- 1Department of Pulmonology, Children's Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 2Department of Endoscopy Center, Children's Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Children's Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Pulmonary mucoepidermoid carcinoma (PMEC) is a rare tumor, particularly in children, and its clinical manifestations vary. When the tumor is small, it may be asymptomatic; however, with larger tumors, patients may present with symptoms such as recurring pneumonia, atelectasis, persistent cough, chest pain, and even hemoptysis. PMEC appears as an exophytic intrabronchial mass. This study aims to report on the clinical manifestations, imaging findings, treatment approaches, and prognosis of two children diagnosed with PMEC at our hospital between January 2018 and December 2022. The age of onset for both children was 9 years, and the masses were located in the right upper lobe bronchi. Following surgical treatment, both patients showed a good prognosis. In addition, we conducted a comprehensive review of the relevant literature to enhance the overall understanding of PMEC.
Introduction
Pulmonary mucoepidermoid carcinoma (PMEC) originates in the submucosal bronchial glands and is a rare malignant tumor. It constitutes 2.5%–7.3% and 0.1%–0.2% of endobronchial adenomas and primary lung cancers, respectively (1). The incidence of PMEC is extremely low in children, with 55 cases reported (1). From January 2018 to December 2022, we identified and analyzed two cases of PMEC that were diagnosed at our hospital. In this study, we emphasize the clinical features, treatment outcomes, and prognosis of these cases.
Case presentation
Case 1
A previously healthy 9-year-old girl was admitted to the hospital with persistent cough and chest pain for over 10 days. Remarkably, she showed no current fever, hemoptysis, nausea, abdominal pain, vomiting, diarrhea, chest pain, palpitations, or weight loss. Moreover, her medical history was devoid of any surgeries, traumas, encounters with sick individuals, aspiration episodes, or exposure to infectious and/or hazardous substances. Furthermore, she had no family history of asthma, diabetes, immunodeficiency, malignancy, or tuberculosis. The patient had encountered a bout of upper right pneumonia more than a year before the current presentation. However, after the patient's respiratory symptoms improved, the chest radiograph was not reviewed, and no further tests were performed. On the fifth day of experiencing chest pain, the patient visited a local hospital. Subsequent chest CT showed atelectasis of the upper lobe of the right lung, and flexible bronchoscopy was recommended. Her parents referred her to our facility for further evaluation and management. Blood routine and biochemical tests were all within normal ranges, and the T-spot test, oncological biomarkers, and purified protein derivative (PPD) test yielded negative outcomes. Chest contrast-enhanced CT revealed a space-occupying lesion at the bronchial opening of the right upper lobe, with uneven enhancement (Figures 1A–C). On the fifth day of hospitalization, flexible bronchoscopy (Figure 1D) and biopsy were performed. However, owing to the challenges linked with performing a biopsy in the right upper lobe, only a few specimens could be obtained. Due to unsatisfactory biopsy results, a multidisciplinary consultation was conducted, and a right superior lobectomy was recommended due to the high possibility of a bronchial tumor being the underlying condition. Eventually, a right upper lobectomy was performed. The lesion was a firm mass with a faint yellow surface. Hematoxylin and eosin (HE) staining of the specimen showed that the tumor cells were arranged in nests or glandular tubes, with abundant cytoplasm and epithelioid appearance, and the area was rich in mucus (Figure 1E). Histopathological examination revealed a PMEC with tumor-free surgical margins and metastasis to the mediastinal lymph node. Immunohistochemical analysis yielded remarkably positive results for the expression of cytokeratin (CK), CK-7, CK-19, and periodic acid-schiff stain (Figure 1F), while showing relatively weaker positivity for epithelial membrane antigen (EMA). Synaptophysin expression was notably absent. No recurrence was observed during the 3-month postoperative follow-up.
