- Pediatric Department, Peking University Third Hospital, Beijing, China
Background: Central venous catheterization (CVC) is broadly used in neonatal intensive care units (NICUs) for efficient vascular access; however, its establishment and maintenance are associated with numerous risks and complications. Here, we focus on investigating the value of point-of-care ultrasound (POCUS) in the early diagnosis and treatment of pericardial effusion associated with CVC and compare the differences in ultrasound and radiography in CVC localization and monitoring in the NICU.
Methods: Twenty-five infants with CVC-associated pericardial effusion (PCE) who were hospitalized in the NICU of Peking University Third Hospital between January 2013 and March 2023 were retrospectively selected for the study. Data concerning their catheterization characteristics, CVC tip position, clinical and imaging manifestations of PCE, treatments, and prognoses were analyzed.
Results: The mean gestational age of our cohort was 29.3 ± 3.1 weeks, and the mean birth weight was 1,211 ± 237 g. The incidence of CVC-associated PCE was 0.65%, and 80% of PCE cases occurred within 4 days of CVC. After PCE, the most common symptoms were tachypnea (44%) and tachycardia (64%). Chest radiographs revealed cardiothoracic enlargement, and only 2 cases (9.10%) showed a “flask heart”. Cardiac ultrasound showed that the catheter tip extended deep into the heart in 72% of infants with PCE. Cardiac insufficiency was observed in 12 cases (48%). Overall, 8 infants (32%) had pericardial tamponade, 7 (87.5%) of whom underwent pericardiocentesis. Overall, 2 (8%) infants died, and the remaining 23 (92%) were cured.
Conclusion: CVC-associated PCE mostly occurs in the early post-catheterization stages (within 4 days) in infants. Some cases may have critical clinical manifestations and progress rapidly, with some even developing pericardial tamponade. A CVC tip being deep into the heart cavity is an important cause of PCE. Compared with chest radiography, point-of-care ultrasound is more accurate for CVC tip positioning and can detect PCE more quickly. Furthermore, it is more advantageous for locating and monitoring CVC-associated PCE. Early identification and diagnosis can effectively reduce fatality rates and improve the prognosis of infants with CVC-associated PCE.
1. Introduction
Central venous catheterization (CVC) allows rapid and effective vascular access and is widely used in neonatal intensive care units (NICUs). It is a minimally invasive, safe, and feasible mode of catheterization for infants in the early neonatal period. CVC can effectively reduce the incidence of repeated puncture injuries and peripheral phlebitis, making it an ideal option for mid-to-long-term intravenous infusions in preterm infants. The umbilical venous catheter (UVC) and peripherally inserted central catheter (PICC) are the most widely used (1, 2). Pericardial effusion (PCE) is a CVC-associated complication that can progress to pericardial tamponade. The accumulation of a large volume of fluid in the pericardium can affect the heart's diastolic function. If not identified, diagnosed, and treated in time, this phenomenon can cause severe hemodynamic disturbances with a high mortality risk. Therefore, locating and monitoring CVC are critical and are mainly performed using x-rays and ultrasound examination.
In this study, we retrospectively analyzed the data of 25 infants with CVC-associated PCE who were treated in the NICU of our hospital over the past 10 years. We analyzed their clinical manifestations, catheterization characteristics, treatment strategies, and prognoses. We also compared the differences and accuracies in ultrasound and radiography, to improve the identification and treatment of CVC-associated PCE and choose the best monitoring technique.
2. Materials and methods
2.1. Study population
This retrospective cohort study included 25 infants who were admitted to the NICU at Peking University Third Hospital after birth and underwent CVC during hospitalization between January 2013 and March 2023. The inclusion criteria were age <28 days and the presence of PCE while a central venous catheter was left in place. The exclusion criteria were PCE detected before catheterization or after catheter removal; the presence of other factors or underlying diseases that led to PCE, such as congenital heart disease, cardiomyopathy, severe anemia, and hypoproteinemia; cardiac insufficiency and rheumatic immune diseases that were present before catheterization; and confirmed intrauterine infections, such as infections caused by Toxoplasma gondii, rubella virus, cytomegalovirus, herpes simplex virus, or parvovirus B19. The studies involving human participants were reviewed and approved by the Peking University Third Hospital Medical Science Research Ethics Committee. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and institutional requirements.
2.2. Data collection
Patient data, including basic demographic information; CVC type; time, site, and length of insertion; and catheter position in ultrasound and radiography, were recorded. Data on the time of PCE discovery, clinical manifestation, management measures, ancillary examination, and prognosis were also collected. Cardiography was performed by pediatric physicians with basic training.
