRALI type II (with ARDS risk factor or with mild existing ARDS) has been proposed (9). Despite mitigation strategies that include exclusion of females from plasma donation or exclusion of females with a history of pregnancy or known anti-leukocyte antibody, as well as the recent update of TRALI definition, this life-threatening condition often remains unrecognized and under-reported (10).
Clinical data on the pediatric population showed that TRALI is relatively common in critically ill children and indicate several independent risk factors for TRALI, such as high Pediatric Risk of Mortality III score on admission, mechanical ventilation, and sepsis (11). Blood derivative transfusion-related risk factors in children with sJIA complicated by MAS are not reported in detail to date. There are no specific transfusion threshold guidelines in patients with this complication either. A long-term extension study of phase III pivotal trials of canakinumab treatment in patients with sJIA and active systemic features recorded one patient diagnosed with TRALI but no additional data on the possible trigger or type of TRALI were provided (12).
We describe an unusual case of a girl previously diagnosed with sJIA complicated by MAS who received whole blood derived filtered and irradiated platelets from a single male donor due to profound thrombocytopenia and developed TRALI. The aim of this case report is to emphasize the diversity of the possible lethal complications during treatment of MAS in patients with sJIA.
2 Case report
Our patient, a 6-year-old girl with a one-year medical history of sJIA diagnosed by the International League of Associations for Rheumatology classification criteria (13) was rehospitalized due to the first relapse of sJIA that developed at 13 months of disease onset. Laboratory evaluation revealed a significant increase in acute phase reactants (erythrocyte sedimentation rate 94 mm/h, C-reactive protein 234 ng/L, procalcitonin 22.96 ng/ml, ferritin 3,272 μg/L), increased D-dimers (4.11 mg/L) and fibrinogen (8.1 g/L), and normal levels of aspartate transaminase, alanine transaminase and lactate dehydrogenase, accompanied by white blood cell and platelet counts, and no anemia. Due to immunosuppressive therapy and systemic inflammatory response, our first aim was to exclude infection; following sampling for blood cultures, polymerase chain reaction for Epstein-Barr virus and cytomegalovirus, empirical treatment was first started with ceftriaxone i.v., later switched first to tazocine, and then to meropenem and vancomycin following consultation with an infectious disease specialist. Early evaluation of antibacterial treatment showed no effect on clinical presentation and inflammatory markers; thus pulse corticosteroid therapy was administered again (30 mg/kg for three days) with only partial effect on the disease presentation and inflammatory markers. Therefore, treatment with an interleukin-1 receptor antagonist (anakinra) was sought from the Committee for Drug Approval, as this drug was not at the time registered for sJIA treatment in our country. Following the Committee approval and signed informed consent by the parents, anakinra was administered at a dose of 100 mg s.c. once daily. This treatment led to normalization of fever and inflammatory markers within 3 days.
