
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 11 August 2023
Sec. Pediatric Obesity
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1226933
A correction has been applied to this article in:
Corrigendum: Association between precocious puberty and obesity risk in children: a systematic review and meta-analysis
Objectives: The aim of this study was to evaluate the potential association between early onset puberty and the risk of different forms of obesity in children.
Methods: The databases PubMed, EMBASE, Web of Science and Cochrane Library were systematically searched for relevant studies. The odds ratio (OR) and 95% confidence interval (CI) of obesity in precocious puberty were calculated using Stata software 14.0. A fixed-effects model was used if P > 0.1 and I2 ≤ 50%. Otherwise, a random-effects model was used. Publication bias was assessed using funnel plots and Egger's test.
Result: The pooling analysis showed that precocious puberty in girls was associated with a higher risk of obesity (OR = 1.98; 95% CI: 1.76–2.24; I2 = 0.00%, P < 0.001). Girls with a history of precocious puberty were found to have an increased risk of general obesity (OR = 2.03; 95% CI: 1.62–2.55; I2 = 22.2%, P < 0.001), central obesity (OR = 1.96; 95% CI: 1.70–2.26; I2 = 0.00%, P < 0.001), and overweight (OR = 2.03; 95% CI: 1.68–2.46; I2 = 5.1%, P < 0.001). The pooled analysis showed that precocious puberty in boys was not associated with an increased risk of obesity (OR = 1.14; 95% CI: 0.86–1.51; I2 = 50.6%, P = 0.369). In boys, the occurrence of precocious puberty was not associated with an elevated risk of general obesity (OR = 0.96; 95% CI: 0.40–2.27; I2 = 79.6%, P = 0.922), central obesity (OR = 1.17; 95% CI: 0.96–1.43; I2 = 0.00%, P = 0.125), or overweight (OR = 1.03; 95% CI: 0.56–1.88; I2 = 74.4%, P = 0.930).
Conclusion: This meta-analysis suggests that the onset of puberty at an early age in girls is associated with an increased risk of obesity, however precocious puberty in boy was not associated with an increased risk of obesity. These findings highlight that precocious puberty should be considered an independent risk factor for obesity in girls.
Systematic Review Registration: CRD42023404479.
Childhood obesity is a complex public health crisis that is prevalent in most developed countries worldwide. The prevalence of childhood obesity has increased faster than adult obesity in many countries since 1980, with more than 70 countries reporting a doubling of obesity rates. In 2015, there were 107.7 million obese children worldwide (1), and approximately 4 million deaths were attributed to high Body Mass Index (BMI), with 70% of these deaths resulting from cardiovascular disease related to high BMI (2). The prevalence of obesity in children was 5%, while the combined prevalence of obesity and overweight was as high as 23% (2). Obese children have a higher risk of cardiometabolic disease compared to their normal weight peers (3–6), and severe obesity is associated with a higher risk of premature death (7).
Pediatric endocrinology identifies sexual precocity as a prevalent endocrine disorder, as indicated by epidemiological surveys that demonstrate a notable and rapid rise in the incidence of precocious puberty among children (8). Epidemiological studies conducted in Denmark have demonstrated a substantial rise in the prevalence of precocious puberty among girls of Danish origin, up to six-fold, and boys of Danish origin, up to fifteen-fold (9). Similarly, a significant increase in the incidence and prevalence of precocious puberty was observed in both genders during an epidemiological survey in Korea from 2008 to 2014 (10). Precocious puberty can pose a psychological burden on affected children, leading to fear, anxiety, low self-esteem and other psychological disorders (11). Furthermore, it increases the risk of developing hypertension, diabetes and infertility in adulthood (8).
Childhood obesity arises from a complex interplay of various environmental and genetic factors (12). It is primarily caused by an energy imbalance, where caloric intake exceeds energy expenditure, leading to an accumulation of excess body weight and adipose tissue (1). Sedentary behavior, insufficient sleep, and consumption of calorie-dense, nutrient-poor diets are common behavioral causes of obesity (13). A meta-analysis revealed that obesity in girls is a risk factor for early onset of puberty (14). Numerous studies have demonstrated a positive association between BMI and early onset of puberty in girls (15, 16). However, it remains unclear whether precocious puberty is a risk factor for obesity in both boys and girls. This novel question lacks a definitive answer, which motivated our systematic review of population-based studies to evaluate the association between precocious puberty and obesity, general obesity, central obesity, and overweight.