2.3. The CVC tip position
Numerous authors have suggested that the CVC line tip should be located outside the heart contour. The advocate placement of the PICC tip in the upper limb is the intersection of the superior vena cava and right atrium, with radiographs from the 4th to the 6th thoracic vertebrae. From the lower extremities, the tip of PICC should be located in the inferior vena cava below the thorax, with radiographs from the 9th to the 11th thoracic vertebrae (3). The tip of a UVC should be positioned at the intersection of the inferior vena cava and right atrium (4, 5). With radiographs, the best position is 0.5–1 cm above the diaphragm or at the 9th thoracic vertebra level (6).
2.4. PCE diagnosis
The diagnosis was based on echocardiography. PCE diagnosis was confirmed when sonolucent fluid was observed in the pericardial cavity by probing the apical four-chamber, parasternal four-chamber, aortic short-axis, left ventricular long-axis, and subxiphoid four-chamber sections (7). The depths of the sonolucent fluid in the anterior and posterior pericardial cavities, outer region of the wall, and apex of the heart were measured at the end of expiration and ventricular diastole.
2.5. PCE grading
PCE grading was based on the results from adults, and the main reference in the neonatal period was PCE distribution. The grading was as follows: mild, when probed in the supine position, with the sonolucent fluid being detected only in the posterior pericardial cavity and not extending to other areas of the pericardial cavity; moderate, when fluid accumulation was observed around the heart, predominantly located in the inferior wall of the left ventricle; and severe, surrounding the entire heart, with a swinging heart sign and clinical signs of cardiac tamponade (8).
2.6. Statistical analysis
All analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA). The Kruskal–Wallis test was used for continuous variables. We calculated the mean (±standard deviation) for continuous variables conforming to a normal distribution.
3. Results
3.1. General information
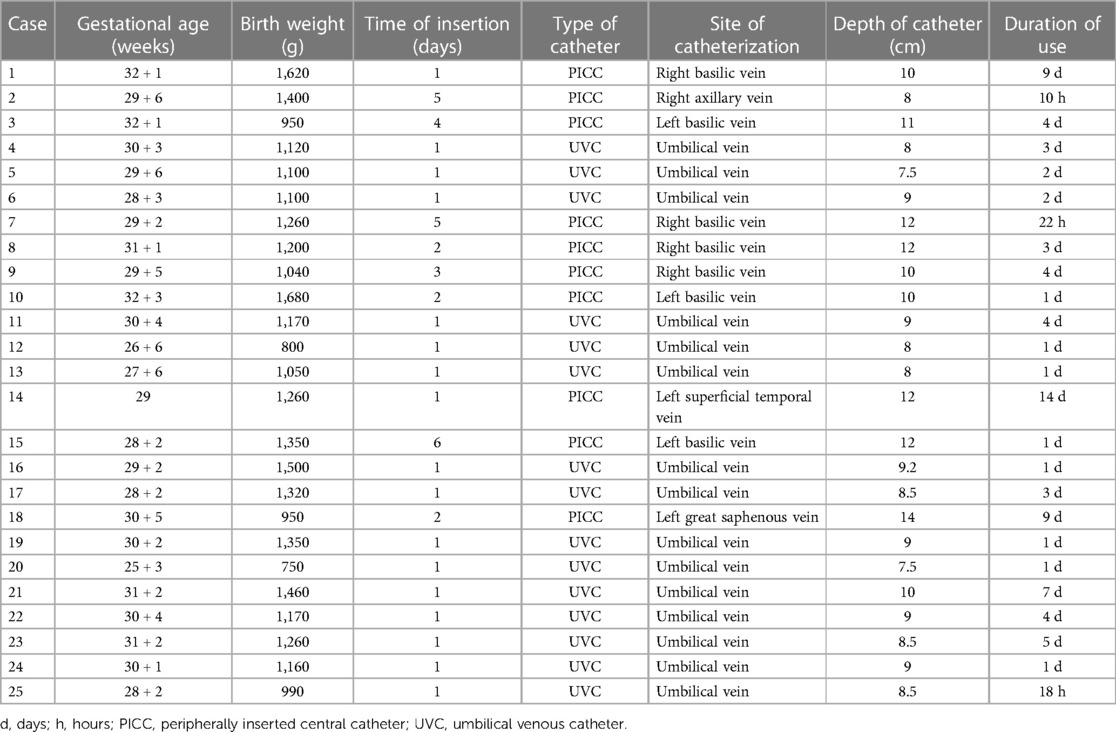
A total of 3,856 CVC cases in the NICU were retrospectively selected between January 2013 and March 2023, including 1,997 UVC and 1,859 PICC cases. Twenty-five cases of CVC-associated PCE occurred, including 15 and 10 cases associated with UVCs and PICC, respectively. Among those cases, 11 (11/25, 44%) and 14 (14/25, 56%) involved male and female infants, respectively. The mean gestational age was 29.3 ± 3.1 weeks (range, 25–32 weeks), and the mean birth weight was 1,211 ± 237 g (range, 750–1,680) g. In total, 80% (20/25) of PCE cases developed within 4 days (range, 1–4 days) of CVC. The PICC was inserted in the upper and lower extremities in nine and one case, respectively (Table 1).
