- 1Blood and Marrow Transplant Unit, Royal Manchester Children’s Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
- 2Division of Infection, Immunity and Respiratory Medicine, Faculty of Biology, School of Biological Sciences, Lydia Becker Institute of Immunology and Inflammation, Medicine and Health, University of Manchester, Manchester, United Kingdom
- 3Transplantation Laboratory, Manchester University NHS Foundation Trust, Manchester, United Kingdom
- 4Manchester Academic Health Science Centre, University of Manchester, Manchester, United Kingdom
Allogeneic hematopoietic stem cell transplantation (HSCT) has been an important and efficacious treatment for acute leukemia in children for over 60 years. It works primarily through the graft-vs.-leukemia (GVL) effect, in which donor T-cells and other immune cells act to eliminate residual leukemia. Cord blood is an alternative source of stem cells for transplantation, with distinct biological and immunological characteristics. Retrospective clinical studies report superior relapse rates with cord blood transplantation (CBT), when compared to other stem cell sources, particularly for patients with high-risk leukemia. Xenograft models also support the superiority of cord blood T-cells in eradicating malignancy, when compared to those derived from peripheral blood. Conversely, CBT has historically been associated with an increased risk of transplant-related mortality (TRM) and morbidity, particularly from infection. Here we discuss clinical aspects of CBT, the unique immunology of cord blood T-cells, their role in the GVL effect and future methods to maximize their utility in cellular therapies for leukemia, honing and harnessing their antitumor properties whilst managing the risks of TRM.
Introduction
For children with high-risk acute leukemia, allogeneic HSCT is an important and effective treatment strategy. HSCT produces potent anti-leukemia activity through the dose intensification of chemotherapy used in conditioning and more importantly, the GVL effect mediated primarily through donor T-cells and other immune cells. Maximizing GVL must occur in balance with prevention of graft-vs.-host disease (GVHD), in which alloimmune T-cell responses are directed against healthy tissues resulting in patient morbidity and mortality in both its acute and chronic forms (1). Conventional HSCT includes bone marrow transplantation (BMT) and peripheral blood stem cell transplantation (PBSCT), often from family or matched unrelated donors (MUD). Cord blood has been used as a source of hematopoietic stem cells (HSCs) in transplantation for acute leukemia for over three decades (2). It has many important differences and consequent advantages compared to conventional stem cell sources, including significantly reduced relapse rates in acute leukemia, particularly for those patients with residual disease pre-transplant, and lower rates of chronic GVHD (3, 4). Here we discuss the key clinical and immunological features of cord blood, and in particular cord blood T-cells, in order to shed light on the mechanisms through which this augmented disease response occurs.
Graft-vs.-Leukemia
T-cells are important mediators of GVL (5). In allogeneic HSCT, T-cell depletion with ex vivo graft manipulation or in vivo with T-cell depleting antibodies, such as anti-thymocyte globulin (ATG) or alemtuzumab, is used to prevent of GVHD. In malignancy, however, the use of T-cell depletion results in an increased risk of relapse (6–9). Conversely, relapse is reduced in those patients who develop GVHD and when relapse occurs, remission can be restored using donor lymphocyte infusions (DLI) (10–15). These clinical observations provide clear evidence for the potent role of T-cells in the GVL effect. In recent years, advancements in adoptive T-cell therapies, particularly chimeric antigen receptor (CAR) T-cells, have allowed sustained remission and cure to be attained in patients with previously incurable hematological malignancies, which further emphasizes the importance of T-cells in controlling malignant disease (16, 17).
T-cells recognize leukemia through interactions between their T-cell receptor (TCR) and human leukocyte antigen (HLA) molecules expressed on the surface of leukemia cells, which present antigenic peptide. HLA molecules are encoded by the human major histocompatibility (MHC) complex, which is a highly polymorphic region of genes located on chromosome 6 (18). CD8+ T-cells recognize peptide bound to HLA class I molecules, which are expressed on all nucleated cells, whereas CD4+ T-cells recognize HLA class II molecules, which are primarily expressed on specialized antigen-presenting cells (APCs). Both CD8+ and CD4+ T-cells mediate GVL reactions through interactions with antigen presented by HLA class I and class II molecules respectively (19).
HLA offers a potential important GVL target. In CBT, HLA-mismatch has shown to correlate with reduced relapse rates, with higher relapse rates following transplant in patients receiving the best matched cord blood units (20–22). Following haploidentical HSCT, loss of the entire mismatched haplotype through uniparental disomy of chromosome 6 p has been described in multiple patients (23). Leukemia relapse occurs as a consequence of this genetic event due to immune evasion. This suggests that in both the CBT and haplo-setting, mismatched HLA is a key target for GVL mediators. Loss of class I HLA expression through focal genetic deletions has also been described following matched allogeneic HSCT (24). In this setting, loss of HLA is likely to prevent presentation of important peptides involved in the GVL effect, for example, minor histocompatibility antigens (miHAs). These peptides are presented by major HLA molecules and differ between donor and recipient due to genetic polymorphisms meaning they can be recognized by engrafting T-cells and used to elicit a GVL effect (25, 26). Leukemia-associated antigens, expressed solely on leukemic cells and not normal tissues, are another potential target for GVL. There has been much work looking at identifying such antigens in order to generate effective, specific antigen-directed immunotherapy (27). Early clinical studies demonstrating that recipients of syngeneic HSCT have a higher incidence of relapse compared to allogeneic HSCT, suggest that it is not solely leukemia-associated antigens involved in the GVL effect, however, and a difference between donor and recipient is required (28). In double CBT (dCBT), in which two cord blood units are infused simultaneously in order to overcome limitations of cell dose, one unit asserts dominance over the other to become the engrafting unit. Higher CD3+ and naïve CD8+ T-cell content of the unit is positively associated with unit dominance in which one rejects the other (29). NK cell alloreactivity is also important in GVL, eliminating leukemia whilst protecting against GVHD (30). Following allogeneic transplant, development of antibodies against specific miHAs is positively correlated with survival, indicating a role of B-cells in the GVL effect also (31).
Cord blood transplantation
The first successful CBT was performed in 1988 for a patient with a diagnosis of Fanconi's anemia (32). Today, there are cord blood banks situated in many countries across the globe with over 800,000 estimated cord blood units stored worldwide in public banks and over 4 million privately stored (33). It is feasible and practicable to collect and store cord blood stem cells through cryopreservation without deleterious effect on their viability (2). This means that cord blood has the advantage of being readily available without the need for stem cell harvest from an adult or sibling donor, which can dramatically reduce donor search time and procurement of stem cells for transplant from 2 to 4 months for bone marrow (BM) or peripheral blood stem cells (PBSCs) to as little as 2 weeks for cord blood (34, 35). This can have important clinical consequences for those patients with high-risk malignancies. In addition to this, cord blood registries are able to provide donors from a larger selection of ethnic backgrounds, which allows those groups who may have previously struggled to find an appropriately matched unrelated donor to be eligible for transplant (1). Despite this, reported rates of cord blood use for allogeneic transplant have been steadily declining with the rise of haploidentical transplants as an alternative option for those without a related or unrelated fully matched donor (33, 36, 37).
Outcomes after cord blood transplantation for acute leukemia
The greatest risk to survival in acute leukemia is relapse. In those patients with residual disease pre-transplant and highest disease risk, use of CBT reduces relapse rates compared to other stem cell sources (3, 4). Superiority of CBT to other cell sources in preventing relapse has been replicated in multiple studies in both adults and children (3, 4, 38–40). In 2007, Eapen et al. showed in a retrospective, registry study, that in children with acute leukemia there was significantly reduced relapse risk following a 5/8 HLA-mismatched CBT compared to BMT (relative risk 0.54; p = 0.0045) (20). Milano et al., reported in 2016, significant superiority of CBT in preventing relapse when compared to MUD and mismatched unrelated donor (MMUD) stem cell sources, in adults with acute leukemia and residual disease pre-transplant, (hazard ratio in the HLA-mismatched group, 3.01; p = 0.02 and hazard ratio in the HLA-matched group, 2.92; p = 0.007) (4). This translated into improved overall survival for CBT recipients with a higher risk of death in the MUD and MMUD groups (hazard ratio in the HLA-mismatched group, 2.92; p = 0.001 and hazard ratio in the HLA-matched group, 1.69; p = 0.08) (4). For those patients without residual disease the benefit from CBT was less evident with lower hazard ratios (hazard ratio in the HLA-mismatched group, 1.36; p = 0.3 and hazard ratio in the HLA-matched group, 0.78; p = 0.33). Ando et al. showed improved 2-year overall survival with CBT compared to BMT and PBSCT, in adults with acute leukemia regardless of disease status pre-transplant (76.4% vs. 62% and 67.2%; p = 0.021) (40). In a large, multi-center, retrospective review of pediatric patients with acute myeloid leukemia (AML), Horgan et al. reported dramatically reduced incidence of relapse at 2-years with CBT in comparison to other cell sources in those patients with detectable MRD (36.2% vs. 66.2%; hazard ratio 0.46; p = 0.007). This promoted improved disease-free survival in the cord blood group (50% vs. 21%; hazard ratio 0.55; p = 0.017) (3). Excellent outcomes have also been reported by Barker et al., in adults using dCBT with 3-year OS and progression-free survival (PFS) at 82% and 76% respectively (41). These results taken together indicate that CBT produces a significant GVL effect, which translates into better relapse and leukemia-free survival outcomes for patients, particularly in those with high-risk disease.
Transplant-related mortality including infectious complications
Historically, CBT has been associated with a higher incidence of TRM and infectious complications in conjunction with delayed neutrophil and platelet engraftment (8, 20). Mortality during CBT in children has reduced over time (42). Early registry-based studies performed by Eapen et al. in both children and adults, described an increased risk of TRM using CBT compared to BM grafts (20, 43). For children receiving CBT with two-allelic mismatches the risk of TRM was greater than matched BM (relative risk 2.31; p = 0.003) and this risk was also evident with one-allelic mismatch (relative risk 1.88; p = 0.0455) (20). In adults receiving 4–6/6 CBT there was also increased TRM seen in comparison to fully-matched BM (relative risk 1.69; p < 0.01) and PBSC (relative risk 1.62; p < 0.01) (43). In comparison to 1 allele mismatched BM and PBSC grafts TRM was similar. In another study from 2001, Rocha et al. reported increased early TRM within the first 100 days post-transplant in patients receiving CBT with ATG serotherapy compared to BMT (hazard ratio 2.13; p < 0.001) (8). Increased incidence of early TRM with CBT has also been described by Weisdorf et al. within the first 3-months post-transplant (hazard ratio 2.83; p < 0.0001) but beyond 3-months TRM rates were similar between CBT and BM groups (hazard ratio 1.00; p = 0.99) (44). Konuma et al. examined outcomes of patients aged 55 and above receiving CBT without serotherapy against BM and PBSC recipients, finding reduced rates of TRM in the latter groups (hazard ratio 0.61; p < 0.001 and 0.63; p < 0.001 respectively). This has also been reported in children receiving T-replete CBT for myeloid malignancy with an increased rate of TRM compared to a comparator arm (hazard ratio 2.04; p = 0.042) (3). Other studies, however, have produced conflicting data with no significant increase in TRM detected with CBT (38, 40, 45–48). Heterogeneity of patient populations, disease groups, conditioning regimens, particularly the use of T-depleting serotherapy and outcome measures confounds direct comparison of these studies. TRM is a significant consideration in CBT and needs to be considered on the basis of risk to the individual patient and the risk of their disease.