The present meta-analysis adhered to the revised Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA 2020) guidelines (17) and was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO) platform with an approval number of CRD42023404479.
A comprehensive systematic search was conducted in major electronic databases including PubMed, Cochrane Library, Embase, and Web of Science up to 1 March 2023, without any restrictions on language, country, or study type. The search strategy utilized both medical subject headings (MeSH) and keywords. The search terms used were “Puberty precocious”, “Pubertas Praecox”, “Obesity”, “Overweight” and their variants. In addition, references of the included studies and other published systematic reviews were thoroughly reviewed to identify any additional relevant studies. The complete search strategy is presented in Supplementary Table S1.
The present study aimed to include eligible studies based on pre-defined criteria, which were (1) case-control study, cohort study or cross-sectional study, (2) investigations of the association between precocious puberty and the risk of obesity, central obesity or overweight, (3) reported risk of obesity as the outcome, presented as an adjusted odds ratio (OR) with its corresponding 95% confidence interval (CI). Obesity was considered the primary outcome, and general obesity, central obesity, or overweight were regarded as secondary outcomes. In cases where multiple studies reported data based on the same population, the study with the longest follow-up or the largest number of individuals was included.
Exclusion criteria were conference abstracts, study protocols, duplicate publications, and studies that had no outcomes of interest.
According to the pre-defined eligibility criteria, two reviewers (Yibu Kong and Xiaofei Xie) were independently selected to screen the literature for the study. Initial screening was performed based on the title and abstract of the articles, and review articles, duplicate publications, animal experiments, and irrelevant articles were excluded. Subsequently, the full-text of the remaining articles was downloaded and carefully read to identify all the studies that fulfilled the criteria. In case of any discrepancy, the reviewers consulted with Professor Yongji Wang.
The process of extracting data was carried out independently by two reviewers (Yibu Kong and Xiaofei Xie) using a pre-designed table. The table was used to extract relevant information such as the first author, year of publication, country or region where the study was conducted, type of study, sample size, study duration, age of participants, gender of population, diagnostic criteria for obesity, type of obesity and confounding variables adjusted for in the analysis. For the meta analytic calculations, adjusted OR at 95% CI for precocious puberty compared to normal development were extracted. When there are multiple subgroups in the same study, each subgroup is analysed as a separate variable. In case of any disagreement, a third reviewer (Yongji Wang) was consulted to reach a consensus.
Two reviewers (Yongfu Song and Yongji Wang) independently assessed the methodological quality and level of evidence of the included studies and resolved differences through discussion. The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) for cohort and case-control studies (18). The NOS evaluates the quality of a study based on three main criteria: study population selection, comparability of groups, and outcome or exposure assessment. The total score ranges from 0 to 9 stars. Based on the total score, the study was categorized as low quality (0–3 stars), moderate quality (4–6 stars), and high quality (7–9 stars).
For cross-sectional studies, the American Agency for Health Care Quality and Research's (AHRQ) tool was used to assess study quality (19). If the answer was “yes”, we assigned 1 point, if the answer was “unclear” or “no”, we assigned 0 point, and finally a maximum of 11 points could be awarded, with more points indicating higher study quality. Based on the scores, the studies were categorized as low quality (0–3 points), moderate quality (4–7 points), and high quality (8–11 points).
The stata software version 14.0 was used to perform the meta-analysis. The adjusted OR and its 95% CI from the included studies were used to calculate the relation between precocious puberty and obesity, general obesity, central obesity or overweight. Heterogeneity was calculated using I2-values. If P > 0.1 and I2 ≤ 50%, we used a fixed-effects model. If I2 > 50%, we used a random-effects model. Considering clinical heterogeneity, a random-effects model was adopted by most studies. The sensitivity analysis was performed to verify the robustness of the overall effects. The funnel plot and Egger's regression test was conducted to statistically assess publication bias.
A total of 2,228 articles were identified from the systematic search conducted before March 1, 2023, out of which 821 duplicate articles were excluded. Based on the screening of the title and abstract, 1,371 articles were excluded. Finally, seven studies were found to be eligible for inclusion in this meta-analysis (20–26). The process of eligible study selection has been depicted in Figure 1.