Three weeks after the introduction of anakinra, the patient became febrile again with chills, mild hepatosplenomegaly, and significantly increased inflammatory markers (C-reactive protein 493.7 ng/L, procalcitonin 50.32 ng/ml). Additional laboratory evaluation confirmed MAS in our patient during the first relapse of sJIA (Table 1, column “Flare 1”) and she was transferred to the pediatric intensive care unit for further treatment under intensive monitoring. Heart ultrasound revealed pericarditis and hypertrophic myocardiopathy accompanied by significant increase in N-terminal prohormone of brain natriuretic peptide of >35,000 ng/L and normal troponin. On the first day at the intensive care unit, she received fresh frozen plasma for correction of hypofibrinogenemia, and on days 2 and 3 whole blood derived filtered and irradiated platelets stored in a single donor plasma from a male donor due to rapid drop in platelet count (from 178 to 36 × 109 /L within 12 h). Approximately one hour post-infusion of platelets on day 3, she started coughing and rapidly became progressively dyspneic. Physical examination of the patient revealed tachycardia, tachypnea, and diffuse bilateral crepitations throughout her lungs. Hypoxemia was evident with peripheral oxygen saturation persistently below 90% and increasing oxygen requirements. The administration of intravenous furosemide and hydrocortisone had no effect on her symptoms. Urgent chest radiograph (Figure 1A) showed bilateral inhomogeneous opacities but no cardiomegaly, and she was promptly intubated and placed on mechanical ventilation for a total of 30 h (Figure 1B). There was no evidence for acute heart failure secondary to ischemic event or circulatory overload, so the diagnosis of transfusion-associated circulatory overload (TACO) was unlikely. Microbial analysis for possible sepsis was negative. Laboratory evaluation revealed transitory leukopenia (Figure 2). The patient's pulmonary condition rapidly improved and a radiograph taken 40-h post-event showed marked resolution of the airspace shadowing (Figure 1C). She was extubated and transferred to the Immunology and Rheumatology Department one day later, with no further pulmonary sequels. Based on the relevant criteria for TRALI at the time of presumed transfusion complication, blood immunology was performed to detect Human Leukocyte Antigen (HLA) class I, class II, and human neutrophil antigen (HNA) antibodies in the donor's and patient's plasma by using the following tests: granulocyte immunofluorescence test, granulocyte