The incidence of primary graft failure following CBT is around 11%–12% in adults and children, compared to 5%–6% with HLA-matched BM and PBSC grafts (49, 50). The risk of graft failure is lower in those patients undergoing transplantation for hematological malignancy (50). In CBT, for deaths primarily attributed to graft failure the TNC dose received is predictive (RR 0.4 for increasing dose; p < 0.001) but level of HLA-mismatch is not (51). Fully-HLA matched CBT is associated with improved neutrophil engraftment (relative risk 1.8; p < 0.001) although there is no significant differences between recipients of grafts with 1- or 2-HLA mismatches (relative risk 1.0; p = 0.896). Patients can be successfully salvaged following graft failure with a second allogeneic HSCT (49, 52, 53).
Infection is a large contributor to non-relapse mortality in all HSCTs including CBT. Viruses in particular can create a higher burden of morbidity and mortality in CBT (54). Members of the herpes virus family including cytomegalovirus (CMV), varicella zoster virus (VZV), human herpes virus 6 (HHV6) have been shown to have higher incidence with CBT (55–57). There are low rates of viral transmission with CBT due to screening, therefore this represents reactivation rather than primary infection. CMV can require longer duration of treatment with CBT (57). Risks from viruses can be mitigated, however, through routine testing, prophylaxis and pre-emptive treatment upon identification and this is an evolving area with improvements in supportive care and new therapies (58, 59). For example use of letermovir for CMV reactivation, has shown promising results that may translate into better outcomes in pediatric CBT in the future (59). The use of anti-thymocyte globulin (ATG) serotherapy can negatively impact infection rates in CBT and development of T-cell responses to common viral and bacterial pathogens (60, 61). In one study, ATG use in CBT was significantly associated with increased risk of CMV, EBV and adenovirus viraemia and death from viral infections (61). This occurred in conjunction with delayed immune reconstitution. Omitting T-cell depleting serotherapy in malignancy, may therefore improve both relapse and non-relapse mortality (62, 63). In adults, particularly elderly patients or those with multiple co-morbidities, reduced intensity conditioning (RIC) regimens used in conjunction with CBT can allow the graft itself to drive engraftment and GVL (64–66). Overall survival in these patients is comparable to those receiving myeloablative conditioning (MAC) regimens, although reduced non-relapse mortality is offset by increased risk of relapse in some studies (64). Other complications associated with CBT include autoimmune cytopenia and gastrointestinal complications including the cord colitis phenomenon (67, 68).
GVHD and tolerance of HLA-mismatch
One advantage of cord blood is its greater tolerance of HLA-mismatch. Outcomes in children with acute leukemia following CBT with mismatch at one or two alleles are at least equivalent to fully matched unrelated donor transplants despite HLA disparity (20, 45). The important composite end point of GVHD-free, relapse-free survival is superior in CBT recipients compared to other stem cell sources (3, 39). Across all HSCTs, the impact of HLA-mismatch on survival also appears to be less pronounced if there is a higher risk of the disease (69).
HLA-mismatch can result in better outcomes when transplanting for malignancy. Use of HLA-mismatch in CBT correlates with reduced relapse risk in both children and adults (20, 21). Sanz et al. demonstrated in adults with AML, that an HLA-mismatch of 2 or more alleles was associated with a lower 5-year incidence of relapse (without an increase in TRM Mismatch ≥2 22%; mismatch <2 44%; p = 0.04) (21). Yokoyama et al. further examined the relationship between HLA-mismatch and outcomes in CBT for acute leukemia, and reported inferior survival only in children receiving CBT with 4 or more allelic mismatches (hazard ratio 2.03; p = 0.011) (22). Overall survival was comparable between all other groups. In addition to this, a significantly higher incidence of relapse was noted in adults who had received a fully HLA-matched 8/8 CBT (hazard ratio 1.53; p = 0.0037). Altogether these are important observations that greater HLA-mismatch can be tolerated by CBT and that this could mediate greater GVL effect without excessive TRM.
The incidence of grade II-IV acute GVHD is higher in CBT than for MSD transplants, reported at around 35%–50% (40, 44, 45, 70). Although there is variation within the literature, the risk of grade II-IV acute GVHD is similar between CBT and MUD transplants if bone marrow is used as the cell source, but lower in CBT if PBSCs are utilized (20, 40, 44). CBT confers lower risk of acute GVHD than MMUD transplants (39, 43). There is reduced risk of severe grade III-IV acute GVHD with CBT compared to both MUD and MMUD transplants (39, 40, 48). Despite the high incidence of acute GVHD with CBT this does not translate into increased rates of chronic GVHD. The risk of developing chronic GVHD is lower in CBT than both MUD and MMUD transplants using both BM and PBSC cell sources (39, 40, 43–45, 70). The incidence of chronic GVHD using CBT is between 5%–28%, compared to 44%–53% for MUD transplants (3, 40, 44). Haploidentical transplantation is another alternative donor stem cell source option, often considered in place of CBT. Lower chronic GVHD rates are seen consistently with CBT in comparison to haploidentical transplantation (71, 72). Table 1 summarizes the literature on acute and chronic GVHD based on donor type and cell source.
There is increased risk of development of chronic GVHD in adults compared to children receiving CBT (relative risk 5.7; p < 0.05) as well as BMT (relative risk 4.8; p < 0.05) and PBSCT (relative risk 10.0; p < 0.05) (74, 75). For children receiving MSD BMT, there is reduced incidence of grade II-IV acute and chronic GVHD in younger patients aged 2–12 years, than those older patients aged 13–18 years (76). This is not seen in CBT with similar rates of acute and chronic GVHD across all age groups of children (77).
It is important to consider when comparing CBT with MSD or MUD transplants that CBT will be HLA-matched at 5 or more alleles, rather than fully HLA-matched at 8 or 10 alleles as when using adult donors, and therefore more GVHD may be expected. In addition to this, the presence of GVHD is associated with reduced risk of leukemia relapse due to the corresponding GVL effect (13, 14). Conversely, the management of severe grade III-IV acute GVHD includes corticosteroids and other immunosuppressive agents (78). Prolonged use of immune suppression may contribute to increased risk of relapse, and its rapid withdrawal can be effective in the management of early relapse (78–80). GVHD itself can also negatively impact immune reconstitution, particularly thymopoiesis (81). Naïve T-cells induce potent alloreactive responses in xenograft studies, and there is clinical data to suggest that graft depletion of naïve T-cells reduces the severity of GVHD (82, 83). A peak of activated CD8+ T-cells expressing activation marker CD38 in peripheral blood has been associated with development of acute GVHD (84). This implies that high numbers of naïve T-cells transferred in cord blood grafts, differentiating into effectors in response to alloantigen could drive GVHD (85).
Research in the field of GVHD biomarkers is rapidly developing, to inform both diagnosis and prognosis of the condition (86). ST2 is one such biomarker that has been associated with development of acute GVHD after day 28 in CBT (87). Analysis of cell-signaling in the pathophysiology of GVHD has highlighted the importance of the rat sarcoma/ mitogen-activated protein kinase kinase/ extracellular receptor kinase (RAS/MEK/ERK) pathways in alloreactive T-cells. Detection of higher levels of phosphorylation of the ERK1/2 pathway in CD4+ T-cells has been described as a biomarker for the development of acute GVHD (88), MEK inhibitors have additionally been shown to preferentially inhibit cytokine production in naïve alloreactive T-cells (89). In the future, stratification of patients on the basis of biomarkers could facilitate decisions around therapeutic interventions for GVHD, allowing GVL to be maximized in those at low risk but earlier intervention in those at high risk of severe GVHD, reducing the burden of TRM in CBT.
The clinical characteristics of CBT for leukemia are summarized in Figure 1. These clinical findings suggest key differences in the immunology of cord blood, and in particular T-cell biology, when compared to adult peripheral blood or bone marrow. These differences are responsible for the observed improved relapse rates, greater tolerance of HLA-mismatch and reduced rates of chronic GVHD. It also highlights the importance of improving the understanding and application of supportive care for patients undergoing CBT, to reduce the burden of TRM and further improve survival outcomes.
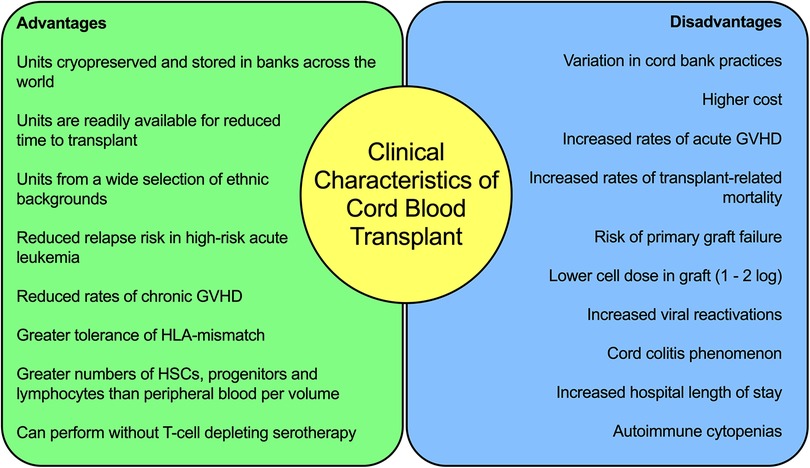
Figure 1. Summary of the clinical characteristics of cord blood transplantation for acute leukaemia.
Clinical protocol for cord blood transplantation for pediatric acute leukemia
Our center is a large children's cord blood transplant center. In AML that is refractory to chemotherapy or relapsed, our first choice would be to select to use an unrelated cord blood donor without T-depleting serotherapy due to the reduced risk of relapse observed by our group and others in this high-risk cohort of patients (3, 4). We recognize the increased procedure-related risk with performing a mismatched unrelated CBT in comparison to a MUD transplant, but with higher risk myeloid leukemia the better associated disease outcomes overcome this consideration (90). Recent American Society for Transplantation and Cellular Therapy guidelines suggest that selection of less well-matched units could be considered for those patients with hematological malignancy (91).
We would consider refractory T-cell leukemia, mixed phenotype acute leukemia and refractory infant leukemia as similar to high-risk myeloid malignancy (90). Our conditioning regimen would typically include myeloablative busulfan if a first allogeneic transplant, without serotherapy. GVHD prophylaxis would include ciclosporin and mycophenolate mofetil, which we would aim to wean early in the absence of GVHD. Single cord blood unit selection follows a nationally defined protocol (92).
In children with relapsed leukemia after an earlier transplant procedure, we have used experimental procedures to augment GVL. This has included our use of granulocyte transfusions in conjunction with T-replete CBT to promote CD8+ T-cell expansion (93).
Cord blood graft composition
Cord blood grafts are relatively enriched for hematopoietic stem cells (HSCs) when compared to BM and in particular PBSC grafts (94). Clinical cord blood units for transplant, however, usually contain 1–2 log lower cell dose than those obtained from peripheral blood or bone marrow donors so the actual number of transferred cells is usually smaller (95). Cord blood HSCs are of a more primitive phenotype with reduced CD38 expression (96). These stem cells have high capability for self-renewal and proliferation (96, 97). Cord blood also contains higher numbers of myeloid and lymphoid progenitors with high replication potential (98, 99). Analysis of lymphocyte subsets also shows higher absolute numbers of T, B and NK cells within a given volume of cord blood when compared to adult peripheral blood samples (100).