3.2. Clinical manifestations
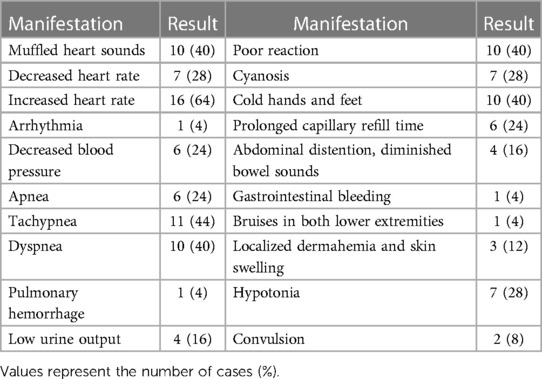
In total, 10 (40%), 14 (56%), 20 (80%), 7 (28%), 4 (16%), and 8 (32%) infants manifested poor reaction, respiratory symptoms, circulatory symptoms, neurological symptoms, digestive symptoms, and other symptoms, respectively. Severe cases may show shock manifestations, such as decreased blood pressure, poor peripheral circulation and perfusion, and multiple organ dysfunction. Five (5/25, 20%) cases had no obvious clinical symptoms or positive signs, and PCE was detected only on routine point-of-care ultrasound (POCUS) examination. Detailed clinical manifestations are presented in Table 2.
3.3. Chest radiography
A total of 96% (24/25) of infants underwent chest radiography after catheterization. Using vertebral-level assessment, the catheter tips were placed in the correct position in 4 (4/24, 16.67%) cases, at the peripheral depth in 2 (2/24, 8.33%) cases, and too deep in 18 (18/24, 75%) cases. The diaphragmatic level was used to evaluate the position of the UVC tip; in 8 (8/15, 53.33%) cases, the position was correct, and the CVC tips were too deep in 7 cases (7/15, 46.67%). The positions of the CVC tips were adjusted in 11 cases according to x-ray positioning. Chest radiographs were re-examined in 22 (22/25, 88%) infants following PCE development. CVCs were removed in 13 (13/22, 59.10%) infants. Using vertebral-level assessment, the catheter tips were correctly placed in three (3/9, 33.33%) cases but were too deep in six (6/9, 66.67%) cases. Using the diaphragmatic level to evaluate the position of the UVC tip, the CVC tips were correctly placed in three (3/6, 50%) cases, too shallow in one (1/9, 11.11%) case, and too deep in two (2/9, 22.22%) cases. The cardiothoracic ratio increased after PCE development, and the difference was significant (0.53 ± 0.05 vs. 0.60 ± 0.08, P < 0.05) (Table 3).
3.4. Echocardiogram
In total, 76% (19/25) of infants underwent chest echocardiography after catheterization, which revealed that the catheter tips were correctly positioned in 5 (5/19, 26.32%) cases, deep into the atrium in 12 (12/19, 63.16%) cases, and in the peripheral vein in 2 (2/19, 10.53%) cases. After PCE, all infants underwent ultrasound examination. The positions of the catheter tips were correct in 5 (5/25, 20%) cases, deep into the atrium in 18 (18/25, 72%) cases, and located in the peripheral vein in 2 (2/25, 8%) cases. The left ventricular systolic function was reduced after PCE (67.38 ± 5.51 vs. 53.84 ± 12.09, P < 0.05). After PCE, echocardiography revealed a median maximum fluid depth of 4.6 mm (range, 2.8–14.0 mm) and a visible swinging heart sign in 6 (6/25, 24%) cases and indicated left ventricular dysfunction in 11 (11/25, 44%) cases (Table 3).
3.5. Clinical treatment
Pericardial tamponade occurred in 8 infants (8/25, 32%, Cases 1–4, 9, 12, 13, and 24). Among them, 7 (7/8, 87.5%) underwent pericardiocentesis. The other 18 (18/25, 72%) received conservative treatments, such as an altered fluid rehydration regimen, CVC removal, fluid volume limiting, and cardiac function enhancement.