This meta-analysis includes four cohort studies (20, 22, 25, 26), and four cross-sectional studies (21–24), encompassing a total of 65,144 participants (24,464 boys and 40,680 girls) published between 2001 and 2022. One of the included studies contained both cross-sectional and cohort studies (22), and we classified compound obesity as central obesity. Although there were slight variations in the confounding factors such as age, gender, exercise, and energy intake, adjusted estimates were reported in all studies. The characteristics of the seven studies are summarized in Tables 1, 2.
According to NOS and AHRQ criteria, the scores of all included cohort and cross-sectional studies are shown in Tables 1, 2. Base on NOS criteria, two studies (20, 22) were scored as 8 (high quality) and two studies (25, 26) were scored as 7 (high quality), and the average score for each study was 7.5, indicating that all cohort studies were of high quality. Base on AHRQ criteria, a study (22) was scored as 7 (moderate quality) and 3 studies (21, 23, 24) were scored as 6 (moderate quality), and the average score for each study was 6.25, representing that all cross-sectional studies were of moderate quality.
The present meta-analysis investigated the association between precocious puberty and the risk of obesity, separately for boys and girls. A total of four studies (21, 22, 24, 25) reported on the association between precocious puberty in girls and the risk of obesity. The pooled analysis demonstrated a significant positive association between precocious puberty in girls and an increased risk of obesity (OR = 1.98; 95% CI: 1.76–2.24; I2 = 0.0%, P < 0.001; Figure 2B). Two studies (22, 24) investigated the association between precocious puberty in boys and the risk of obesity, and the pooled analysis revealed that precocious puberty in boys was not significantly associated with an increased risk of obesity (OR = 1.14; 95% CI: 0.86–1.51; I2 = 50.6%, P = 0.369; Figure 2A).
Four studies (21, 22, 24, 25) were included to investigate the association between precocious puberty in girls and the risk of general obesity. The pooled analysis indicated that precocious puberty in girls was significantly associated with a higher risk of general obesity (OR = 2.03; 95% CI: 1.62–2.55; I2 = 22.2%, P < 0.001; Figure 3B). Only one included study (22) assessed the relationship between precocious puberty in girls and the risk of central obesity, and it demonstrated that precocious puberty in girls was associated with an increased risk of central obesity (OR = 1.96; 95% CI: 1.70–2.26; I2 = 0.00%, P < 0.001; Figure 3B).
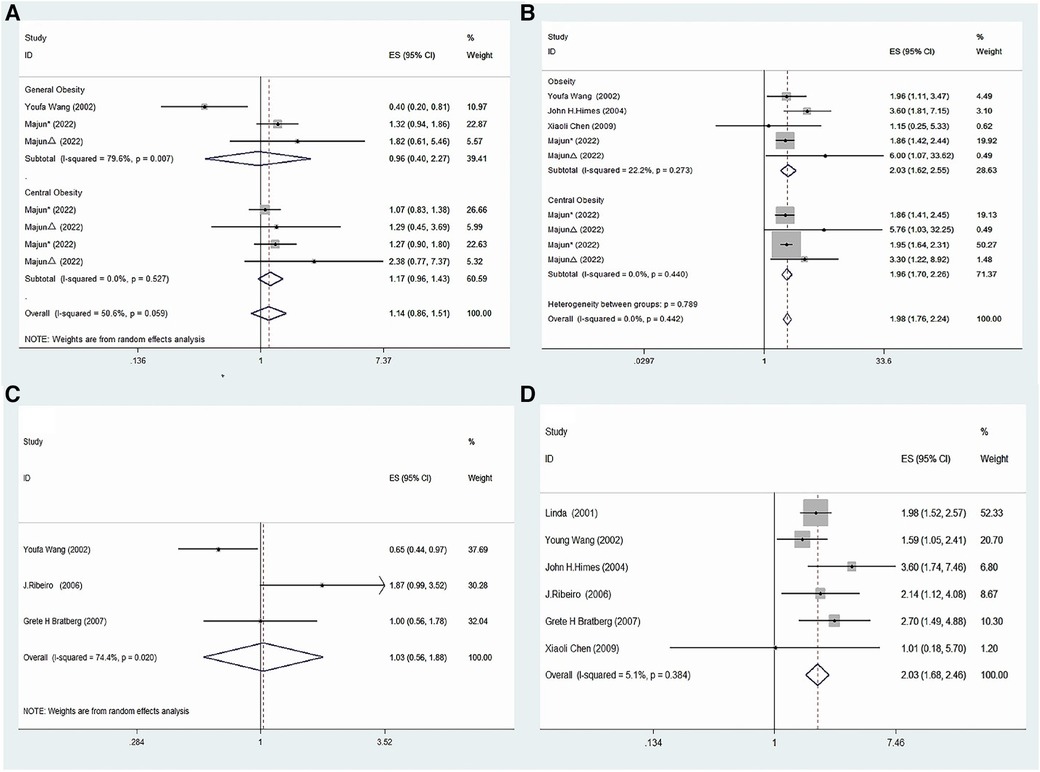
Figure 3. Meta-analysis of specific subgroups. (A) Precocious puberty in boys and the risk of various forms of obesity. (B) Precocious puberty in boys and the risk of various forms of obesity. (C) Precocious puberty in boys and the risk of overweight. (D) Precocious puberty in girls and the risk of overweight.