Lymphocyte subsets within cord blood
Lymphocyte subsets within cord blood differ from those in adult peripheral blood both in number and phenotype. Within the CD3+ compartment of cord blood there is a higher proportion of CD4+ to CD8+ T-cells when compared to those in adult peripheral blood (100, 101). Cord blood T-cells consist predominantly of the naïve (CD45RA+/CD45RO−) phenotype, whilst adult peripheral blood is mainly comprised of memory (CD45RA−/CD45RO+) T-cells (100). Cytotoxic CD8+ T-cell populations are absent in cord blood with lower numbers of effector T-cells and those expressing activation markers such as HLA-DR (100). Whilst naïve T-cells are the most numerous subset in cord blood, some antigen-experienced T-cells are also present. The most notable of these includes those specific for maternal minor histocompatibility antigens (102).
Gamma delta (γδ) T-cells are a distinct subset of T lymphocytes defined by their expression of γδ T-cell receptors. They have effector capabilities and can promote inflammation (103). In peripheral blood, they make up only a small proportion of around 1%–5% of all T-cells with conventional αβ T-cells comprising the majority of all those in circulation (104). They are abundant at barrier sites. Absolute numbers of γδ T-cells are negligible in cord blood when compared to adult peripheral blood (100, 105, 106). Cord blood γδ T-cells are primarily of the Vδ1 subtype and have a naïve phenotype in comparison to Vγ9Vδ2 T-cells, which are the most numerous in adult peripheral blood (107, 108). The small number of Vγ9Vδ2 T-cells within cord blood are functionally immature (109). Cord blood γδ T-cells, however, have a highly diverse polyclonal repertoire (107, 108, 110). Receptor diversity is increasingly restricted with age and very limited in adult γδ T-cells (107, 108). In vitro, cord blood γδ T-cells readily expand and differentiate, becoming functionally cytotoxic (108). These characteristics in combination, mean that cord blood γδ T-cells are being further investigated for use in cancer immunotherapy (108).
Invariant Natural Killer T (iNKT) cells are a specialized subset of T-cells that crossover between innate and adaptive immunity (111). They are defined by their restricted TCR that can solely recognize lipid antigen presented by CD1d (111). Numbers remain stable throughout life from birth to adulthood but are highly variable between individuals (111, 112). They comprise a comparatively small proportion of all T-cells at approximately 0.1%–0.2% on average in both cord and peripheral blood. In murine HSCT models, adoptive transfer of iNKT cells can protect against GVHD, whilst preserving the GVL effect through inhibition of alloreactive T-cell expansion and activation and induction of donor regulatory T-cell expansion (113, 114). The scarcity of iNKT cells in peripheral blood compounds their utility in clinical applications, however. An early study of iNKT cells engineered from cord blood HSCs has shown promising pre-clinical results in amelioration of GVHD whilst preserving GVL (115).
NK cells are lymphocytes that form part of the innate immune system. They elicit anti-cancer effects through release of cytotoxic granules as well as activation of apoptotic pathways and can produce inflammatory cytokines (116). Cord blood grafts are relatively enriched for NK cells, which constitute around 20%–30% of the lymphocyte population in comparison to 10%–15% in peripheral blood (101). NK cells can be divided into two sub-populations, which are CD56dimCD16bright and CD56brightCD16dim (116, 117). These sub-populations have distinct phenotypic properties with CD56dimCD16bright NK cells mediating cytotoxicity through granzyme B and perforin production and CD56brightCD16dim NK cells mainly producing inflammatory cytokines such as IFNγ and TNFα (118). The predominant population of NK cells in cord blood are CD56brightCD16dim with reduced cytotoxic capabilities and a more immature phenotype (119, 120). These cord blood NK cells have reduced expression of granzyme B and killer immunoglobulin-like receptors (KIR) and higher expression of the inhibitory receptor NKG2A (120, 121). Within cord blood, however, a distinct NK cell progenitor exists that can readily differentiate into functional, mature NK cells with the ability to produce IFN-γ, TNFα, IL-10, and GM-CSF and lyse cells in vitro (122). Cord blood NK cells also express higher amounts of CXCR4 suggesting superior bone marrow homing when compared to peripheral blood NK cells (120). Cord blood is a rich source of NK cells for use in immunotherapy, with encouraging early results (123).
B-cells are immunoglobulin producing lymphocytes that play a very important role in adaptive immunity. B-cells make up a larger proportion of the lymphocyte population in cord blood compared to peripheral blood, at around 15%–20% (101). Characteristics of B-cells differ between cord and peripheral adult blood. Cord blood contains a higher percentage of B-cell progenitors in comparison to adult and pediatric bone marrow (124). Most B-cells within cord blood do exhibit the same CD20+/CD5− phenotype as B-cells found in adult peripheral blood (125, 126). There is, however, a distinct CD20+/CD5+ population of B-cells within cord blood (127). These CD5+ B-cells are specifically derived from fetal and neonatal progenitors and are characterized by IgM production that is polyreactive and indeed autoreactive with low affinity binding (126, 128). Additionally, cord blood B-cells produce accelerated responses to stimulation with distinct transcriptional pathways (125).
Cord T-cell biology
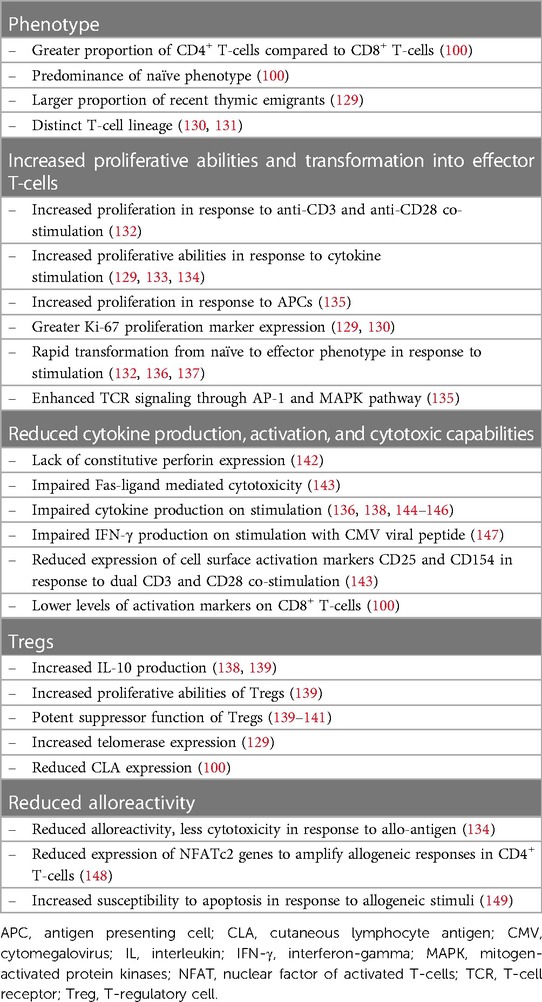
Cord blood and adult peripheral blood T-cells have different functional properties, which are summarized in Table 2.
In vitro studies show that cord blood T-cells have a greatly increased capacity for proliferation in response to multiple stimuli and lymphopenia. Cord blood T-cells proliferate more rapidly than those in adult peripheral blood in response to cytokine stimulation (129, 133, 134). In assays analyzing these responses, IL-7 produced greater proliferation in cord blood T-cells, particularly the CD4+ population whereas IL-15 stimulated the greatest proliferation in cord blood CD8+ T-cells (129). Clinically in BMT, low serum levels of IL-15 are associated with an increased risk of post-transplant relapse in AML indicating its potential role in the GVL effect, perhaps through induction of CD8+ T-cell responses (150). CD8+ T-cells in neonates, also exhibit an intrinsic ability to rapidly proliferate in response to in response to CD3 and CD28 co-stimulation (130, 132). Cord blood CD4+ T-cells proliferate more than adult peripheral blood T-cells when stimulated with self-APCs (135). This occurs in conjunction with enhanced TCR signaling and upregulation of the MAPK signaling cell-cycle pathway (135). This greater proliferative potential is thought to arise, in part, secondary to distinct lineages of fetal and adult progenitor cells and a transcriptional program geared towards lymphopenia-induced proliferation (130, 131). In xenograft studies, greater levels of expression of the proliferation marker Ki-67 is also seen in both cord blood CD4+ and CD8+ T-cells, in comparison to those in adult peripheral blood. This indicates that there is also a higher proportion of proliferating cord blood T-cells in vivo (129, 130). Cord blood CD8+ T-cells in particular have higher levels of Ki-67 expression (129). Additionally, spontaneous telomerase expression occurs in cord blood T-cells to allow proliferation without telomere shortening, aiding longevity of the T-cell progeny (129).
In response to antigenic stimulation, fetal CD8+ T-cells preferentially become terminally differentiated, short lived effectors rather than memory T-cells as seen in adults (130). Naïve CD4+ T-cells in the newborn expressing CD45RA are also able to much more rapidly transform to CD45RO effector memory phenotype on stimulation when compared to naïve adult peripheral blood CD4+ T-cells (136).
CD4+ helper T-cells Th1 and Th2 are broadly defined by their ability to produce IFNγ/TNFα and IL-4/IL-5/IL-13 as well as the transcription factors T-bet and GATA-3, respectively. Cord blood T-cells produce less inflammatory cytokines overall in response to stimulation when compared to adult peripheral blood (129, 144, 145). Both CD4+ and CD8+ subsets produce less IFNγ and TNFα as well as lower levels of IL-2 and IL-4 (136, 144, 145). This includes in response to CMV peptide stimulation (147). Reduced expression of the transcription factor nuclear factor of activated T-cells c2 (NFATc2), which upregulates the expression of many cytokines involved in T-cell inflammatory responses, has been hypothesized as a mechanism for this observation (148). Low production of IFNγ specifically by CD4+ T-cells in the neonate, has been associated with hypermethylation of the IFNγ promoter (151). Neonatal naïve T-cells that have been allowed to mature in vitro, however, acquire the ability to secrete IFNγ and IL-2 on secondary stimulation (136).
Cord blood T-cells display less alloreactivity when compared to peripheral blood T-cells, which may contribute to the reduced rates of GVHD seen clinically (134). Cord blood T-cells more readily undergo apoptosis in response to alloantigen (143, 149). Levels of constitutive perforin expression in cord blood CD8+ T-cells are low in comparison to those in adult peripheral blood, and cytotoxicity mediated through the FAS-ligand pathway is impaired (142, 143). Cord blood T-cells co-cultured with immature adult dendritic cells produced greater IL-10 and reduced IFNγ than adult T-cells, which is a regulatory cytokine profile (138). Cutaneous lymphoid antigen (CLA) is expressed on T-cells involved in migration to areas of skin inflammation. There is no expression of CLA on cord blood T-cells, which could be significant in the lower incidence of chronic skin GVHD with CBT (100).