3.6. Laboratory findings of the pericardial fluid aspirated through pericardiocentesis
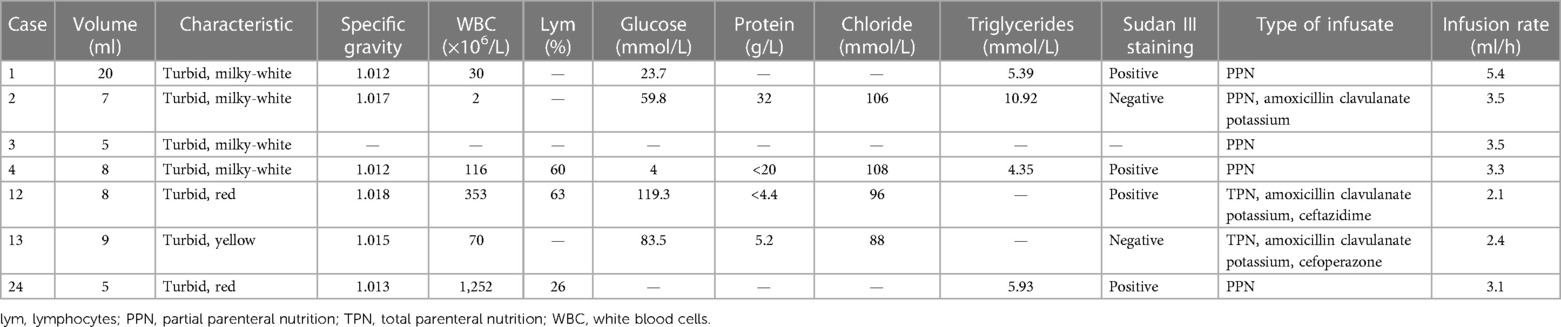
In this study, 7 infants (7/25, 28%) underwent pericardiocentesis. The volume of aspirated pericardial fluid was 5–20 ml, and it had a milky-white, red, and light-yellow color in four, two, and one case, respectively. Laboratory tests for the aspirated fluid in Case 4 suggested an increased cell count with mainly lymphocytes and elevated triglyceride levels. In the other 5 cases, laboratory test results suggested a low cell count and lymphocyte percentage, and the glucose concentration was significantly higher than that in the serum (Table 4). All infants were provided constant partial parenteral nutrition infusion by CVC, and antibiotics were administered by CVC to three infants with no history of fluid resuscitation within 12 h.
3.7. Prognoses and outcomes
Two of the 25 (2/25, 8%) infants died, one of whom had coexisting severe metabolic acidosis, internal environmental disturbances, and acute liver and kidney failure; the infant died 44 h after the disease condition deteriorated. The other one had comorbid respiratory and circulatory failure, severe coagulation disorder, and anemia and died within 3 h of pericardial tamponade development. Among the 23 (23/25, 92%) surviving infants, 6 had pericardial fluid absorption within 4 days after pericardiocentesis. Among the 17 infants treated conservatively, the pericardial fluid was absorbed within 17 days (range, 1–17 days). None of the surviving infants showed structural or functional heart damage during the subsequent follow-up period.
4. Discussion
CVC-associated PCE and pericardial tamponade most commonly occur in preterm infants hospitalized in the NICU and are the most common causes of acquired chylous serous cavity effusion (9). In this study, we demonstrate that POCUS is superior to radiographs in terms of CVC tip localization and monitoring, accuracy of identifying complications, and effect of guiding pericardiocentesis. Therefore, monitoring of CVC by POCUS should be popularized in the NICU.
In this study, the incidence rate of CVC-associated PCE was 0.65% (0.75% for UVC and 0.54% for PICC). The mortality rate of infants with pericardial tamponade was 25% (2/8), accounting for 0.02% of CVC cases. Therefore, PCE is a rare complication of CVC, and progression to pericardial tamponade indicates poor prognosis (10). In this study, all neonates were preterm infants with gestational ages of 25–32 weeks and birth weight of 800–1,680 g, suggesting that low birthweight preterm infants are more prone to PCE and/or pericardial tamponade. The pathological mechanism is related to the thin and immature cardiac tissue. In our study, PCE occurred between 10 h and 9 days after birth, and 80% (20/25) of cases developed within 4 days of CVC catheterization, indicating that PCE is more likely to occur in the early post-catheterization stage. Although the CVC tips were too deep based on the radiographic findings in 18 cases, the position of the CVC tip was adjusted in 11 (11/25, 44%) infants after catheterization. However, in 15 (15/25, 60%) infants, the position of the CVC tip had changed during the application. This may have been caused by factors resulting in a large fluctuation in the abdominal circumference in preterm infants, such as physiological weight loss, feeding intolerance, and application of a ventilator. Moreover, limb movement, umbilical cord contracture, and heartbeat can affect the position of the catheter tip (11, 12). Therefore, catheter tip displacement is inevitable, and CVC tip displacement can also lead to PCE development. In this study, echocardiography indicated that the CVC tip was too deep in 18 (18/25, 72%) infants when PCE occurred. CVC-associated PCE can be caused by atrial wall perforation resulting from mechanical injury caused by the CVC tip, but it is most commonly caused by erosion of the right atrial wall (13–15). In this study, the CVC tips were correctly positioned in 5 (5/25, 20%) infants when PCE occurred, with persistent infusion of partial parenteral nutrition, suggesting that persistent infusion of high permeability liquid can damage the endothelial cells and endocardium. The aforementioned can affect vascular permeability and eventually cause PCE (16). Four (4/25, 16%) infants had histories of repeated catheterization, suggesting that repeated penetration of the vena cava by the catheter and multiple irritations of the atrial wall can lead to injury and even micro-perforations, which can ultimately trigger PCE development (15). Therefore, a greater catheter depth is one of the main causes of PCE; parenteral nutrition and repeated catheterization are additional risk factors. Radiographs provide a static, single image to demonstrate the CVC tip position, and it is difficult for infants to maintain an ideal position during the procedure; therefore, detecting alterations of the CVC tip