Two studies (22, 24) were included to evaluate the relationship between precocious puberty in boys and the risk of general obesity, and the analysis indicated that there was no significant increase in the risk of general obesity for boys with a history of precocious puberty (OR = 0.96; 95% CI: 0.40–2.27; I2 = 79.6%, P = 0.922; Figure 3A). Furthermore, one study (22) was included to assess the association between precocious puberty in boys and the risk of central obesity, which indicated that the boys with a history of precocious puberty did not have an increased risk of central obesity (OR = 1.17; 95% CI: 0.96–1.43; I2 = 0.0%, P = 0.125; Figure 3A).
Six included studies (20, 21, 23–26) assessed the relation between precocious puberty in girls and the risk of overweight and found that precocious puberty in girls had a higher risk of overweight (OR = 2.03; 95% CI: 1.68–2.46; I2 = 5.1%, P < 0.001; Figure 3D).
Three studies (20, 23, 24) were included in this meta-analysis, which evaluated the association between precocious puberty in boys and the risk of overweight. The pooled analysis revealed that the history of precocious puberty in boys was not associated with an increased risk of overweight (OR = 1.03; 95% CI: 0.56–1.88; I2 = 74.4%, P = 0.930; Figure 3C).
A visual inspection of the funnel plot showed no evidence of a significant publication bias in the outcome of precocious puberty in girls and the risk of obesity (Figure 4). Egger's regression test (P = 0.087) likewise indicated no publication bias in our meta-analysis.
In this meta-analysis of 65,144 individuals, including 24,464 boys and 40,680 girls, we investigated the association between precocious puberty and the risk of obesity in girls. Our findings suggest a significant increase in the risk of general obesity, central obesity, or overweight among individuals with precocious puberty in girls. The overall risk increased by 2.03-fold, 1.96-fold, and 2.03-fold for general obesity, central obesity, and overweight, respectively, compared with non-precocious puberty controls. These results suggest that precocious puberty in girls is an independent risk factor for obesity. In contrast, our results show that precocious puberty in boys did not increase the risk of general obesity or overweight. We found a 0.96-fold, 1.14-fold, and 1.01-fold incidence of general obesity, central obesity, or overweight, respectively, among individuals with precocious puberty in boys. These findings suggest that precocious puberty in boys is not a risk factor for general obesity or overweight.
A systematic review and meta-analysis (27) investigated the association between precocious puberty and obesity. The findings showed that girls with precocious puberty had an increased risk of obesity. However, the relationship between precocious puberty and specific types of obesity was not consistent. Some studies reported no significant association between precocious puberty and obesity in boys, while others showed a positive association. Several cross-sectional studies suggested a strong association between precocious puberty and overweight in girls, but limited research has been conducted in boys (24, 26). Our current analysis included more recent and relevant studies and we performed a separate analysis for general obesity, central obesity, and overweight, providing robust evidence regarding the association between precocious puberty and the risk of obesity.