agglutination test, monoclonal immobilization of granulocyte antigen, and enzyme immunoassay kit (Lifecodes Quick-screen and B-screen, Immucor GTI Diagnostics, Wisconsin, USA) (14). Anti-HLA class I, class II and/or HNA antibodies were not detectable either in donor's or patient's plasma, suggesting that in our patient, TRALI may have resulted from a non-immune mechanism in which the recipient had previous and/or existing inflammatory pathological condition favoring the development of type II TRALI (9).
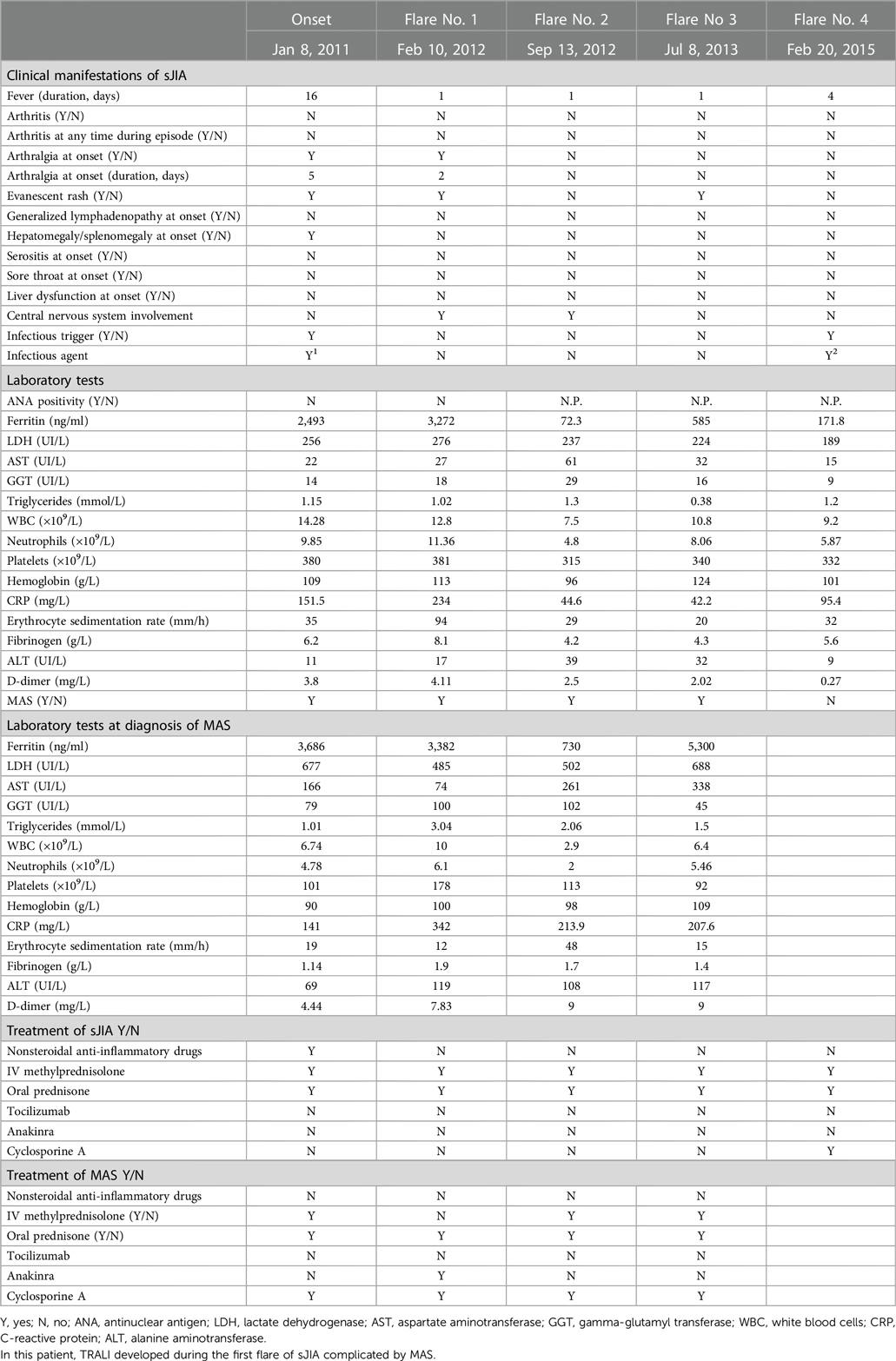
Table 1. Clinical manifestations, laboratory values and treatment administered for systemic juvenile idiopathic arthritis (sJIA), in sJIA flare, and following diagnosis of macrophage activation syndrome (MAS).
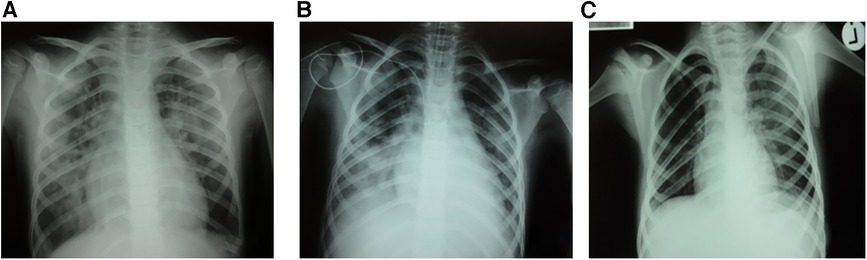
Figure 1. Bilateral pulmonary infiltrates post-transfusion (A) and immediately after intubation (B) with significant radiological improvement 40 hours post-event (C).
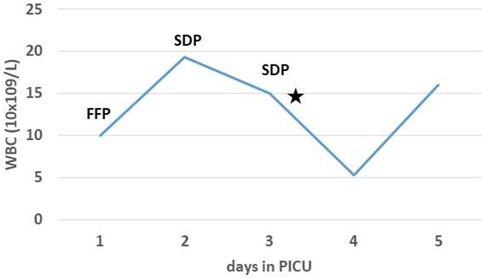
Figure 2. Laboratory investigations showed transitory leukopenia (⋆) following transfusion of single donor platelets (SDP). WBC, white blood cells; FFP, fresh frozen plasma.
Following clinical suspicion of TRALI in our patient, the Croatian Hemovigilance Network (CHN) members immediately contacted all medical centers in our country to see if any other patient having received blood components from the same male donor had TRALI, but no such complication was reported. Treatment of MAS was continued with repeated pulse dose of steroids for three days, followed by prednisolone p.o. and cyclosporine A, and this approach led to successful control of sJIA and MAS as its complication within 10 days. According to the recommendation obtained from Transfusion Service, physicians were instructed to pay due attention to the patient's risk factors for acute lung injury and implement evidence-based national transfusion practices avoiding transfusions when they were not vitally indicated (15).
During 12-year follow-up, our patient had a total of four relapses of sJIA (Table 1). Following the first relapse complicated with MAS and suspected type II TRALI, additional two flares of sJIA with MAS were promptly recognized and treated. The patient's parents refused therapy with anakinra, so treatment with steroids and cyclosporine A was administered during these flares. Our patient had no need of transfusion of blood derivatives during the follow-up. Based on the severe and relapsing presentation of the disease complicated by MAS, which could be considered as refractory sJIA (16), additional evaluation for familial hemophagocytic lymphohistiocytosis was performed at Karolinska University Hospital, Huddinge, Sweden, but the NK cell count, NK cell-mediated cytotoxicity, degranulation of NK- and T cells, perforin and SLAM-associated protein were all normal. The patient has been symptom-free and without any therapy for the last eight years.