via radiography is not always appropriate. In radiography, inaccurate determinations of the CVC tip may result from difficulties in detecting the landmarks of the superior and inferior vena cava (17). However, infants have great acoustic windows for the examination of the right atrium and a large portion of the superior and inferior vena cava. The relationship of the CVC tip with the heart can be accurately assessed (18). Compared with radiography, echocardiography provides real-time information on CVC tip position, helping to accurately show the catheter tip and the range of position changes associated with limb movement in the ultrasound image (13, 17). Ultrasonic verification of the CVC tip position requires less time and is repeatable and easy to perform (11). This study demonstrates the advantages of echocardiography in accurately detecting the position of PICC tips within the heart. Therefore, we suggest that POCUS can be used for the initial confirmation and follow-up of the CVC tip position.
The increase in the pericardial cavity pressure resulted in a limitation in the cardiac diastolic function, a decrease in cardiac output, and an accumulation of a large fluid volume in the pericardial cavity within a short period, leading to rapid symptom onset. In this study, infants mainly manifested respiratory and circulatory symptoms, rapid progression to multi-organ and multi-system involvement, and internal environmental disturbances (14). Both infants who eventually died had convulsions, which were considered to be related to the development of severe hypoxia, irreversible metabolic acidosis, and internal environmental disturbances. Owing to the lack of typical clinical manifestations in the early stages, PCE identification is often difficult (18). Furthermore, owing to the rapid progression of pericardial tamponade, radiography could not be completed for most infants immediately, resulting in a delayed diagnosis. In this study, radiography suggested that the cardiothoracic ratio of the infants increased after PCE development. However, the majority of the infants had a normal cardiac silhouette and did not show a typical flask-shaped heart. Therefore, radiography is inaccurate and time-consuming, which limits the identification and diagnosis of PCE (19). Conventional radiology cannot be considered the “gold standard” for ECC tip location (20). In contrast, bedside echocardiography can help visualize the catheter position in relation to the heart, allow real-time localization of the catheter tip, identify and evaluate the degree of PCE to confirm the diagnosis, and identify the presence of cardiac compression with pericardial tamponade (21).
In this study, radiographic findings revealed that the catheter was correctly placed in 5 (5/25, 20%) infants, and echocardiography suggested that the tip was deep in the right atrium. The results of the two methods differed, consistent with previous findings (22). Chinese guidelines for central vein catheterization recommend that the central vein catheter tip should preferentially be positioned with radiography, with the landmarks being the thoracic vertebral bodies and the diaphragm. The correct position of the UVC tip is usually 0.5–1.0 cm above the diaphragm or at the 9th thoracic vertebra level (6). These two landmarks are often different, which can be confusing for clinicians. Moreover, the diaphragm is a movable structure that is influenced by factors, such as spontaneous breathing, pulmonary disease, ventilator parameters, and intra-abdominal pressure. These factors contribute to a wide range of motion. Ades et al. reported that catheters properly placed at the right atrial/inferior vena cava junction or in the inferior vena cava, as documented by echocardiography, were located at a wide range of vertebral bodies by the cardiothoracic ratio (T6–T11) (22). The wide variability in atrial size, position, and redundancy of the atrial septum has resulted in no fixed position relationship with the thoracic vertebra. In very low birth weight newborns, correct placement of the UVC tip during radiography does not avoid misplacement by echocardiography (13). Unnecessary removal and attempted replacement of the UVC may result in increased manipulation of the infant, possible loss of central access, and increased radiation exposure. Considering radiation exposure, protection, temperature, and position management, chest radiography in combination with lateral x-ray may be challenging to perform. Therefore, radiography alone is insufficient for determining the adequate position of a CVC, especially in premature infants (13, 22). Ultrasonography enables the quantification of the difference between the tip position, as observed in echocardiography and radiography. POCUS can help visualize the position of the catheter in the heart, allowing the real-time position of the catheter to be identified and evaluated, which has more advantages in CVC positioning (23).