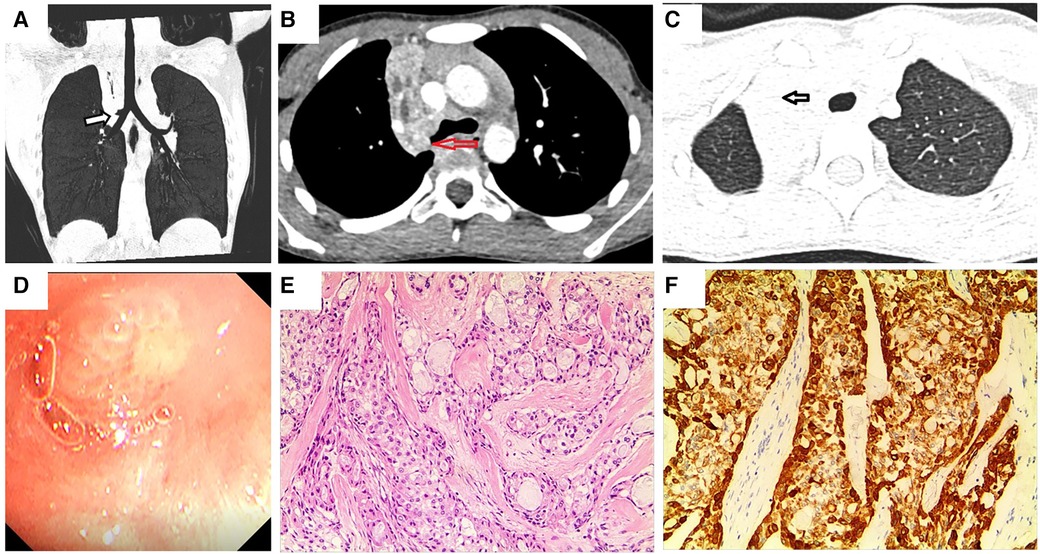
Figure 1. Chest enhanced CT, bronchoscopy, pathological image, and immunohistochemistry assay of case 1. (A,B) Contrast-enhanced CT images of the chest show a space-occupying lesion at the right upper lobe bronchial opening. Arrows indicate space-occupying lesions. (C) Arrow indicates right upper lobe atelectasis. (D) Visual depiction of flexible bronchoscopy, confirming the presence of a ball-shaped soft solid tumor originating from the right upper lobe bronchus. (E) Photomicrograph (HE × 100) shows the confirmed diagnosis of pulmonary mucoepidermoid carcinoma. (F) Photomicrograph shows positive immunohistochemical staining for CK (magnification × 100).
Case 2
A previously healthy 9-year-old boy was admitted to the hospital owing to two instances of hemoptysis within 4 weeks. The volume of expectorated blood was small, appearing as dark red blood clots. Except for a paroxysmal cough before hemoptysis, the patient did not manifest any cough at presentation. He also was devoid of experiencing any fever, shortness of breath, chest tightness, epistaxis, vomiting, or abdominal pain. No abnormalities were detected by physical examinations. Blood routine as well as biochemical tests yielded results that were within normal ranges. Similarly, assessments such as the T-spot test, oncological biomarkers, and PPD test yielded negative results. Chest enhanced CT revealed the presence of a mass in the right upper lobe, invading the right upper lobe bronchus, causing occlusion of the upper lobe bronchus. This radiological finding raised suspicions of malignancy, supported by concurrent mediastinal lymph node metastasis and atelectasis of the right upper lobe (Figures 2A–C). Flexible bronchoscopy (Figure 2D) and biopsy revealed a mucoepidermoid carcinoma in the right superior lobe bronchus. The patient subsequently received a thoracoscopic right upper lobectomy and dissection of the hilar lymph node. HE staining of the excised tissue specimens showed a variety of mucoid cells, epidermoid cells, and intermediate cells, some of which were solid and others were arranged in a glandular tube configuration (Figure 2E). Pathological results confirmed the diagnosis of PMEC, further confirming the absence of tumor involvement at the surgical margins and the presence of mediastinal lymph node metastasis. Immunohistochemical analysis yielded remarkably positive results for the expression of CK, CK-7, CK-19 and EMA (Figure 2F), while showing weaker positivity for chromogranin A and CK-20. Synaptophysin expression was absent. No recurrence was observed during the postoperative follow-up period of 3.5 years.