3 Discussion
Transfusion-related acute lung injury is the leading cause of transfusion-associated mortality (10). TRALI was initially defined based on clinical and radiological parameters at the 2004 Consensus Conference organized by the National Heart, Lung and Blood Institute Working Group on TRALI, as a newly developed acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) within six hours of blood product transfusion (8). Most cases of TRALI have been reported in adult patients, but rising awareness of TRALI in children, especially in the setting of hematologic diseases, has led to its recognition in children as well (17, 18).
Various mechanisms have been proposed for the pathogenesis of TRALI. The priming step consists of previous and/or existing inflammatory pathological conditions or external factors attracting leukocytes to lung vessels and creating conditions favorable for the second step, in which anti-HLA, anti-HNA antibodies, various biologically active mediators secreted by cells or present in transfused blood products lead to the stress of leukocytes and consequent inflammation of lung epithelia and pulmonary edema. In addition, monocyte and macrophage activation has also been implicated in the pathophysiology of TRALI. Several activation mechanisms of these cells have been described, such as those mediated by pathogen-associated and damage-associated molecular patterns and their receptors, antibodies, complement, as well as neutrophil extracellular traps (10, 19–21).
The most common pulmonary complications in sJIA patients include pleural effusion and pleuritis. More recently, pulmonary artery hypertension, interstitial lung disease and alveolar proteinosis have been reported in these patients with increased frequency and being associated with mortality (22, 23). However, our patient had no signs and symptoms of respiratory disease prior to MAS onset, which may have contributed to ALI development or mimic TRALI.
Based on a recent significant progress in defining the factors contributing to TRALI pathophysiology, patients with sJIA complicated by MAS have many risk factors for TRALI onset since these conditions share many overlapping mechanisms. An immune feature of MAS in patients with sJIA is excessive activation and proliferation of macrophages and T lymphocytes. Massive hypercytokinemia is strongly associated with MAS pathogenesis, particularly the overproduction of cytokines such as interleukin (IL)-1, IL-6, IL-18, interferon-γ, and tumor necrosis factor-α. Inadequate production of IL-10, a regulatory cytokine to counter-regulate interferon-γ, might be related to the development of MAS (24). Several of these cytokines (IL-6, IL-10) are also important in the pathophysiology of TRALI (21). Based on the several overlapping mechanisms involved in the pathophysiology of MAS in sJIA and those identified as triggers/risks for TRALI, our patient had significant recipient-related risk factors that could have contributed to the onset of TRALI.
The management of TRALI is mainly supportive by using oxygen and ventilatory support, and most cases show improvement within the first few hours and completely resolve within 1–4 days (10). Our patient improved markedly after 40 h of oxygen therapy. Plasma-containing components from female donors with leukocyte antibodies were responsible for the majority of immune (antibody mediated) TRALI fatalities before mitigation strategies had been implemented. Since 2006, the CHN has introduced mitigation approach for TRALI in our country following the international recommendations. Serology investigation for anti-leukocyte antibodies in suspected TRALI cases has been implemented in the Croatian Institute of Transfusion Medicine since 2008, according to the International Society of Blood Transfusion Granulocyte Working Party recommendation (14). As already described, our patient received platelets stored in a single donor plasma. Starting from 2015, transfusion with platelets suspended in platelet additive solution has been introduced in our country, as this additional strategy can reduce TRALI-associated morbidity (25).
Based on the clinical presentation of new ARDS within six hours of platelet transfusion in our patient, we were able to initiate necessary investigations and identify the possible implicated mechanism of TRALI. This analysis revealed no presence of anti-HNA or anti-HLA class I and class II antibodies in either donor's or patient's plasma, suggesting that in our patient, TRALI may have resulted from a non-immune mechanism.
TRALI has been recently described in an adult patient with systemic lupus erythematosus complicated by autoimmune hemolytic anemia following infusion of packed red blood cells and intravenous immunoglobulins (26). This report further emphasizes the possible common pathophysiological pathway of TRALI with autoimmune and/or autoinflammatory diseases.