In this study, 8 infants (8/25, 32%) developed pericardial tamponade with severe hemodynamic disturbances, 2 (2/8, 25%) died, and 6 (6/8, 75%) were cured. If an infant with an indwelling CVC develops reduced cardiopulmonary function that cannot be explained by other causes, the possibility of PCE and pericardial tamponade should be considered. Pericardiocentesis can effectively reduce the fatality rate of pericardial tamponade and prevent progression to cardiac arrest (11). In this study, cases of pericardial tamponade were all found by POCUS examination. Seven infants underwent pericardiocentesis under POCUS guidance with an aspirated fluid volume of 5–20 ml. When pericardial tamponade occurred, all infants were provided partial parenteral nutrition infusion at a low speed (2.1–5.4 ml/h) and had no history of liquid expansion in the last 12 h, glucose concentration ≤15%, and osmotic pressure ≤900 mOsm/L. Six infants (6/7, 85.71%) who underwent pericardiocentesis only demonstrated pericardial fluid, and the laboratory test results revealed a low cell count and lymphocyte percentage but a higher glucose concentration than that in the serum, which was inconsistent with the typical characteristics of chyle. Moreover, the effusion did not progress after pericardiocentesis or removal of the central venous catheter. Combined with the pathophysiological findings reported in the literature, these are thought to be associated with the leakage of parenteral nutrient solutions caused by CVC (24). In this study, 17 infants (17/25, 68%) received conservative treatment only, and PCE was absorbed in 1–17 days, suggesting that cases of moderate or low volume PCE with stable hemodynamics can be treated conservatively, with several procedures, such as fluid restriction, applied diuretics, and cardiac stimulants. The surviving infants exhibited no heart structural or functional damage in the long-term follow-up period. Therefore, pericardial effusion can be asymptomatic or a life-threatening event. The abrupt onset of hemodynamic instability without an obvious cause with a CVC in situ should raise suspicion of pericardial effusions (20, 25). POCUS can also be used as a localization tool to assist in pericardiocentesis and reduce the exposure of infants to radiation (26). Concurrently, it can reveal changes in ventricular size and cardiac output and identify other heart diseases, such as congestive heart failure. Early identification, diagnosis, and intervention result in positive outcomes and prognoses (27).
Our echocardiography procedure, performed by pediatric physicians with extensive formal training, added strength and high accuracy to our study results. This also revealed that with basic training, implementing POCUS in NICUs is feasible. POCUS can improve the rapid diagnosis of PCE and pericardial tamponage and other critical conditions, reduce radiation exposure, strengthen the continuous monitoring of CVCs (28). Ultrasound can be performed in real time and is the optimal technique for CVC tip positioning in infants (29, 30). Neonatologists can accurately localize the CVC tip and evaluate the hemodynamic and cardiac function (31). However, one of the obstacles to its widespread use is its high operability or dependence. Similar to other technical skills, proper and normalized training and certifications are essential (11). POCUS has not been widely adopted in NICUs in China owing to issues concerning the ultrasound equipment and personnel qualifications, which are also issues in many developing countries. Collaboration with pediatric cardiologists and robust training and accreditation programs are essential to ensure safety and quality service. Therefore, it is essential to provide basic technical training for neonatal physicians, improve cooperation with pediatric cardiology ultrasound specialists, promote the wider application of POCUS in the NICU, and improve its safety in clinical practice.
Our study was limited by its moderate sample size, single-center retrospective nature, and low incidence of CVC-related PCE in the NICU, which may have introduced bias in infant selection and study results. Further research with large samples from multiple centers should be conducted to verify our findings.
In conclusion, CVC-associated PCE mostly occurs in preterm infants with young gestational ages and low birth weights and tends to occur within 4 days after catheterization. It is mostly associated with the CVC tip being located deep inside the atria and has varying clinical manifestations that can rapidly progress to circulatory failure. CVC tip displacement cannot be completely avoided, complications may be fatal, and it is essential to strengthen monitoring. Echocardiography is superior to radiography in the localization and monitoring of CVC. It can obtain anatomical or physiological information to help in making physiology-based decisions or target specific interventions. Regarding CVC and related pericardial effusion, POCUS performed by neonatologists in the NICU offers advantages, such as safety, convenience, rapidity, sensitivity, and high repeatability. POCUS resulted in rapid identification, diagnosis, and timely symptomatic management, significantly reducing diagnosis and treatment duration and improving the clinical prognosis. Thus, POCUS performed by neonatologists in NICUs should become a routine part of clinical practice after standardized training and assessment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Medical Science Research, Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Patient consent was waived because of the retrospective nature of this study and because most of the guardians and parents of the patients resided out of town and it was difficult to obtain their signatures for the informed consent form. Written informed consent was not obtained from the minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article because Patient consent was waived because of the retrospective nature of this study and because most of the guardians and parents of the patients resided out of town and it was difficult to obtain their signatures for the informed consent form.