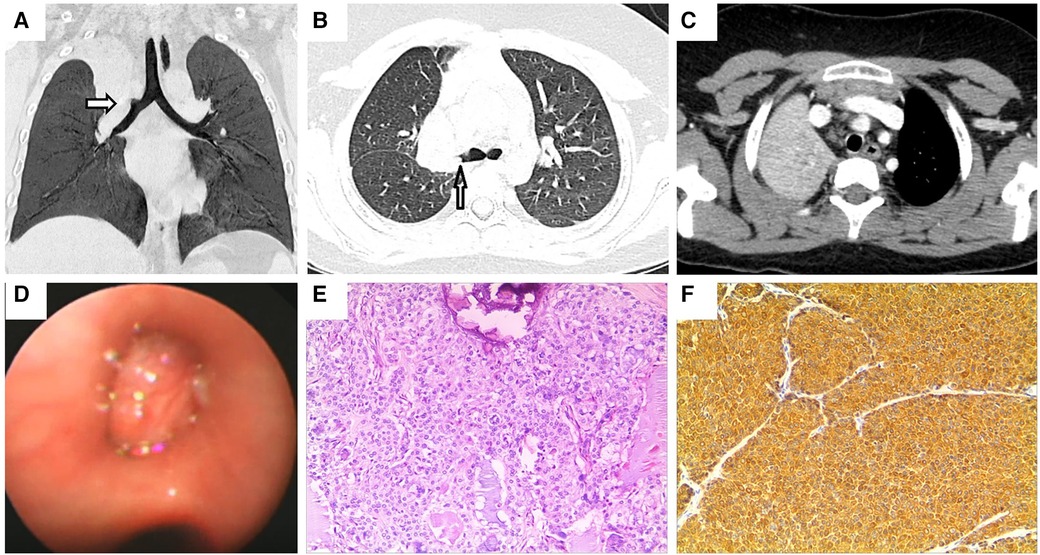
Figure 2. Chest enhanced CT, bronchoscopy, pathological image and immunohistochemistry assay of case 2. Contrast-enhanced CT of the chest shows (A,B) a blockage of the right superior lobe bronchus. Arrows indicate space-occupying lesions. (C) Atelectasis of the right superior lobe. (D) Visual representation of flexible bronchoscopy, confirming the presence of a mass in the right superior lobular bronchus. (E) Photomicrograph (HE × 100) shows the confirmed diagnosis of pulmonary mucoepidermoid carcinoma. (F) Photomicrograph shows positive immunohistochemical staining for CK (magnification × 100).
Discussion
Primary neoplasms originating in the tracheobronchial tree and lungs are rare, with the majority being malignant. PMEC tumor, is hallmarked by a combination of squamous, mucus-secreting, and intermediate cell types, and is defined by the World Health Organization (2). The incidence of PMEC constitutes approximately 0.1%–0.2% of all primary lung cancers (3). Although PMEC can manifest across all age groups (range, 3–78 years), it is more common in individuals aged 30–50 years (4). A study conducted in Taiwan has confirmed a higher incidence in males than in females (5), whereas other reports have demonstrated an equal distribution between the two genders (6, 7). In our report, we presented two cases, involving one male and one female patient, each. The incidence of PMEC in children is rare, with approximately 55 cases only reported currently (1). In the past 5 years, only two cases have been diagnosed in our hospital, both of which occurred in 9-year-old patients.
PMEC typically presents as an exophytic intrabronchial mass that can have intact or ulcerated bronchial mucosa. The tumors are situated in the submucosal layer of the larger bronchi under microscopy (8). When the tumor is small, patients might not experience typical symptoms. However, as the tumor gradually increases in size, symptoms such as cough, expectoration, fever, and hemoptysis may manifest. In more advanced cases, obstruction of the bronchial lumen can lead to additional changes. Therefore, chest CT and flexible bronchoscopy should be promptly performed when patients present with recurrent cough, sputum, hemoptysis, or atelectasis. These tumors usually originate in the bronchial mucous glands located in the main bronchial trunk or the proximal bronchus of a lobe and are less frequently found in the segmental bronchus and trachea. The growth pattern involves polypoid formations in the bronchus, covered by normal respiratory epithelium (1, 9). Therefore, bronchial lavage and brushing are rarely used for diagnosis, and forceps biopsy is a necessary approach (10). In our study, all the observed lesions were located in the right upper lobe bronchi.