There are currently no specific international transfusion recommendations for children with sJIA complicated by MAS. A recently published EULAR/American College of Rheumatology report provides the points to consider as guidance for supportive therapy based on the available data and expert opinion, including blood product transfusion, and suggests implementation of national recommendations and guidelines (27).
In conclusion, our findings underline the importance of clinical diagnosis of TRALI in a life-threatening condition, i.e., sJIA complicated by MAS, in case of a new-onset ALI during the treatment of MAS with blood derivatives. Further research is necessary to determine whether specific transfusion threshold guidelines in these critically ill children are required. Until these data are available, the critical and restrictive approach for transfusion of blood products according to the local/national recommendations should be followed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because According to the law in our country, ethical approval is not required for retrospective study. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from laboratory findings which were necessary for diagnosis and treatment, no additional samples were taken from this patient. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
AG contributed to data collection, data analysis, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Dr. Yenan Bryceson at Karolinska University Hospital, Huddinge, Sweden, for his contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cimaz R. Systemic-onset juvenile idiopathic arthritis. Autoimmun Rev. (2016) 15:931–4. doi: 10.1016/j.autrev.2016.07.004
2. Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. (2019) 46:190–7. doi: 10.3899/jrheum.180168
3. Onel KB, Horton DB, Lovell DJ, Shenoi S, Cuello CA, Angeles-Han ST, et al. 2021 American college of rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol. (2022) 74:553–69. doi: 10.1002/art.42037
4. Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European league against rheumatism/American college of rheumatology/paediatric rheumatology international trials organisation collaborative initiative. Ann Rheum Dis. (2016) 75:481–9. doi: 10.1136/annrheumdis-2015-208982
5. Minoia F, Davì S, Horne A, Bovis F, Demirkaya E, Akikusa J, et al. Dissecting the heterogeneity of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Rheumatol. (2015) 42(6):994–1001. doi: 10.3899/jrheum
6. Boom V, Anton J, Lahdenne P, Quartier P, Ravelli A, Wulffraat NM, et al. Evidence-based diagnosis and treatment of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Pediatr Rheumatol Online J. (2015) 13:55. doi: 10.1186/s12969-015-0055-3
7. Jelušić M, Kronja M, Frković M, Sršen S, Huljev Frković S, Štekić Novački K. Comparison of different diagnostic guidelines for the diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: single center experience. Acta Clin Croat. (2018) 57:307–11. doi: 10.20471/acc.2018.57.02.11
8. Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. (2004) 44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x
9. Vlaar APJ, Toy P, Fung M, Looney MR, Juffermans NP, Bux J, et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion. (2019) 59:2465–76. doi: 10.1111/trf.15311
10. Kuldanek SA, Kelher M, Silliman CC. Risk factors, management and prevention of transfusion-related acute lung injury: a comprehensive update. Expert Rev Hematol. (2019) 12:773–85. doi: 10.1080/17474086.2019.1640599
11. Mulder HD, Augustijn QJ, van Woensel JB, Bos AP, Juffermans NP, Wösten-van Asperen RM. Incidence, risk factors, and outcome of transfusion-related acute lung injury in critically ill children: a retrospective study. J Crit Care. (2015) 30:55–9. doi: 10.1016/j.jcrc.2014.10.005
12. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat NM, Horneff G, et al. Canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic features: results from the 5-year long-term extension of the phase III pivotal trials. Ann Rheum Dis. (2018) 77:1710–9. doi: 10.1136/annrheumdis-2018-213150
13. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31:390–2.14760812
14. ISBT Working Party on Granulocyte Immunobiology, Bierling P, Bux J, Curtis B, Flesch B, Fung L, et al. Recommendations of the ISBT working party on granulocyte immunobiology for leucocyte antibody screening in the investigation and prevention of antibody-mediated transfusion-related acute lung injury. Vox Sang. (2009) 96:266–9. doi: 10.1111/j.1423-0410.2008.01144.x
15. Tomičić M, Vuk T, Hundrić-Hašpl Ž. Indikacije i kontraindikacije za primjenu trombocitnih transfuzija u bolesnika s trombocitopenijom. Lijec Vjesn. (2014) 136:90–3. (in Croatian).