Author contributions
YZ and AY collected data; YZ prepared the original draft; YL reviewed and edited the manuscript and supervised and provided the methodology. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the Beijing Natural Science Foundation (M22018).
Acknowledgments
We thank all the staff, infants, and their families who participated in this study. We also thank Editage for the language improvement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wen J, Yu Q, Chen H, Chen N, Huang S, Cai W. Peripherally inserted central venous catheter-associated complications exert negative effects on body weight gain in neonatal intensive care units. Asia Pac J Clin Nutr. (2017) 26:1–5. doi: 10.6133/apjcn.112015.07
2. Oestreich AE. Umbilical vein catheterization–appropriate and inappropriate placement. Pediatr Radiol. (2010) 40:1941–9. doi: 10.1007/s00247-010-1840-2
3. Gorski LA. Infusion nursing standards of practice. J Infus Nurs. (2007) 30:151–2. doi: 10.1097/01.NAN.0000270673.13439.95
4. Yoder D. Cardiac perforation and tamponade: the deadly duo of central venous catheters. Int J Trauma Nurs. (2001) 7:108–12. doi: 10.1067/mtn.2001.117434
5. Concepcion NDP, Laya BF, Lee EY. Current updates in catheters, tubes and drains in the pediatric chest: a practical evaluation approach. Eur J Radiol. (2017) 95:409–17. doi: 10.1016/j.ejrad.2016.06.015
6. Neonatal Group of Pediatric Branch of Chinese Medical AssociationHospital Infection Control Committee of China Maternal and Child Health Care Association, National Children’s Medical Center, Beijing Children's Hospital affiliated to Capital Medical University. Guidelines for the prevention and control of complications related to umbilical venous catheterization in neonates. Chin J Neonatol (2021) 36:1–9. doi: 10.3760/cma.j.issn.2096-2932.2021.02.001
7. Expert group of the clinical application of bedside ultrasound in emergency medicine and intensive care medicine. Expert consensus on the clinical application of bedside ultrasound in emergency medicine and intensive care medicine. Chin J Emerg. (2016) 25:10–21. doi: 10.3760/cma.j.issn.1671-0282.2016.01.005
8. Zhu XM, Xie MX, Zhang XS. Guidelines for clinical ultrasound measurement [M]. Jiangsu Science and Technology Press. (2012):182–3.
9. Lu DF, Tong XM. A clinical analysis of neonatal chylous effusions. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22:328–33. doi: 10.7499/j.issn.1008-8830.1910018
10. Liz CF, Domingues S, Pinho L, Lopes L, Carvalho C, Magalhães M, et al. Neonatal pericardial effusion: case report and review of the literature. J Pediatr Neonatal Individ Med. (2020) 9:e090111. doi: 10.7363/090111
11. Zaghloul N, Watkins L, Choi-Rosen J, Perveen S, Kurepa D. The superiority of point of care ultrasound in localizing central venous line tip position over time. Eur J Pediatr. (2019) 178:173–9. doi: 10.1007/s00431-018-3269-9
12. He X, Wu S, Zhang F, Ge W, Wu D, Chen M, et al. Assessing peripherally inserted central catheter tip location in multiple postures: a case report. Asia Pac J Oncol Nurs. (2023) 10:100238. doi: 10.1016/j.apjon.2023.100238
13. Karber BC, Nielsen JC, Balsam D, Messina C, Davidson D. Optimal radiologic position of an umbilical venous catheter tip as determined by echocardiography in very low birth weight newborns. J Neonatal Perinatal Med. (2017) 10:55–61. doi: 10.3233/NPM-1642
14. Tseng MH, Lin SH, Chung HT, Hsu TC, Lien R, Huang JL, et al. Severe hyponatremia secondary to peripherally inserted central catheter in a neonate. Pediatr Neonatol. (2016) 57:541–3. doi: 10.1016/j.pedneo.2015.08.010
15. Jumani K, Advani S, Leacy F, Gosey L, Milstone A. Epidemiology and risk factors for complications necessitating peripherally inserted central venous catheter (PICC) removal in hospitalized children. Infectious Diseases Society of America 2011 Annual Meeting. (2011).