According to classification, PMEC can be low or high grade, depending on nuclear pleomorphism, mitotic activity, and the presence or absence of necrosis. Generally, low-grade tumors are more common in children (1, 3, 4, 9, 11–16). Until the year 2000, only 54 cases of PMEC in children had been reported, of which 92.5% (50/54) were low-grade cancers (9, 15). Conversely, high-grade tumors are more common in adults (5, 17–19). Hsieh et al. (5) reported 41cases in adults, among which 10 patients (24.4%) had low-grade tumors, whereas, the rest (75.6%) had high-grade tumors. Among the 41 patients, 22 patients were older, with only one patient developing low-grade tumor; whereas the rest 21 older patients were all diagnosed with high-grade tumors. Jiang et al. (18) confirmed that 25 of the 34 (73.5%) adult patients had low-grade tumors.
Fluoro-18-fluoro-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) combines anatomical position location and morphological date from CT with functional data availed by PET, significantly influencing lung cancer diagnosis and staging (20). The use of PET was first reported in a 5-year-old girl with low-grade PMEC (21). The maximum standardized uptake value (SUVmax) is a common semi-quantitative parameter that shows tumor glucose metabolism and estimates tumor proliferation on PET and CT scans. Park et al. (22) employed 18F-FDG PET/CT to predict PMEC individuals’ prognosis and the pathological grade. The authors affirmed that patients with SUVmax higher than 6.5 were predisposed to lymph node metastasis, high-grade PMECs, and recurrences, based on an analysis of 23 patients. Jindal et al. (23) showed that SUVmax values on 18F-FDG PET-CT scans ranged from 0 to 6.2 and 2.86 to 23.4 in low-grade and high-grade PMEC cases, respectively. The authors suggested that PET/CT might play a role in tumor differentiation by predicting the histopathological prognosis. However, these two studies mainly involved adults, and only rare cases of 18F-FDG PET/CT use in children with PMEC have been reported. The comprehensive 18F-FDG PET imaging findings for PMEC in children have not been well established as per our knowledge. Low-grade PMEC was reported in a 15-year-old girl and an 11-year-old boy, yielding SUVmax values of 6.2 and 3.8, respectively (24, 25).
PMEC exhibits diverse immunohistochemical staining patterns, and no single stain can be definitively attributed to its pathogenesis. Tumors showed positive staining for markers such as p40, CK 5/6, and p63. In addition, staining with a keratin cocktail, CK 7, Muc5AC, and CEA may also yield positive results (26). In a study conducted by Andy et al. (27) involving 25 patients with PMEC, all patients showed expression of p63. Hu et al. (28) reported that the positive percentages for p63, CK7, Muc5AC, p40, and CK5/6 were 100%, 100%, 100%, 96.3%, and 50%, respectively. Human epidermal growth factor receptor 2, Napsin A and TTF-1, were all negative (27, 28). In our two patients, both CK and CK7 were determined to be positive.
The most common genetic change in PMEC is the t(11; 19) (q21; p13) translocation, which culminates in the generation of the fusion protein mucoepidermoid carcinoma translocated 1–mammalian mastermind like 2 (MECT::MAML2) genes (3, 28, 29). This genetic change involves the fusion of exon 1 of a novel gene on chromosome 19, mucoepidermoid carcinoma translocated 1 (MECT1), with exons 2–5 of a gene located on chromosome 11 that is linked to the Notch signaling pathway, known as mastermind-like 2 (MAML2) (30). Achcar et al. (19) showed MAML2 rearrangement in 13 out of 17 (77%) cases of PMEC. Subsequent research has indicated that the MECT::MAML2 fusion product is specific to PMEC and associated with a subset of tumors that exhibit a more favorable prognosis owing to their extended clinical course (31). In our study, the detection of MECT::MAML2 was not feasible owing to limitations in specimen collection. The precise role of this reciprocal translocation and resultant fusion protein in the development of PMEC warrants further investigation in a large number of patients, especially among children.
Surgical resection is the recommended approach for PMEC, with an emphasis on achieving complete excision through lobectomy, sleeve resection, or other surgical methods based on the location of the tumor (8, 18, 32, 33). In low-grade PMEC cases, efforts are made to minimize the removal of normal lung tissue. Prognostic factors that predict unfavorable survival encompass the histological tumor grade, TNM stage, patient age, and the extent of resection (5, 34, 35). Jiang et al. pointed out that lymph node metastasis was the sole independent prognostic factor (18).