Cord blood is a rich source of CD4+/CD25+/FoxP3+ regulatory T-cells (Tregs), which are inherently programmed to immune tolerance with low levels of immunological memory (140). Cord blood Tregs have strong suppressive capabilities against alloreactive T-cells with high levels of IL-10 production in vitro, following activation and expansion (139, 141). This suppressive effect is potent and occurs consistently, inhibiting production of T-cell activation-dependent cytokines such as IL-2, IFN-γ, TNFα and GM-CSF in mixed lymphocyte reaction assays (141). In comparison to Tregs isolated from peripheral blood, cord blood Tregs also have much greater capacity for expansion with higher Ki-67 expression and a gene expression profile that favors proliferation and chromatin modification (139, 152). Cord blood Tregs also express higher levels of the chemokines CCR9 and CCR7 than their peripheral blood counterparts, which regulate trafficking to the gut and lymph nodes respectively (152). The potential of cord-derived Tregs to prevent GVHD whilst preserving the GVL effect has been established in xenograft models (153). Further work has optimized the purification and expansion process to allow for use in clinical settings (154, 155). There are promising results from early clinical trials using cord-derived Tregs as preventative therapy for GVHD with reduced rates of acute and chronic GVHD without an increase in relapse (154, 156).
Cord blood antitumor effects in xenograft models
The superior clinical outcomes in malignancy have been further supported in pre-clinical studies, investigating cord blood T-cells as the primary mediators of enhanced antitumor activity. In xenograft models, very successful antitumor effects of cord blood T-cells have been demonstrated (137, 157). In one study, cord blood mononuclear cells induced dramatic remission in mice with lung and cervical cancers, with high levels of tumor infiltration by CD3+ T-cells shown (157). Further in vitro assays demonstrated tumor specific antigen cytotoxicity. Comparative analysis of cord blood and peripheral blood T-cells, also shows that cord blood T-cells produce a greatly superior antitumor response (137). In an Epstein-Barr virus (EBV)-driven human B-cell lymphoblastoid tumor mouse model, cord blood T-cells had enhanced antitumor effect with rapid infiltration and induction of remission. Cord blood T-cells expressed enhanced levels of the tumor-homing receptor CCR7. In contrast, peripheral blood T-cells were slower to infiltrate and had reduced antitumor activity with tumor progression. Analysis of the tumor infiltrating cord blood lymphocytes showed them to be primarily CD8+ T-cells that had converted from naïve to central and effector memory phenotype. Importantly, these cord blood T-cells were able to mediate a greatly augmented GVL effect without exerting xenoreactivity, mirroring the clinical picture seen with CBT. Effector memory CD4+ T-cells have also been shown to exert effective GVL effects without GVHD in the mismatched HSCT setting in mouse models of chronic myelogenous leukemia (158).
In summary, T-cells contained within a cord blood graft are a distinct entity from those derived from peripheral blood and bone marrow. They are predominantly CD4+ T-cells and both CD4+ and CD8+ T-cells are naïve (100). These subsets possess the ability, however, to quickly transform into effector T-cells with cytotoxic potential (132, 137). Both CD4+ and CD8+ cord blood T-cells are capable of rapid proliferation in response to lymphopenia, cytokines and APCs, more so than adult T-cells. There is some suggestion, however, that there is impairment in the production of inflammatory cytokines and mediation of cytotoxicity through certain cellular pathways (129, 143). There is, therefore, much more to be understood about how an enhanced GVL effect is mediated during cord blood transplantation. Cord blood CD8+ T-cells are endowed with enhanced tumor-homing capabilities due to high levels of CCR7 expression, which in conjunction with greater proliferation may contribute to the superior GVL effect (135, 137). Additionally, the primitive nature of cord blood itself could potentially reduce susceptibility to inhibitory co-signaling and induction of an exhausted T-cell state (130, 143). There are also differences in clinical characteristics of CBT including greater use of HLA-mismatch and omission of T-cell depleting serotherapy, which likely contribute to the phenomenon seen.
Mechanism of T-cell reconstitution following HSCT
Following transplant conditioning there is a period of profound lymphopenia and aplasia. T-cell reconstitution occurs through two separate pathways. In the first, there is thymus-independent peripheral expansion of T-cells transplanted within the graft or of residual recipient T-cells that have escaped transplant conditioning, driven by exposure to antigen and cytokines. This is the pathway that predominates in the early post-transplant phase (159). Distinct from this, restoration of thymopoiesis from hematopoietic progenitors occurs in parallel but at a much slower rate. This thymus-dependent pathway utilizes progenitors transferred within the graft or those derived from donor hematopoietic stem cells to produce naïve T-cells with a greater TCR repertoire, thus promoting longevity of T-cell reconstitution with increased functionality (81, 160).
Within a lymphopenic environment, homeostatic proliferation of naïve T-cells occurs during which they acquire the phenotype and functional properties of effector T-cells (161, 162). Interaction of cells with peptide bound to MHC molecules and IL-7 is essential to the survival and expansion of naïve CD4+ and CD8+ T-cell populations (159, 163). With lymphopenia there is reduced competition for APCs and cytokines allowing greater expansion to occur.
Reliance on the thymus-independent pathway alone for T-cell reconstitution can result in a reduced TCR repertoire that is skewed and oligoclonal (147). Restoration of thymopoiesis is required for the formulation of de novo naïve T-cells following HSCT. This is necessary to restore a complete and long-lasting T-cell compartment capable of responding to a wide range of antigens (147, 159, 164). Thymopoiesis is the process through which progenitors derived from HSCs in the bone marrow (or in the context of transplant, infused with the graft) proliferate and mature within the thymus and become committed to the T lineage. It is in this manner that TCR specificity is generated (159). One technique to assess reestablishment of thymopoiesis, is detection of T-cell receptor rearrangement excision circles (TRECs), which are extra-chromosomal fragments of DNA that are generated as by-products of the TCR gene rearrangement process within the thymus, and can be used to identify recent thymic emigrants (RTEs) (164). They exist stably within the cytoplasm but are not replicated during cell division, which means they can be measured to quantify functionality of the thymus (159).
T-cell reconstitution after cord blood transplantation
Cord blood T-cell reconstitution is CD4+ biased, which differs from BM and PBSC cell sources in which CD8+ T-cells predominate (135, 165–170). Cord blood transplant without T-cell depleting serotherapy has been shown to result in rapid CD4+ reconstitution in both adults and children (135, 165, 171). This is initially attained through the thymus-independent pathway based on the low number of detectable TRECs and may be secondary to the increased proliferative capabilities of cord blood CD4+ T-cells in lymphopenic environments (135). Successful CD8+ T-cell reconstitution can be slower in cord blood transplant recipients than BM and PBSCs (166, 171–173). The most prevalent early reconstituting CD4+ and CD8+ cord blood T-cells are effector memory, with an increase in naïve T-cells occurring from 6 to 9 months suggesting thymus-dependent recovery at this time point (171). Further phenotypic analysis of early reconstituting CD8+ T-cells in CBT suggests significantly greater proportions of highly activated effector memory cells expressing CD38 than in PBSCT recipients (173). Marked CD8+ T-cell expansion can occur with CMV reactivation following CBT (171). A polyclonal TCR repertoire with normal spectratyping has been observed early in some patients within the first month post-CBT without T-cell depleting serotherapy (165). Conversely, delayed restoration of TCR diversity has been reported in the ATG setting (81).
There is efficient recovery of thymopoiesis seen with cord blood transplant in the T-replete setting with TRECs detectable within the first 3 months post-transplant and reaching normal levels within 6 months (165, 174). This is initially comparable to haploidentical and MSD transplants but is in fact superior in cord blood at 2-years post-transplant with higher TREC numbers and greater TCR repertoire indicating efficient regeneration of thymic function with CBT (81, 174). This occurs despite the much lower cell dose contained within cord blood units than BM or PBSC grafts. Higher numbers of lymphomyeloid progenitors (LMPs) contained within cord blood grafts may contribute to this finding, but it may also suggest that cord blood LMPs themselves are superior in their ability to reconstitute thymopoiesis in comparison to those in other graft sources (160). With use of ATG serotherapy, however, CD4+ reconstitution following cord blood transplant is significantly delayed (147, 166, 168, 175, 176). ATG serum concentration modelling during HSCT shows cord blood CD4+ T-cells are particularly susceptible to ATG exposure even at low levels (62, 63, 177). Further research is warranted to define when polarization into CD4+ T helper cell subsets occurs following CBT and how this may differ from other stem cell sources.
Sustained CD4+ reconstitution has been associated with improved OS children with leukemia, independent of cell source (178). In CBT specifically, CD4+ T-cell reconstitution has been positively associated with improved overall and leukemia-free survival in children and adults (62, 171). Early post-transplant, higher numbers of cytotoxic, effector CD8+ T-cells are also associated with improved OS and reduced NRM (40).
NK cell and B-cell reconstitution after cord blood transplantation
NK cells are the first lymphocyte to engraft following CBT, achieving normal levels within the first month (166, 175, 179, 180). In the post-transplant period, T-cell lymphopenia is associated with a compensatory increase in NK cells to above physiological levels (147). NK cell reconstitution occurs more rapidly with CBT than PBSC or BM cell sources (167, 178, 179, 181). Despite their initially immature phenotype, NK cells can quickly attain their innate cytolytic abilities following CBT meaning they are crucial in exerting a GVL effect in the early post-transplant period for acute leukemia (116, 167). NK cells in CBT recipients show significantly higher expression of activation markers CD69 and NKP30 in the first few weeks, than those receiving PBSCs (173).
Significant B-cell recovery starts around 3–4 months after HSCT reaching normal levels by 6–12 months (168, 182). Adequate immunoglobulin production, however, can take several years to be achieved (183). B-cell recovery after CBT is comparatively enhanced and occurs much earlier than with BMT (166, 167, 178, 184). Similarly to NK cells, B-cell numbers have also been seen to reach higher than physiological levels following CBT, perhaps as a compensatory measure for T-cell lymphopenia (147). Immunoglobulin levels in response to commonly encountered antigens approach normal levels within the first year of CBT (95, 170). In adults, IgM recovery is comparable between dCBT and PBSCT recipients. Normal levels of IgG, however, occur at 5–6 months following dCBT, which is not seen at 12 months following PBSCT (185). Higher numbers of naïve B-cells expressing CD127 are seen with CBT recipients (172). Stromal cells contribute to B-cell development and therefore the greater numbers of mesenchymal stem cells found in in cord blood might contribute to this quick recovery and early functionality (96, 186).
Future directions to augment GVL effect with cord blood transplantation
We have established that T-cells are important mediators in GVL and that CBT is associated with reduced relapse rates, secondary to the superior antitumor properties of cord blood T-cells. The exact mechanism of this is yet to be elucidated, but may involve a combination of factors including the differing composition of cord blood grafts with predominance of naïve T-cells; the innate biology of cord blood T-cells themselves, particularly their ability to proliferate and transform into short-lived effectors; their greater tolerance of HLA-mismatch, allowing this to be utilized in conjunction with omission of T-cell depleting serotherapy in conditioning regimens; as well as the differing pattern of immune reconstitution following CBT, with earlier restoration of thymopoiesis due to greater numbers of LMPs. Therefore, future directions must look at further defining and augmenting the GVL effect of CBT.