16. Unal S, Arifoglu I, Celik IH, Yilmaz O, Bas AY, Demirel N. Pleural and pericardiac effusion as a complication of properly placed umbilical venous catheter. J Neonatal Surg. (2017) 6:34. doi: 10.21699/jns.v6i2.508
17. Tauzin L, Sigur N, Joubert C, Parra J, Hassid S, Moulies ME. Echocardiography allows more accurate placement of peripherally inserted central catheters in low birthweight infants. Acta Paediatr. (2013) 102:703–6. doi: 10.1111/apa.12245
18. Barreiros LL, Andrade FM, Torres RA, Magalhães LVB, Farnetano BDS, Fiorelli RKA. Cardiac tamponade by peripherally inserted central catheter in preterm infants: role of bedside ultrasonography and therapeutic approach. Rev Col Bras Cir. (2018) 45:e1818. doi: 10.1590/0100-6991e-20181818
19. Barone G, Pittiruti M, Biasucci DG, Elisei D, Iacobone E, La Greca A, et al. Neo-ECHOTIP: a structured protocol for ultrasound-based tip navigation and tip location during placement of central venous access devices in neonates. J Vasc Access. (2022) 23:679–88. doi: 10.1177/11297298211007703
20. Zareef R, Anka M, Hatab T, El Rassi I, Yunis K, Bitar F, et al. Tamponade and massive pleural effusions secondary to peripherally inserted central catheter in neonates-A complication to be aware of. Front Cardiovasc Med. (2023) 10:1092814. doi: 10.3389/fcvm.2023.1092814
21. Razak HA, Abdullah N, Alias EY, Mat Bah MN. Umbilical venous catheter-associated cardiac tamponade: a rare complication. 22nd Annual Congress of Perinatal Society of Malaysia. (2015). doi: 10.13140/RG.2.2.26136.21762
22. Ades A, Sable C, Cummings S, Cross R, Markle B, Martin G. Echocardiographic evaluation of umbilical venous catheter placement. J Perinatol. (2003) 23:24–8. doi: 10.1038/sj.jp.7210851
23. Katheria AC, Fleming SE, Kim JH. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J Perinatol. (2013) 33:791–4. doi: 10.1038/jp.2013.58
24. Warren M, Thompson KS, Popek EJ, Vogel H, Hicks J. Pericardial effusion and cardiac tamponade in neonates: sudden unexpected death associated with total parenteral nutrition via central venous catheterization. Ann Clin Lab Sci. (2013) 43:163–71.23694791
25. Paladini A, D'Andrea V, Bottoni A, Purcaro V, Prontera G, Costa S, et al. Ultrasound-guided diagnostic pericardiocentesis in preterm infants: a case report. J Matern Fetal Neonatal Med. (2023) 36:2212831. doi: 10.1080/14767058.2023.2212831
26. Zarkesh MR, Haghjoo M. Neonatal cardiac tamponade, a life-threatening complication secondary to peripherally inserted central catheter: a case report. J Med Case Rep. (2022) 16:305. doi: 10.1186/s13256-022-03506-4
27. Singh Y, Bhombal S, Katheria A, Tissot C, Fraga MV. The evolution of cardiac point of care ultrasound for the neonatologist. Eur J Pediatr. (2021) 180:3565–75. doi: 10.1007/s00431-021-04153-5
28. Casani A, Tozzi N, Cocca F. The impact of neonatologist performed echocardiography in an Italian neonatal unit. J Neonatal Perinatal Med. (2022) 15:237–42. doi: 10.3233/NPM-210811
29. D'Andrea V, Cascini V, Russo R, Perri A, Prontera G, Ancora G, et al. The role of ultrasound in epicutaneo-caval catheter insertion in neonates: systematic review, meta-analysis and future perspectives. Diagnostics (Basel. (2023) 13(17):2850. doi: 10.3390/diagnostics13172850
30. D'Andrea V, Prontera G, Cota F, Russo R, Barone G, Vento G. Real-time ultrasound tip location reduces malposition and radiation exposure during epicutaneo-caval catheter placement in neonates. Am J Perinatol. (2023. doi: 10.1055/s-0043-1760744
Keywords: pericardial effusion, pericardial tamponade, peripherally inserted central catheter, point-of-care ultrasound, umbilical venous catheter
Citation: Zhang Y, Yan A and Liu Y (2023) The advantage of point-of-care ultrasound in central venous catheterization and related pericardial effusion in infants in the NICU. Front. Pediatr. 11:1228070. doi: 10.3389/fped.2023.1228070
Received: 24 May 2023; Accepted: 27 October 2023;
Published: 10 November 2023.
Edited by:
Fahri Ovalı, Istanbul Medeniyet University, TürkiyeReviewed by:
Vito D'Andrea, Agostino Gemelli University Polyclinic (IRCCS), ItalySadık Yurttutan, Kahramanmaras Sütçü Imam University, Türkiye
© 2023 Zhang, Yan, Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfeng Liu bGl1eXVuZmVuZzk2QDE2My5jb20=
Abbreviations CVC, central venous catheterization; NICU, neonatal intensive care unit; PCE, pericardial effusion; PICC, peripherally inserted central catheter; POCUS, point-of-care ultrasound; UVC, umbilical venous catheter.
 Yahui Zhang
Yahui Zhang Aijing Yan
Aijing Yan Yunfeng Liu
Yunfeng Liu