In children, PMEC tends to show low-grade malignancy, and the long-term prognosis after surgical resection is excellent (1, 3, 9, 11, 13, 16, 36–38). Granata et al. (3) confirmed that among 51 pediatric patients, 49 patients with low-grade tumors experienced no tumor recurrence or metastasis after surgery (mean follow-up, 5 years and 3 months; range, 8 months to 23 years). Among the remaining two patients with high-grade tumors, one was lost to follow-up, whereas the other remained disease-free after a 6-year follow-up. A study involving 34 adults with PMEC revealed that 7 patients experienced tumor recurrence or metastasis, including 4 who died (mean follow-up period, 63 months). All patients’ 5-year overall survival and progression-free survival rates, were 84.6% and 81.6%, correspondingly. In our study, neither of the two patients experienced recurrence, and the longest follow-up period was 3.5 years. Overall, children with PMEC show a better prognosis compared to adults.
In conclusion, PMEC is a rare malignant neoplasm, especially in children. When patients present with repeated cough, sputum production, hemoptysis, or atelectasis, chest CT and flexible bronchoscopy should be performed promptly to facilitate timely diagnosis and consideration of PMEC. Surgical resection is an effective treatment strategy for managing patients with PMEC. However, further studies are warranted to better understand PMEC, particularly in children.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies were reviewed and approved by Children's Hospital Affiliated to Zhejiang University School of Medicine. Written informed consent was obtained from the participants' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH was responsible for data collection, initial drafting, and completion of the first draft. YF, BX, and JS examined the manuscript. LW and L-fT supervised this study to guarantee its authenticity as well as its practicability. All authors critically reviewed, revised, approved the final manuscript, and agreed to be responsible for all aspects of this study. All authors were rigorously vetted and approved the final manuscript and were responsible for all aspects of the study. All authors contributed to the article and approved the submitted version.
Funding
The Zhejiang Province Public Welfare Technology Application Research Project (No. LGF22H010002) supported this study, which was awarded to LW.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Al-Qahtani AR, Di Lorenzo M, Yazbeck S. Endobronchial tumors in children: institutional experience and literature review. J Pediatr Surg. (2003) 38(5):733–6. doi: 10.1016/jpsu.2003.50195
2. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: IARC Press (2015).
3. Granata C, Battistini E, Toma P, Balducci T, Mattioli G, Fregonese B, et al. Mucoepidermoid carcinoma of the bronchus: a case report and review of the literature. Pediatr Pulmonol. (1997) 23(3):226–32. doi: 10.1002/(SICI)1099-0496(199703)23:3%3C226::AID-PPUL10%3E3.0.CO;2-9
4. Kuzucuoglu M, Karamustafaoglu YA, Cicin I, Yoruk Y. A rarely seen mucoepidermoid carcinoma of the left main bronchus. J Cancer Res Ther. (2014) 10(2):384–6. doi: 10.4103/0973-1482.136665
5. Hsieh CC, Sun YH, Lin SW, Yeh YC, Chan ML. Surgical outcomes of pulmonary mucoepidermoid carcinoma: a review of 41 cases. PLoS One. (2017) 12(5):e0176918. doi: 10.1371/journal.pone.0176918