16. Ambler WG, Nanda K, Onel KB, Shenoi S. Refractory systemic onset juvenile idiopathic arthritis: current challenges and future perspectives. Ann Med. (2022) 54:1839–50. doi: 10.1080/07853890.2022.2095431
17. Church GD, Price C, Sanchez R, Looney MR. Transfusion-related acute lung injury in the paediatric patient: two case reports and a review of the literature. Transfus Med. (2006) 16:343–8. doi: 10.1111/j.1365-3148.2006.00683.x
18. Lieberman L, Petraszko T, Yi QL, Hannach B, Skeate R. Transfusion-related lung injury in children: a case series and review of the literature. Transfusion. (2014) 54:57–64. doi: 10.1111/trf.12249
19. Guo K, Ma S. The immune system in transfusion-related acute lung injury prevention and therapy: update and perspective. Front Mol Biosci. (2021) 8:639976. doi: 10.3389/fmolb.2021.639976 (Erratum in: Front Mol Biosci. (2021) 8:720653).33842545
20. Tung JP, Chiaretti S, Dean MM, Sultana AJ, Reade MC, Fung YL. Transfusion-related acute lung injury (TRALI): potential pathways of development, strategies for prevention and treatment, and future research directions. Blood Rev. (2022) 53:100926. doi: 10.1016/j.blre.2021.100926
21. Yu Y, Lian Z. Update on transfusion-related acute lung injury: an overview of its pathogenesis and management. Front Immunol. (2023) 14:1175387. doi: 10.3389/fimmu.2023.1175387
22. Kimura Y, Weiss JE, Haroldson KL, Lee T, Punaro M, Oliveira S, et al. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). (2013) 65:745–52. doi: 10.1002/acr.21889
23. Saper VE, Chen G, Deutsch GH, Guillerman RP, Birgmeier J, Jagadeesh K, et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis. (2019) 78:1722–31. doi: 10.1136/annrheumdis-2019-216040
24. Shimizu M. Macrophage activation syndrome in systemic juvenile idiopathic arthritis. Immunol Med. (2021) 44:237–45. doi: 10.1080/25785826.2021.1912893
25. Mathur A, Swamy N, Thapa S, Chakraborthy S, Jagannathan L. Adding to platelet safety and life: platelet additive solutions. Asian J Transfus Sci. (2018) 12:136–40. doi: 10.4103/ajts.AJTS_150_17
26. Pelechas E, Gerolymatou N, Voulgari PV, Drosos AA. Transfusion-related acute lung injury in a patient with systemic lupus erythematosus. Clin Exp Rheumatol. (2017) 35:353.28134082
27. Shakoory B, Geerlinks A, Wilejto M, Kernan K, Hines M, Romano M, et al. The 2022 EULAR/ACR points to consider at the early stages of diagnosis and management of suspected haemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS). Ann Rheum Dis. (2023) 82:1271–85. doi: 10.1136/ard-2023-224123
Keywords: transfusion-related acute lung injury (TRALI), systemic juvenile idiopathic arthritis (sJIA), macrophage activation syndrome (MAS), cytopenia, thrombocytopenia, platelets
Citation: Gagro A, Tomičić M, Škarić I and Dawidowsky B (2024) Case report: Suspected transfusion-related acute lung injury type II in a child with refractory systemic juvenile idiopathic arthritis complicated by macrophage activation syndrome. Front. Pediatr. 11:1237111. doi: 10.3389/fped.2023.1237111
Received: 8 June 2023; Accepted: 15 December 2023;
Published: 8 January 2024.
Edited by:
John-Paul Tung, Australian Red Cross Lifeblood, AustraliaReviewed by:
Stefan Franciscus Van Wonderen, Amsterdam University Medical Center, NetherlandsSentot Santoso, University of Giessen, Germany
© 2024 Gagro, Tomičić, Škarić and Dawidowsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alenka Gagro YWxlbmthLmdhZ3JvQGdtYWlsLmNvbQ==
 Alenka Gagro
Alenka Gagro Maja Tomičić3
Maja Tomičić3 Ivančica Škarić
Ivančica Škarić Barbara Dawidowsky
Barbara Dawidowsky