Our group previously described significant T-cell expansion driven by granulocyte transfusions in conjunction with CBT (187). This expansion was predominantly of CD8+ T-cells and importantly was associated with prolonged remission in patients with acute leukemia. On the basis of these findings, we have conducted an early phase I/II study in which we have trialed the use of third-party, pooled granulocyte transfusions in conjunction with CBT to promote CD8+ expansion in children with high-risk myeloid malignancy (93). We have reported significant and reproducible T-cell expansion occurring in association with a cytokine release syndrome, in all patients excluding one with primary graft failure. Expanded T-cells were CD8+ and effector memory or terminally differentiated effector memory (TEMRA) phenotype and exhibited canonical markers of activation and cytotoxicity. The phenotype of expanded T-cells appears to be similar to that of the highly effective tumor-eradicating T-cells from xenograft models (137). In this high-risk cohort of patients with MRD positive acute leukemia, 90% of patients achieved hematological remission, 80% became MRD negative and 50% of patients are alive and in disease remission with over one year median follow up (93).
Further research into the mechanisms of GVL are necessary to better understand and advance this field. We speculate that cord blood T-cells are biologically distinct from adult T-cells, and that the observed improved GVL without chronic GVHD likely reflects this distinct biology. Understanding this relationship between T-cell biology and clinical outcomes might allow the T-cell responses we have reported, to be improved further yet or re-directed. Specifically identifying targets of cord blood T-cells, separating this entity from GVHD and understanding the role of HLA-mismatch, will mean that T-cell techniques can be honed, and potentially improve patient outcomes.
Universal or “off-the shelf”, allogeneic CAR T-cells are also being trialed in numerous studies, in order to overcome the limitations of using autologous CAR T-cells, which rely upon the functionality of the recipient T-cell pool and can have prolonged manufacture time with high costs (188). Cord blood T-cells could be ideal candidates for this technology due to their naïve phenotype, increased replicative capabilities and reduced alloreactivity (188, 189).
The lack of availability of DLI with CBT has previously been seen as a disadvantage, but with newer methods of T-cell separation, cryopreservation and cord unit expansion, theoretically, cord blood T-cells could be stored for DLI in the future (190, 191). Improvements in supportive care and utilization of targeted or less toxic conditioning regimens could dramatically reduce the burden of TRM associated with CBT. The advent of new treatments for acute GVHD, which may include cord blood-derived Tregs, and anti-viral drugs such as letermovir will be crucial in these efforts (59, 154).
Conclusion
In conclusion, cord blood transplantation offers an enhanced GVL effect that has shown to be particularly effective in difficult-to-treat leukemia, without the risk of chronic GVHD. This effect can be further enhanced through amelioration of the effects of TRM and trials to appropriately risk stratify patients. Cord blood T-cells are a phenotypically distinct entity from those in peripheral adult blood or bone marrow, but the exact mechanisms through which they elicit superior antitumor effects remain to be elucidated. Further research is required but cord blood offers exciting opportunities in the field of cellular immunology and the landscape of CBT is likely to rapidly expand and evolve in coming years, giving rise to novel and innovative treatment modalities.
Author contributions
All authors contributed to the design of the review. RB wrote the initial manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. (2014) 371(4):339–48. doi: 10.1056/NEJMsa1311707
2. Gluckman E. History of cord blood transplantation. Bone Marrow Transplant. (2009) 44(10):621–6. doi: 10.1038/bmt.2009.280
3. Horgan C, Mullanfiroze K, Rauthan A, Patrick K, Butt NA, Mirci-Danicar O, et al. T-replete cord transplants give superior outcomes in high risk and relapsed/refractory paediatric myeloid malignancy. Blood Adv. (2023) 7(10):2155–65. doi: 10.1182/bloodadvances.2022009253
4. Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. (2016) 375(10):944–53. doi: 10.1056/NEJMoa1602074
5. Sweeney C, Vyas P. The graft-versus-leukemia effect in AML. Front Oncol. (2019) 9. doi: 10.3389/fonc.2019.01217
6. Apperley JF, Jones L, Hale G, Waldmann H, Hows J, Rombos Y, et al. Bone marrow transplantation for patients with chronic myeloid leukaemia: T-cell depletion with Campath-1 reduces the incidence of graft-versus-host disease but may increase the risk of leukaemic relapse. Bone Marrow Transplant. (1986) 1(1):53–66.3332120
7. Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. (2001) 98(12):3192–204. doi: 10.1182/blood.V98.12.3192
8. Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. (2001) 97(10):2962–71. doi: 10.1182/blood.V97.10.2962
9. Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. (1990) 75(3):555–62. doi: 10.1182/blood.V75.3.555.555
10. Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. (2000) 96(8):2712–6. doi: 10.1182/blood.V96.8.2712
11. Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia working party. J Clin Oncol. (2007) 25(31):4938–45. doi: 10.1200/JCO.2007.11.6053
12. Orti G, Barba P, Fox L, Salamero O, Bosch F, Valcarcel D. Donor lymphocyte infusions in AML and MDS: enhancing the graft-versus-leukemia effect. Exp Hematol. (2017) 48:1–11. doi: 10.1016/j.exphem.2016.12.004
13. Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the acute leukemia working party of the European group for blood and marrow transplantation. Leukemia. (2012) 26(12):2462–8. doi: 10.1038/leu.2012.135
14. Kato M, Kurata M, Kanda J, Kato K, Tomizawa D, Kudo K, et al. Impact of graft-versus-host disease on relapse and survival after allogeneic stem cell transplantation for pediatric leukemia. Bone Marrow Transplant. (2019) 54(1):68–75. doi: 10.1038/s41409-018-0221-6
15. Valcárcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. (2008) 26(4):577–84. doi: 10.1200/JCO.2007.11.1641
16. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222
17. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. (2015) 385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3
18. Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA Variation and disease. Nat Rev Immunol. (2018) 18(5):325–39. doi: 10.1038/nri.2017.143
19. Matte-Martone C, Liu J, Jain D, McNiff J, Shlomchik WD. CD8+ but Not CD4+ T cells require cognate interactions with target tissues to mediate GVHD across only minor H antigens, whereas both CD4+ and CD8+ T cells require direct leukemic contact to mediate GVL. Blood. (2008) 111(7):3884–92. doi: 10.1182/blood-2007-11-125294
20. Eapen M, Rubinstein P, Zhang M-J, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. (2007) 369(9577):1947–54. doi: 10.1016/S0140-6736(07)60915-5
21. Sanz J, Jaramillo F, Planelles D, Montesinos P, Lorenzo I, Moscardó F, et al. Impact on outcomes of HLA matching by allele-level typing in adults with acute myeloid leukemia undergoing umbilical cord blood transplantation. Biol Blood Marrow Transplant. (2014) 20:106–10. doi: 10.1016/j.bbmt.2013.10.016
22. Yokoyama H, Morishima Y, Fuji S, Uchida N, Takahashi S, Onizuka M, et al. Impact of HLA allele mismatch at HLA-A, -B, -C, and -DRB1 in single cord blood transplantation. Biol Blood Marrow Transplant. (2020) 26(3):519–28. doi: 10.1016/j.bbmt.2019.11.001
23. Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. (2009) 361(5):478–88. doi: 10.1056/NEJMoa0811036
24. Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, et al. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation. Blood Adv. (2019) 3(14):2199–204. doi: 10.1182/bloodadvances.2019000445
25. Griffioen M, van Bergen CA, Falkenburg JH. Autosomal Minor histocompatibility antigens: how genetic variants create diversity in immune targets. Front Immunol. (2016) 7:100. doi: 10.3389/fimmu.2016.00100
26. Summers C, Sheth VS, Bleakley M. Minor histocompatibility antigen-specific T cells. Front Pediatr. (2020) 8. doi: 10.3389/fped.2020.00284
27. Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. (2012) 26(10):2186–96. doi: 10.1038/leu.2012.145
28. Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb H-J, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. (1990) 75(3):555–62. doi: 10.1182/blood.V75.3.555.555
29. Milano F, Heimfeld S, Gooley T, Jinneman J, Nicoud I, Delaney C. Correlation of infused CD3+CD8+ cells with single-donor dominance after double-unit cord blood transplantation. Biol Blood Marrow Transplant. (2013) 19(1):156–60. doi: 10.1016/j.bbmt.2012.09.004
30. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. (2002) 295(5562):2097–100. doi: 10.1126/science.1068440
31. Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. (2005) 105(7):2973–8. doi: 10.1182/blood-2004-09-3660
32. Gluckman E, Broxmeyer HE, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. (1989) 321(17):1174–8. doi: 10.1056/NEJM198910263211707
33. Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry: an evolving treatment landscape. Stem Cells Transl Med. (2018) 7(9):643–50. doi: 10.1002/sctm.17-0244
34. Gupta AO, Wagner JE. Umbilical cord blood transplants: current status and evolving therapies. Front Pediatr. (2020) 8. doi: 10.3389/fped.2020.570282
35. Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. (2002) 8(5):257–60. doi: 10.1053/bbmt.2002.v8.pm12064362
36. Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Cámara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. (2021) 56(7):1651–64. doi: 10.1038/s41409-021-01227-8
37. Ruggeri A. Optimizing cord blood selection. Hematology. (2019) 2019(1):522–31. doi: 10.1182/hematology.2019000056
38. Gutman JA, Leisenring W, Appelbaum FR, Woolfrey AE, Delaney C. Low relapse without excessive transplant-related mortality following myeloablative cord blood transplantation for acute leukemia in complete remission: a matched cohort analysis. Biol Blood Marrow Transplant. (2009) 15(9):1122–9. doi: 10.1016/j.bbmt.2009.05.014
39. Mehta RS, Holtan SG, Wang T, Hemmer MT, Spellman SR, Arora M, et al. GRFS And CRFS in alternative donor hematopoietic cell transplantation for pediatric patients with acute leukemia. Blood Adv. (2019) 3(9):1441–9. doi: 10.1182/bloodadvances.2018030171
40. Ando T, Tachibana T, Tanaka M, Suzuki T, Ishiyama Y, Koyama S, et al. Impact of graft sources on immune reconstitution and survival outcomes following allogeneic stem cell transplantation. Blood Adv. (2020) 4(2):408–19. doi: 10.1182/bloodadvances.2019001021