6. Bishnoi S, Puri HV, Asaf BB, Pulle MV, Kumar A, Kumar A. Lung preservation in mucoepidermoid carcinoma of tracheobronchial tree: a case series. Lung India. (2021) 38(1):18–22. doi: 10.4103/lungindia.lungindia_511_20
7. Lee GD, Kang DK, Kim HR, Jang SJ, Kim YH, Kim DK, et al. Surgical outcomes of pulmonary mucoepidermoid carcinoma: a review of 23 cases. Thorac Cardiovasc Surg. (2014) 62(2):140–6. doi: 10.1055/s-0033-1342943
8. Liu XL, Adams AL. Mucoepidermoid carcinoma of the bronchus—a review. Arch Pathol Lab Med. (2007) 131(9):1400–4. doi: 10.5858/2007-131-1400-MCOTBA
9. Dinopoulos A, Lagona E, Stinios I, Konstadinidou A, Kattamis C. Mucoepidermoid carcinoma of the bronchus. Pediatr Hematol Oncol. (2000) 17(5):401–8. doi: 10.1080/08880010050034346
10. Torres AM, Ryckman FC. Childhood tracheobronchial mucoepidermoid carcinoma: a case report and review of the literature. J Pediatr Surg. (1988) 23(4):367–70. doi: 10.1016/S0022-3468(88)80211-2
11. Chan EY, MacCormick JA, Rubin S, Nizalik E. Mucoepidermoid carcinoma of the trachea in a 4-year-old boy. J Otolaryngol. (2005) 34(4):235–8. doi: 10.2310/7070.2005.34404
12. Kose M, Bilgin M, Kontas O, Ozturk S, Doganay S, Ozdemir MA. A case of mucoepidermoid carcinoma of the bronchus presented with hydropneumothorax in a child. Pediatr Pulmonol. (2014) 49(3):E86–9. doi: 10.1002/ppul.22938
13. Li S, Zhang Z, Tang H, He Z, Gao Y, Ma W, et al. Pathological complete response to gefitinib in a 10-year-old boy with EGFR-negative pulmonary mucoepidermoid carcinoma: a case report and literature review. Clin Respir J. (2017) 11(3):346–51. doi: 10.1111/crj.12343
14. Lee EY, Vargas SO, Sawicki GS, Boyer D, Grant FD, Voss SD. Mucoepidermoid carcinoma of bronchus in a pediatric patient: (18)F-FDG PET findings. Pediatr Radiol. (2007) 37(12):1278–82. doi: 10.1007/s00247-007-0607-x
15. Vaos G, Zavras N, Priftis K, Micahil-Strantzia C, Antypas G. Bronchotomy in the treatment of a low-grade bronchial mucoepidermoid carcinoma in a child. J Thorac Cardiovasc Surg. (2004) 128(5):782–3. doi: 10.1016/j.jtcvs.2004.03.026
16. Wu M, Wang Q, Xu XF, Xiang JJ. Bronchial mucoepidermoid carcinoma in children. Thorac Cardiovasc Surg. (2011) 59(7):443–5. doi: 10.1055/s-0030-1250389
17. Shen C, Che G. Clinicopathological analysis of pulmonary mucoepidermoid carcinoma. World J Surg Oncol. (2014) 12:33. doi: 10.1186/1477-7819-12-33
18. Jiang L, Li P, Xiao Z, Qiu H, Zhang X, Xiao Y, et al. Prognostic factors of primary pulmonary mucoepidermoid carcinoma: a clinical and pathological analysis of 34 cases. Int J Clin Exp Pathol. (2014) 7(10):6792–9.25400760
19. Achcar RO, Nikiforova MN, Dacic S, Nicholson AG, Yousem SA. Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinoma. Hum Pathol. (2009) 40(6):854–60. doi: 10.1016/j.humpath.2008.11.007
20. Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann Transl Med. (2018) 6(5):95. doi: 10.21037/atm.2018.01.25
21. Kinoshita H, Shimotake T, Furukawa T, Deguchi E, Iwai N. Mucoepidermal carcinoma of the lung detected by positron emission tomography in a 5-year-old girl. J Pediatr Surg. (2005) 40(4):E1–3. doi: 10.1016/j.jpedsurg.2005.01.025
22. Park B, Kim HK, Choi YS, Kim J, Zo JI, Choi JY, et al. Prediction of pathologic grade and prognosis in mucoepidermoid carcinoma of the lung using (1)(8)F-FDG PET/CT. Korean J Radiol. (2015) 16(4):929–35. doi: 10.3348/kjr.2015.16.4.929
23. Jindal T, Kumar A, Kumar R, Dutta R, Meena M. Role of positron emission tomography-computed tomography in bronchial mucoepidermoid carcinomas: a case series and review of the literature. J Med Case Rep. (2010) 4:277. doi: 10.1186/1752-1947-4-277