41. Barker JN, Devlin SM, Naputo KA, Skinner K, Maloy MA, Flynn L, et al. High progression-free survival after intermediate intensity double unit cord blood transplantation in adults. Blood Adv. (2020) 4(23):6064–76. doi: 10.1182/bloodadvances.2020003371
42. Spees LP, Martin PL, Kurtzberg J, Stokhuyzen A, McGill L, Prasad VK, et al. Reduction in mortality after umbilical cord blood transplantation in children over a 20-year period (1995–2014). Biol Blood Marrow Transplant. (2019) 25(4):756–63. doi: 10.1016/j.bbmt.2018.11.018
43. Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang M-J, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. (2010) 11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3
44. Weisdorf D, Eapen M, Ruggeri A, Zhang M-J, Zhong X, Brunstein C, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. (2014) 20(6):816–22. doi: 10.1016/j.bbmt.2014.02.020
45. Keating AK, Langenhorst J, Wagner JE, Page KM, Veys P, Wynn RF, et al. The influence of stem cell source on transplant outcomes for pediatric patients with acute myeloid leukemia. Blood Adv. (2019) 3(7):1118–28. doi: 10.1182/bloodadvances.2018025908
46. Peffault de Latour R, Brunstein CG, Porcher R, Chevallier P, Robin M, Warlick E, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. (2013) 19(9):1355–60. doi: 10.1016/j.bbmt.2013.06.006
47. Shim YJ, Lee JM, Kim HS, Jung N, Lim YT, Yang EJ, et al. Comparison of survival outcome between donor types or stem cell sources for childhood acute myeloid leukemia after allogenic hematopoietic stem cell transplantation: a multicenter retrospective study of study alliance of Yeungnam pediatric hematology-oncology. Pediatr Transplant. (2018) 22(6):e13249. doi: 10.1111/petr.13249
48. Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. (2007) 109(3):1322–30. doi: 10.1182/blood-2006-04-020172
49. Ruggeri A, Labopin M, Sormani MP, Sanz G, Sanz J, Volt F, et al. Engraftment kinetics and graft failure after single umbilical cord blood transplantation using a myeloablative conditioning regimen. Haematologica. (2014) 99(9):1509–15. doi: 10.3324/haematol.2014.109280
50. Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. (2013) 48(4):537–43. doi: 10.1038/bmt.2012.239
51. Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. (2010) 115(9):1843–9. doi: 10.1182/blood-2009-07-231068
52. Baron F, Ruggeri A, Peczynski C, Labopin M, Bourhis J-H, Michallet M, et al. Outcomes of graft failure after umbilical cord blood transplantation in acute leukemia: a study from eurocord and the acute leukemia working party of the EBMT. Bone Marrow Transplant. (2023) 58(8):936–41. doi: 10.1038/s41409-023-02000-9
53. Singh H, Nikiforow S, Li S, Ballen KK, Spitzer TR, Soiffer R, et al. Outcomes and management strategies for graft failure after umbilical cord blood transplantation. Am J Hematol. (2014) 89(12):1097–101. doi: 10.1002/ajh.23845
54. Mehta RS, Bejanyan N, Cao Q, Luo X, Brunstein C, Cooley S, et al. Immune reconstitution after umbilical cord blood versus peripheral blood progenitor cell transplantation in adults following myeloablative conditioning. Blood. (2016) 128(22):2246. doi: 10.1182/blood.V128.22.2246.2246
55. Vandenbosch K, Ovetchkine P, Champagne MA, Haddad E, Alexandrov L, Duval M. Varicella-zoster virus disease is more frequent after cord blood than after bone marrow transplantation. Biol Blood Marrow Transplant. (2008) 14(8):867–71. doi: 10.1016/j.bbmt.2008.05.006
56. Sashihara J, Tanaka-Taya K, Tanaka S, Amo K, Miyagawa H, Hosoi G, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood. (2002) 100(6):2005–11. doi: 10.1182/blood.V100.6.2005
57. Mikulska M, Raiola AM, Bruzzi P, Varaldo R, Annunziata S, Lamparelli T, et al. CMV Infection after transplant from cord blood compared to other alternative donors: the importance of donor-negative CMV serostatus. Biol Blood Marrow Transplant. (2012) 18(1):92–9. doi: 10.1016/j.bbmt.2011.05.015
58. Dumas PY, Ruggeri A, Robin M, Crotta A, Abraham J, Forcade E, et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: a Eurocord and Société Française de Greffe de Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant. (2013) 48(2):253–6. doi: 10.1038/bmt.2012.117
59. Shigle TL, Handy VW, Chemaly RF. Letermovir and its role in the prevention of cytomegalovirus infection in seropositive patients receiving an allogeneic hematopoietic cell transplant. Ther Adv Hematol. (2020) 11:2040620720937150. doi: 10.1177/2040620720937150
60. Saliba RM, Rezvani K, Leen A, Jorgensen J, Shah N, Hosing C, et al. General and virus-specific immune cell reconstitution after double cord blood transplantation. Biol Blood Marrow Transplant. (2015) 21(7):1284–90. doi: 10.1016/j.bbmt.2015.02.017
61. Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. (2014) 123(1):126–32. doi: 10.1182/blood-2013-05-502385
62. Admiraal R, Chiesa R, Lindemans CA, Nierkens S, Bierings MB, Versluijs AB, et al. Leukemia-free survival in myeloid leukemia, but not in lymphoid leukemia, is predicted by early CD4+ reconstitution following unrelated cord blood transplantation in children: a multicenter retrospective cohort analysis. Bone Marrow Transplant. (2016) 51(10):1376–8. doi: 10.1038/bmt.2016.116
63. Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. (2016) 128(23):2734–41. doi: 10.1182/blood-2016-06-721936
64. Baron F, Ruggeri A, Beohou E, Labopin M, Sanz G, Milpied N, et al. RIC Versus MAC UCBT in adults with AML: a report from eurocord, the ALWP and the CTIWP of the EBMT. Oncotarget. (2016) 7(28):43027–38. doi: 10.18632/oncotarget.9599
65. Ponce DM, Sauter C, Devlin S, Lubin M, Gonzales AM, Kernan NA, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. (2013) 19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007
66. Sheth V, Volt F, Sanz J, Clement L, Cornelissen J, Blaise D, et al. Reduced-intensity versus myeloablative conditioning in cord blood transplantation for acute myeloid leukemia (40–60 years) across highly mismatched HLA barriers—on behalf of eurocord and the cellular therapy & immunobiology working party (CTIWP) of EBMT. Biol Blood Marrow Transplant. (2020) 26(11):2098–104. doi: 10.1016/j.bbmt.2020.07.025
67. Sanz J, Arango M, Carpio N, Montesinos P, Moscardó F, Martín G, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant. (2014) 49(8):1084–8. doi: 10.1038/bmt.2014.107
68. Herrera AF, Soriano G, Bellizzi AM, Hornick JL, Ho VT, Ballen KK, et al. Cord colitis syndrome in cord-blood stem-cell transplantation. N Engl J Med. (2011) 365(9):815–24. doi: 10.1056/NEJMoa1104959
69. Crocchiolo R, Ciceri F, Fleischhauer K, Oneto R, Bruno B, Pollichieni S, et al. HLA Matching affects clinical outcome of adult patients undergoing haematopoietic SCT from unrelated donors: a study from the Gruppo Italiano Trapianto di Midollo Osseo and Italian Bone Marrow Donor Registry. Bone Marrow Transplant. (2009) 44(9):571–7. doi: 10.1038/bmt.2009.67
70. Konuma T, Tsukada N, Kanda J, Uchida N, Ohno Y, Miyakoshi S, et al. Comparison of transplant outcomes from matched sibling bone marrow or peripheral blood stem cell and unrelated cord blood in patients 50 years or older. Am J Hematol. (2016) 91(5):E284–92. doi: 10.1002/ajh.24340
71. Konuma T, Kanda J, Yamasaki S, Harada K, Shimomura Y, Terakura S, et al. Single cord blood transplantation versus unmanipulated haploidentical transplantation for adults with acute myeloid leukemia in complete remission. Transplant Cell Ther. (2021) 27(4):334.e1–e11. doi: 10.1016/j.jtct.2021.01.023
72. Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. (2015) 29(9):1891–900. doi: 10.1038/leu.2015.98
73. Wagner JE Jr, Ballen KK, Zhang M-J, Allbee-Johnson M, Karanes C, Milano F, et al. Comparison of haploidentical and umbilical cord blood transplantation after myeloablative conditioning. Blood Adv. (2021) 5(20):4064–72. doi: 10.1182/bloodadvances.2021004462
74. Flowers MED, Inamoto Y, Carpenter PA, Lee SJ, Kiem H-P, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to national institutes of health consensus criteria. Blood. (2011) 117(11):3214–9. doi: 10.1182/blood-2010-08-302109
75. Lazaryan A, Weisdorf DJ, DeFor T, Brunstein CG, MacMillan ML, Bejanyan N, et al. Risk factors for acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation with umbilical cord blood and matched sibling donors. Biol Blood Marrow Transplant. (2016) 22(1):134–40. doi: 10.1016/j.bbmt.2015.09.008
76. Qayed M, Wang T, Hemmer MT, Spellman S, Arora M, Couriel D, et al. Influence of age on acute and chronic GVHD in children undergoing HLA-identical sibling bone marrow transplantation for acute leukemia: implications for prophylaxis. Biol Blood Marrow Transplant. (2018) 24(3):521–8. doi: 10.1016/j.bbmt.2017.11.004
77. Alsultan A, Giller RH, Gao D, Bathurst J, Hild E, Gore L, et al. GVHD After unrelated cord blood transplant in children: characteristics, severity, risk factors and influence on outcome. Bone Marrow Transplant. (2011) 46(5):668–75. doi: 10.1038/bmt.2010.174
78. Soiffer RJ, Chen Y-B. Pharmacologic agents to prevent and treat relapse after allogeneic hematopoietic cell transplantation. Hematology. (2017) 2017(1):699–707. doi: 10.1182/asheducation-2017.1.699
79. Inamoto Y, Flowers ME, Lee SJ, Carpenter PA, Warren EH, Deeg HJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. (2011) 118(2):456–63. doi: 10.1182/blood-2011-01-330217
80. Natasha K, Haesook TK, Gita T, Philippe A, Joseph HA, Corey C, et al. Efficacy of immune suppression tapering in treating relapse after reduced intensity allogeneic stem cell transplantation. Haematologica. (2015) 100(9):1222–7. doi: 10.3324/haematol.2015.129650
81. Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. (2002) 99(4):1458–64. doi: 10.1182/blood.V99.4.1458
82. Bleakley M, Sehgal A, Seropian S, Biernacki MA, Krakow EF, Dahlberg A, et al. Naive T-cell depletion to prevent chronic graft-versus-host disease. J Clin Oncol. (2022) 40(11):1174–85. doi: 10.1200/JCO.21.01755
83. Dutt S, Tseng D, Ermann J, George TI, Liu YP, Davis CR, et al. Naive and memory T cells induce different types of graft-versus-host disease1. J Immunol. (2007) 179(10):6547–54. doi: 10.4049/jimmunol.179.10.6547
84. Khandelwal P, Lane A, Chaturvedi V, Owsley E, Davies SM, Marmer D, et al. Peripheral blood CD38 bright CD8+ effector memory T cells predict acute graft-versus-host disease. Biol Blood Marrow Transplant. (2015) 21(7):1215–22. doi: 10.1016/j.bbmt.2015.04.010
85. Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells. (2018) 36(3):434–45. doi: 10.1002/stem.2759
86. Crossland RE, Perutelli F, Bogunia-Kubik K, Mooney N, Milutin Gašperov N, Pučić-Baković M, et al. Potential novel biomarkers in chronic graft-versus-host disease. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.602547
87. Ponce DM, Hilden P, Mumaw C, Devlin SM, Lubin M, Giralt S, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. (2015) 125(1):199–205. doi: 10.1182/blood-2014-06-584789
88. Itamura H, Shindo T, Yoshioka S, Ishikawa T, Kimura S. Phosphorylated ERK1/2 in CD4 T cells is associated with acute GVHD in allogeneic hematopoietic stem cell transplantation. Blood Adv. (2020) 4(4):667–71. doi: 10.1182/bloodadvances.2019000343
89. Shindo T, Kim TK, Benjamin CL, Wieder ED, Levy RB, Komanduri KV. MEK inhibitors selectively suppress alloreactivity and graft-versus-host disease in a memory stage-dependent manner. Blood. (2013) 121(23):4617–26. doi: 10.1182/blood-2012-12-476218
90. Wynn R, Nataraj R, Nadaf R, Poulton K, Logan A. Strategies for success with umbilical cord haematopoietic stem cell transplantation in children with malignant and non-malignant disease indications. Front Cell Dev Biol. (2022) 10. doi: 10.3389/fcell.2022.836594
91. Politikos I, Davis E, Nhaissi M, Wagner JE, Brunstein CG, Cohen S, et al. Guidelines for cord blood unit selection. Biol Blood Marrow Transplant. (2020) 26(12):2190–6. doi: 10.1016/j.bbmt.2020.07.030
92. Hough R, Danby R, Russell N, Marks D, Veys P, Shaw B, et al. Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, donor selection algorithms and conditioning protocols. Br J Haematol. (2016) 172(3):360–70. doi: 10.1111/bjh.13802
93. Borrill R, Poulton K, Kusyk L, Routledge A, Bonney D, Hanasoge-Nataraj R, et al. Granulocyte transfusion during cord blood transplant for relapsed, refractory AML is associated with massive CD8+ T-cell expansion, significant cytokine release syndrome and induction of disease remission. Br J Haematol. (2023) 202(3):589–98. doi: 10.1111/bjh.18863
94. Wang JCY, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. (1997) 89(11):3919–24. doi: 10.1182/blood.V89.11.3919
95. Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol. (2014) 5. doi: 10.3389/fimmu.2014.00068
96. Hordyjewska A, Popiołek Ł, Horecka A. Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology. (2015) 67(3):387–96. doi: 10.1007/s10616-014-9796-y
97. Lu L, Xiao M, Shen RN, Grigsby S, Broxmeyer HE. Enrichment, characterization, and responsiveness of single primitive CD34 human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood. (1993) 81(1):41–8. doi: 10.1182/blood.V81.1.41.41
98. Broxmeyer HE, Hangoc G, Cooper S, Ribeiro RC, Graves V, Yoder M, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A. (1992) 89(9):4109–13. doi: 10.1073/pnas.89.9.4109
99. Lu L, Xiao M, Shen R-N, Grigsby S, Broxmeyer HE. Enrichment, characterization, and responsiveness of single primitive CD34+++ human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood. (1993) 81(1):41–8. doi: 10.1182/blood.V81.1.41.41
100. Szabolcs P, Park K-D, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naïve phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. (2003) 31(8):708–14. doi: 10.1016/S0301-472X(03)00160-7
101. Yun HD, Varma A, Hussain MJ, Nathan S, Brunstein C. Clinical relevance of immunobiology in umbilical cord blood transplantation. J Clin Med. (2019) 8(11):1968. doi: 10.3390/jcm8111968
102. Mommaas B, Stegehuis-Kamp JA, van Halteren AG, Kester M, Enczmann J, Wernet P, et al. Cord blood comprises antigen-experienced T cells specific for maternal minor histocompatibility antigen HA-1. Blood. (2005) 105(4):1823–7. doi: 10.1182/blood-2004-07-2832
103. Wo J, Zhang F, Li Z, Sun C, Zhang W, Sun G. The role of gamma-delta T cells in diseases of the central nervous system. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.580304
104. Andreu-Ballester JC, García-Ballesteros C, Benet-Campos C, Amigó V, Almela-Quilis A, Mayans J, et al. Values for αβ and γδ T-lymphocytes and CD4+, CD8+, and CD56+ subsets in healthy adult subjects: assessment by age and gender. Cytometry B Clin Cytom. (2012) 82B(4):238–44. doi: 10.1002/cyto.b.21020
105. de Witte MA, Sarhan D, Davis Z, Felices M, Vallera DA, Hinderlie P, et al. Early reconstitution of NK and gamma-delta T cells and its implication for the design of post-transplant immunotherapy. Biol Blood Marrow Transplant. (2018) 24(6):1152–62. doi: 10.1016/j.bbmt.2018.02.023
106. Placido R, Auricchio G, Gabriele I, Galli E, Brunetti E, Colizzi V, et al. Characterization of the immune response of human cord-blood derived gamma/delta T cells to stimulation with aminobisphosphonate compounds. Int J Immunopathol Pharmacol. (2011) 24(1):101–10. doi: 10.1177/039463201102400112
107. Kalyan S, Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. (2013) 10(1):21–9. doi: 10.1038/cmi.2012.44
108. Tan KW, Li Z, Lai J, Guo D, Linn YC, Hwang WY, et al. Single cell immune profiling reveals distinct T cell clones and functional states in in-vitro expanded cord blood derived gamma delta T cells. Blood. (2020) 136(Suppl. 1):35–6. doi: 10.1182/blood-2020-140667
109. Campos Alberto EJ, Shimojo N, Aoyagi M, Kohno Y. Differential effects of tumour necrosis factor-alpha and interleukin-12 on isopentenyl pyrophosphate-stimulated interferon-gamma production by cord blood Vgamma9 T cells. Immunology. (2009) 127(2):171–7. doi: 10.1111/j.1365-2567.2008.02983.x
110. Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, et al. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc Natl Acad Sci U S A. (2015) 112(6):E556–65. doi: 10.1073/pnas.1412058112
111. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. (2013) 13(2):101–17. doi: 10.1038/nri3369
112. Bienemann K, Iouannidou K, Schoenberg K, Krux F, Reuther S, Feyen O, et al. iNKT cell frequency in peripheral blood of Caucasian children and adolescent: the absolute iNKT cell count is stable from birth to adulthood. Scand J Immunol. (2011) 74(4):406–11. doi: 10.1111/j.1365-3083.2011.02591.x
113. Schneidawind D, Pierini A, Alvarez M, Pan Y, Baker J, Buechele C, et al. CD4+ invariant Natural killer T cells protect from murine GVHD lethality through expansion of donor CD4+CD25+FoxP3+ regulatory T cells. Blood. (2014) 124(22):3320–8. doi: 10.1182/blood-2014-05-576017
114. Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow Transplantation1. J Immunol. (2007) 178(10):6242–51. doi: 10.4049/jimmunol.178.10.6242
115. Li YR, Zeng S, Dunn ZS, Zhou Y, Li Z, Yu J, et al. Off-the-shelf third-party HSC-engineered iNKT cells for ameliorating GvHD while preserving GvL effect in the treatment of blood cancers. iScience. (2022) 25(9):104859. doi: 10.1016/j.isci.2022.104859
116. Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. (2017) 8. doi: 10.3389/fimmu.2017.00329
117. Amand M, Iserentant G, Poli A, Sleiman M, Fievez V, Sanchez IP, et al. Human CD56dimCD16dim cells as an individualized natural killer cell subset. Front Immunol. (2017) 8. doi: 10.3389/fimmu.2017.00699
118. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. (2001) 22(11):633–40. doi: 10.1016/S1471-4906(01)02060-9
119. Verneris MR, Miller JS. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br J Haematol. (2009) 147(2):185–91. doi: 10.1111/j.1365-2141.2009.07768.x
120. Luevano M, Daryouzeh M, Alnabhan R, Querol S, Khakoo S, Madrigal A, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol. (2012) 73(3):248–57. doi: 10.1016/j.humimm.2011.12.015
121. Wang Y, Xu H, Zheng X, Wei H, Sun R, Tian Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell Mol Immunol. (2007) 4(5):377–82.17976318
122. Perez SA, Sotiropoulou PA, Gkika DG, Mahaira LG, Niarchos DK, Gritzapis AD, et al. A novel myeloid-like NK cell progenitor in human umbilical cord blood. Blood. (2003) 101(9):3444–50. doi: 10.1182/blood-2002-05-1501
123. Zhao X, Cai L, Hu Y, Wang H. Cord-bBlood natural killer cell-based immunotherapy for cancer. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.584099
124. Arakawa-Hoyt J, Dao MA, Thiemann F, Hao QL, Ertl DC, Weinberg KI, et al. The number and generative capacity of human B lymphocyte progenitors, measured in vitro and in vivo, is higher in umbilical cord blood than in adult or pediatric bone marrow. Bone Marrow Transplant. (1999) 24(11):1167–76. doi: 10.1038/sj.bmt.1702048
125. Budeus B, Kibler A, Brauser M, Homp E, Bronischewski K, Ross JA, et al. Human cord blood B cells differ from the adult counterpart by conserved ig repertoires and accelerated response dynamics. J Immunol. (2021) 206(12):2839–51. doi: 10.4049/jimmunol.2100113
126. Paloczi K. Immunophenotypic and functional characterization of human umbilical cord blood mononuclear cells. Leukemia. (1999) 13(1):S87–9. doi: 10.1038/sj.leu.2401318
127. Rabian-Herzog C, Lesage S, Gluckman E, Charron D. Characterization of lymphocyte subpopulations in cord blood. J Hematother. (1993) 2(2):255–7. doi: 10.1089/scd.1.1993.2.255
128. Hardy RR, Li Y-S, Hayakawa K. Distinctive developmental origins and specificities of the CD5+ B-cell subset. Semin Immunol. (1996) 8(1):37–44. doi: 10.1006/smim.1996.0006
129. Schönland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, et al. Homeostatic control of T-cell generation in neonates. Blood. (2003) 102(4):1428–34. doi: 10.1182/blood-2002-11-3591
130. Wang J, Wissink EM, Watson NB, Smith NL, Grimson A, Rudd BD. Fetal and adult progenitors give rise to unique populations of CD8+ T cells. Blood. (2016) 128(26):3073–82. doi: 10.1182/blood-2016-06-725366
131. Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. (2010) 330(6011):1695–9. doi: 10.1126/science.1196509
132. Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, et al. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol. (2014) 193(1):177–84. doi: 10.4049/jimmunol.1400553
133. Kwoczek J, Riese SB, Tischer S, Bak S, Lahrberg J, Oelke M, et al. Cord blood–derived T cells allow the generation of a more naïve tumor-reactive cytotoxic T-cell phenotype. Transfusion. (2018) 58(1):88–99. doi: 10.1111/trf.14365
134. Risdon G, Gaddy J, Stehman FB, Broxmeyer HE. Proliferative and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cell Immunol. (1994) 154(1):14–24. doi: 10.1006/cimm.1994.1053
135. Hiwarkar P, Hubank M, Qasim W, Chiesa R, Gilmour KC, Saudemont A, et al. Cord blood transplantation recapitulates fetal ontogeny with a distinct molecular signature that supports CD4+ T-cell reconstitution. Blood Adv. (2017) 1(24):2206–16. doi: 10.1182/bloodadvances.2017010827
136. Early E, Reen DJ. Rapid conversion of naive to effector T cell function counteracts diminished primary human newborn T cell responses. Clin Exp Immunol. (2001) 116(3):527–33. doi: 10.1046/j.1365-2249.1999.00920.x
137. Hiwarkar P, Qasim W, Ricciardelli I, Gilmour K, Quezada S, Saudemont A, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood. (2015) 126(26):2882–91. doi: 10.1182/blood-2015-06-654780
138. Liu E, Law HKW, Lau Y-L. Tolerance associated with cord blood transplantation may depend on the state of host dendritic cells. Br J Haematol. (2004) 126(4):517–26. doi: 10.1111/j.1365-2141.2004.05061.x
139. Torelli GF, Maggio R, Peragine N, Chiaretti S, De Propris MS, Lucarelli B, et al. Functional analysis and gene expression profile of umbilical cord blood regulatory T cells. Ann Hematol. (2012) 91(2):155–61. doi: 10.1007/s00277-011-1288-y