24. Ishizumi T, Tateishi U, Watanabe S, Maeda T, Arai Y. F-18 FDG PET/CT imaging of low-grade mucoepidermoid carcinoma of the bronchus. Ann Nucl Med. (2007) 21(5):299–302. doi: 10.1007/s12149-007-0018-y
25. Xie J, Ma C, Tang J. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography findings in a pediatric mucoepidermoid carcinoma and differential diagnosis. Hell J Nucl Med. (2017) 20(2):172–5.28697196
26. Kalhor N, Moran CA. Pulmonary mucoepidermoid carcinoma: diagnosis and treatment. Expert Rev Respir Med. (2018) 12(3):249–55. doi: 10.1080/17476348.2018.1428563
27. Roden AC, Garcia JJ, Wehrs RN, Colby TV, Khoor A, Leslie KO, et al. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol. (2014) 27(11):1479–88. doi: 10.1038/modpathol.2014.72
28. Hu S, Gong J, Zhu X, Lu H. Pulmonary salivary gland tumor, mucoepidermoid carcinoma: a literature review. J Oncol. (2022) 2022:9742091. doi: 10.1155/2022/9742091
29. Tonon G, Gehlhaus KS, Yonescu R, Kaye FJ, Kirsch IR. Multiple reciprocal translocations in salivary gland mucoepidermoid carcinomas. Cancer Genet Cytogenet. (2004) 152(1):15–22. doi: 10.1016/j.cancergencyto.2003.10.007
30. Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. (2003) 33(2):208–13. doi: 10.1038/ng1083
31. Okabe M, Miyabe S, Nagatsuka H, Terada A, Hanai N, Yokoi M, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. (2006) 12(13):3902–7. doi: 10.1158/1078-0432.CCR-05-2376
32. Martin-Ucar AE, Rocco G. Mucoepidermoid carcinoma in unilateral hypoplastic lung: a rare tumor in a rarer condition. Ann Thorac Surg. (2003) 75(3):1020–1. doi: 10.1016/S0003-4975(02)04770-7
33. Tsuchiya H, Nagashima K, Ohashi S, Takase Y. Childhood bronchial mucoepidermoid tumors. J Pediatr Surg. (1997) 32(1):106–9. doi: 10.1016/S0022-3468(97)90109-3
34. Zhu F, Liu Z, Hou Y, He D, Ge X, Bai C, et al. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thorac Oncol. (2013) 8(12):1578–84. doi: 10.1097/JTO.0b013e3182a7d272
35. Xi JJ, Jiang W, Lu SH, Zhang CY, Fan H, Wang Q. Primary pulmonary mucoepidermoid carcinoma: an analysis of 21 cases. World J Surg Oncol. (2012) 10:232. doi: 10.1186/1477-7819-10-232
36. Welsh JH, Maxson T, Jaksic T, Shahab I, Hicks J. Tracheobronchial mucoepidermoid carcinoma in childhood and adolescence: case report and review of the literature. Int J Pediatr Otorhinolaryngol. (1998) 45(3):265–73. doi: 10.1016/S0165-5876(98)00120-7
37. Serra A, Schackert HK, Mohr B, Weise A, Liehr T, Fitze G. t(11;19)(q21;p12∼p13.11) and MECT1-MAML2 fusion transcript expression as a prognostic marker in infantile lung mucoepidermoid carcinoma. J Pediatr Surg. (2007) 42(7):E23–9. doi: 10.1016/j.jpedsurg.2007.04.031
Keywords: pulmonary mucoepidermoid carcinoma (PMEC), children, flexible bronchoscopy, atelectasis, surgery
Citation: Huang Y, Fu Y, Sun J, Xu B, Wu L and Tang L-f (2023) Pulmonary mucoepidermoid carcinoma in children: two case reports and a review of the literature. Front. Pediatr. 11:1232185. doi: 10.3389/fped.2023.1232185
Received: 31 May 2023; Accepted: 29 August 2023;
Published: 12 September 2023.
Edited by:
Jing Liu, Capital Medical University, ChinaReviewed by:
Wei-Chin Chang, Mackay Memorial Hospital, TaiwanHaifeng Zong, Southern Medical University, China
© 2023 Huang, Fu, Sun, Xu, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan-fang Tang NjE5NTAwN0B6anUuZWR1LmNu Lei Wu emp1ZW50QHpqdS5lZHUuY24=
 Yuan Huang
Yuan Huang Yong Fu
Yong Fu Jing Sun
Jing Sun Bin Xu
Bin Xu Lei Wu
Lei Wu Lan-fang Tang
Lan-fang Tang