140. Tsuda S, Nakashima A, Shima T, Saito S. New paradigm in the role of regulatory T cells during pregnancy. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00573
141. Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, et al. Cord blood CD4+CD25+-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. (2005) 105(2):750–8. doi: 10.1182/blood-2004-06-2467
142. Berthou C, Legros-Maïda S, Soulié A, Wargnier A, Guillet J, Rabian C, et al. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. (1995) 85(6):1540–6. doi: 10.1182/blood.V85.6.1540.bloodjournal8561540
143. Sato K, Nagayama H, Takahashi TA. Aberrant CD3- and CD28-mediated signaling events in cord blood T cells are associated with dysfunctional regulation of fas ligand-mediated cytotoxicity. J Immunol. (1999) 162(8):4464. doi: 10.4049/jimmunol.162.8.4464
144. Chalmers IMH, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. (1998) 92(1):11–8. doi: 10.1182/blood.V92.1.11.413a39_11_18
145. Krampera M, Tavecchia L, Benedetti F, Nadali G, Pizzolo G. Intracellular cytokine profile of cord blood T-, and NK- cells and monocytes. Haematologica. (2000) 85(7):675–9.10897117
146. Nitsche A, Zhang M, Clauss T, Siegert W, Brune K, Pahl A. Cytokine profiles of cord and adult blood leukocytes: differences in expression are due to differences in expression and activation of transcription factors. BMC Immunol. (2007) 8(1):18. doi: 10.1186/1471-2172-8-18
147. Komanduri KV, St. John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. (2007) 110(13):4543–51. doi: 10.1182/blood-2007-05-092130
148. Kaminski BA, Kadereit S, Miller RE, Leahy P, Stein KR, Topa DA, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. (2003) 102(13):4608–17. doi: 10.1182/blood-2003-05-1732
149. Hagihara M, Chargui J, Gansuvd B, Tsuchida F, Sato T, Hotta T, et al. Umbilical cord blood T lymphocytes are induced to apoptosis after being allo-primed in vitro. Bone Marrow Transplant. (1999) 24(11):1229–33. doi: 10.1038/sj.bmt.1702050
150. Thiant S, Yakoub-Agha I, Magro L, Trauet J, Coiteux V, Jouet JP, et al. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant. (2010) 45(10):1546–52. doi: 10.1038/bmt.2010.13
151. Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. (2005) 141(1):10–8. doi: 10.1111/j.1365-2249.2005.02799.x
152. Matos TR, Hirakawa M, Alho AC, Neleman L, Graca L, Ritz J. Maturation and phenotypic heterogeneity of human CD4+ regulatory T cells from birth to adulthood and after allogeneic stem cell transplantation. Front Immunol. (2021) 11. doi: 10.3389/fimmu.2020.570550
153. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. (2003) 9(9):1144–50. doi: 10.1038/nm915
154. Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood–derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. (2016) 127(8):1044–51. doi: 10.1182/blood-2015-06-653667
155. McKenna DH Jr, Sumstad D, Kadidlo DM, Batdorf B, Lord CJ, Merkel SC, et al. Optimization of cGMP purification and expansion of umbilical cord blood-derived T-regulatory cells in support of first-in-human clinical trials. Cytotherapy. (2017) 19(2):250–62. doi: 10.1016/j.jcyt.2016.10.011
156. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. (2011) 117(3):1061–70. doi: 10.1182/blood-2010-07-293795
157. Lee Y-S, Kim T-S, Kim D-K. T lymphocytes derived from human cord blood provide effective antitumor immunotherapy against a human tumor. BMC Cancer. (2011) 11(1):225. doi: 10.1186/1471-2407-11-225
158. Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. (2008) 111(4):2476–84. doi: 10.1182/blood-2007-08-109678
159. Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. (2007) 19(5):318–30. doi: 10.1016/j.smim.2007.10.004
160. Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. (2014) 124(22):3201–11. doi: 10.1182/blood-2014-07-589176
161. Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. (2002) 2(8):547–56. doi: 10.1038/nri853
162. Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc Natl Acad Sci U S A. (2002) 99(5):2989–94. doi: 10.1073/pnas.052714099
163. Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. Differential survival of naive CD4 and CD8 T cells. J Immunol. (2000) 165(7):3689. doi: 10.4049/jimmunol.165.7.3689
164. Klein AK, Patel DD, Gooding ME, Sempowski GD, Chen BJ, Liu C, et al. T-cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. (2001) 7(8):454–66. doi: 10.1016/S1083-8791(01)80013-6
165. Chiesa R, Gilmour K, Qasim W, Adams S, Worth AJJ, Zhan H, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. (2012) 156(5):656–66. doi: 10.1111/j.1365-2141.2011.08994.x
166. Rénard C, Barlogis V, Mialou V, Galambrun C, Bernoux D, Goutagny MP, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br J Haematol. (2011) 152(3):322–30. doi: 10.1111/j.1365-2141.2010.08409.x
167. Moretta A, Maccario R, Fagioli F, Giraldi E, Busca A, Montagna D, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. (2001) 29(3):371–9. doi: 10.1016/S0301-472X(00)00667-6
168. Giraud P, Thuret I, Reviron D, Chambost H, Brunet C, Novakovitch G, et al. Immune reconstitution and outcome after unrelated cord blood transplantation: a single paediatric institution experience. Bone Marrow Transplant. (2000) 25(1):53–7. doi: 10.1038/sj.bmt.1702089
169. Chaudhry MS, Velardi E, Malard F, van den Brink MRM. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: time to T up the thymus. J Immunol. (2017) 198(1):40. doi: 10.4049/jimmunol.1601100
170. Fujimaki K, Maruta A, Yoshida M, Kodama F, Matsuzaki M, Fujisawa S, et al. Immune reconstitution assessed during five years after allogeneic bone marrow transplantation. Bone Marrow Transplant. (2001) 27(12):1275–81. doi: 10.1038/sj.bmt.1703056
171. Politikos I, Lavery JA, Hilden P, Cho C, Borrill T, Maloy MA, et al. Robust CD4+ T-cell recovery in adults transplanted with cord blood and no antithymocyte globulin. Blood Adv. (2020) 4(1):191–202. doi: 10.1182/bloodadvances.2019000836
172. Yoshida H, Koike M, Tada Y, Nakata K, Hino A, Fuji S, et al. Different immune reconstitution between cord blood and unrelated bone marrow transplantation with relation to chronic graft-versus-host disease. Int J Hematol Oncol Stem Cell Res. (2020) 14(1):1–10.32337009
173. Zhao X, Wang W, Nie S, Geng L, Song K, Zhang X, et al. Dynamic comparison of early immune reactions and immune cell reconstitution after umbilical cord blood transplantation and peripheral blood stem cell transplantation. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1084901
174. Clave E, Lisini D, Douay C, Giorgiani G, Busson M, Zecca M, et al. Thymic function recovery after unrelated donor cord blood or T-cell depleted HLA-haploidentical stem cell transplantation correlates with leukemia relapse. Front Immunol. (2013) 4. doi: 10.3389/fimmu.2013.00054
175. Thomson BG, Robertson KA, Gowan D, Heilman D, Broxmeyer HE, Emanuel D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. (2000) 96(8):2703–11. doi: 10.1182/blood.V96.8.2703.h8002703_2703_2711
176. Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis. (2011) 13(5):456–65. doi: 10.1111/j.1399-3062.2011.00632.x
177. Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. (2015) 2(5):e194–203. doi: 10.1016/S2352-3026(15)00045-9
178. Bartelink IH, Belitser SV, Knibbe CAJ, Danhof M, de Pagter AJ, Egberts TCG, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. (2013) 19(2):305–13. doi: 10.1016/j.bbmt.2012.10.010
179. Beziat V, Nguyen S, Lapusan S, Hervier B, Dhedin N, Bories D, et al. Fully functional NK cells after unrelated cord blood transplantation. Leukemia. (2009) 23(4):721–8. doi: 10.1038/leu.2008.343
180. Mehta RS, Shpall EJ, Rezvani K. Cord blood as a source of natural killer cells. Front Med (Lausanne). (2016) 2:93. doi: 10.3389/fmed.2015.00093
181. Nguyen S, Achour A, Souchet L, Vigouroux S, Chevallier P, Furst S, et al. Clinical impact of NK-cell reconstitution after reduced intensity conditioned unrelated cord blood transplantation in patients with acute myeloid leukemia: analysis of a prospective phase II multicenter trial on behalf of the Société Française de Greffe de Moelle Osseuse et Thérapie Cellulaire and Eurocord. Bone Marrow Transplant. (2017) 52(10):1428–35. doi: 10.1038/bmt.2017.122
182. Niehues T, Rocha V, Filipovich AH, Chan KW, Porcher R, Michel G, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children—a eurocord analysis. Br J Haematol. (2001) 114(1):42–8. doi: 10.1046/j.1365-2141.2001.02900.x
183. Servais S, Hannon M, Peffault de Latour R, Socie G, Beguin Y. Reconstitution of adaptive immunity after umbilical cord blood transplantation: impact on infectious complications. Stem Cell Investig. (2017) 4:40. doi: 10.21037/sci.2017.05.03
184. Nakatani K, Imai K, Shigeno M, Sato H, Tezuka M, Okawa T, et al. Cord blood transplantation is associated with rapid B-cell neogenesis compared with BM transplantation. Bone Marrow Transplant. (2014) 49(9):1155–61. doi: 10.1038/bmt.2014.123
185. Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. (2012) 18(4):565–74. doi: 10.1016/j.bbmt.2011.08.018
186. Kurosaka D, LeBien TW, Pribyl JA. Comparative studies of different stromal cell microenvironments in support of human B-cell development. Exp Hematol. (1999) 27(8):1271–81. doi: 10.1016/S0301-472X(99)00067-3
187. Hiwarkar P, Adams S, Gilmour K, Nataraj R, Bonney D, Poulton K, et al. Cord blood CD8+ T-cell expansion following granulocyte transfusions eradicates refractory leukemia. Blood Adv. (2020) 4(17):4165–74. doi: 10.1182/bloodadvances.2020001737
188. Martínez Bedoya D, Dutoit V, Migliorini D. Allogeneic CAR T cells: an alternative to overcome challenges of CAR T cell therapy in glioblastoma. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.640082
189. Liu D-D, Hong W-C, Qiu K-Y, Li X-Y, Liu Y, Zhu L-W, et al. Umbilical cord blood: a promising source for allogeneic CAR-T cells. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.944248
190. Cohen S, Roy J, Lachance S, Delisle J-S, Marinier A, Busque L, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol. (2020) 7(2):e134–45. doi: 10.1016/S2352-3026(19)30202-9
Keywords: cord blood, cord blood transplantation, acute leukemia, graft-vs.-leukemia, pediatric hematology, T-cells
Citation: Borrill R, Poulton K and Wynn R (2023) Immunology of cord blood T-cells favors augmented disease response during clinical pediatric stem cell transplantation for acute leukemia. Front. Pediatr. 11:1232281. doi: 10.3389/fped.2023.1232281
Received: 31 May 2023; Accepted: 22 August 2023;
Published: 13 September 2023.
Edited by:
Tayfun Güngör, University Children’s Hospital Zurich, SwitzerlandReviewed by:
Su Han Lum, Great North Children’s Hospital, United KingdomJeffrey J. Bednarski, Washington University in St. Louis, United States
Jean Villard, University of Geneva, Switzerland
© 2023 Borrill, Poulton and Wynn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roisin Borrill cm9pc2luLmJvcnJpbGxAZG9jdG9ycy5vcmcudWs=
 Roisin Borrill
Roisin Borrill Kay Poulton
Kay Poulton Robert Wynn
Robert Wynn