To date, few studies have explored the underlying pathophysiological mechanism linking precocious puberty and obesity. Previous studies have suggested that precocious puberty is associated with early activation of the hypothalamic-pituitary-gonadal (HPG) axis, and identified mutations in the kisspeptin system, AMPK/SIRT signaling, mTOR signaling, and hypothalamic ceramide in precocious puberty (28, 29). Females have a higher number of kiss1 neurons in the anterior ventral periventricular nucleus, which is essential in establishing positive feedback between ovarian steroids and gonadotropin-releasing hormone surge generators and makes females more sensitive to certain metabolic signals than males (29–31). One study suggested that early menarche in girls may lead to obesity (32), possibly due to excessive levels of sex steroids caused by precocious puberty (33). Obesity is associated with leptin, which is directly proportional to body fat stores and acts on certain hypothalamic neurons (30). Leptin can initiate precocious puberty by causing a massive release of nocturnal gonadotropins (34), but excess leptin has been found to inhibit male reproductive function (35).
In the present study, we conducted a subgroup analysis to investigate the association between precocious puberty and obesity, and our results indicate that girls with precocious puberty have a significantly higher risk of general obesity, central obesity or overweight than boys with precocious puberty. Our findings confirm the results of previous reports suggesting that precocious puberty is associated with an increased risk of obesity in girls, while the relationship between precocious puberty and obesity in boys remains inconclusive according to previous studies (25, 36, 37). Previous studies have also shown that boys with precocious puberty are associated with a higher BMI, although the BMI threshold for puberty development in boys is higher than that in girls (38). During pubertal development, significant sex differences exist between obese girls and boys, with increased peripheral conversion of low-potency androgens to estrogens by adipose tissue-aromatase and increased insulin resistance being two potential contributing factors (39). These findings provide further evidence for sex differences in the relationship between precocious puberty and obesity.
In the analyzed studies, we observed significant heterogeneity in the results, which could be attributed to various factors. Firstly, small sample sizes in three studies may have influenced the precision of the results (21, 23, 25), thus requiring larger sample sizes to confirm the relationship between precocious puberty and obesity. Secondly, differences in diagnostic criteria for obesity in the included studies, such as the use of BMI or Waist To Height Ratio (WHTR), as well as varying methods of examination or questionnaire, may have led to discrepancies in the results. Additionally, the diagnosis of obesity was mainly based on electronic health records in some included studies, which may have further contributed to variability in the findings. Thirdly, the geographic distribution of the studies conducted in America, Europe, and Asia may have introduced regional bias, which may affect the generalizability of the results.
This meta-analysis aimed to investigate the association between precocious puberty and the risk of obesity. The results suggest that precocious puberty in girls should be considered an independent risk factor for obesity. This finding highlights the importance of identifying high-risk groups of obesity in girls with precocious puberty. However, this meta-analysis also found that the history of precocious puberty in boys does not increase the risk of general obesity, central obesity, or overweight. This suggests that precocious puberty in boys is not a risk factor for these types of obesity.
There are several limitations to this meta-analysis that should be considered. Firstly, only seven studies were included, which may limit the ability to perform subgroup analyses for other types of obesity. Secondly, although the included studies adjusted for confounders, the lack of covariate analyses in this meta-analysis should be acknowledged. Thirdly, the sample size of overweight boys included in the study was small, and larger sample sizes are needed to determine the relationship between early maturity and overweight in boys (20, 23, 24). Fourthly, the diagnostic criteria for obesity varied among the included studies, which may have influenced the final results. Specifically, BMI has been found to be positively correlated with precocious puberty, and as a result, children with precocious puberty may be misclassified as obese or overweight (21). This issue has been previously addressed by the World Health Organization (WHO) Expert Committee on Use of Anthropometrics (40), which provided corrections and evaluated them (41). However, the misclassification of adolescents as obese due to adult-related BMI classification errors can have psychological effects on children (42). Therefore, it is crucial for pediatricians to carefully distinguish between pubertal development and obesity and to select appropriate treatment methods.
Our study indicates that girls with a history of precocious puberty are at an increased risk of obesity. In contrast, boys with precocious puberty do not appear to have an increased risk of general obesity, central obesity, or overweight, although this finding should be interpreted with caution. However, the precise pathophysiological mechanisms underlying this association require further investigation through additional studies. The results of our meta-analysis can provide valuable insight into the prevention and treatment strategies for obesity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YS, YK, and YW conceived the study. YS, YK, and XX collected the data. YS conducted analysis and drafted the manuscript. YK and YW contributed to data interpretation and revised the manuscript. NW conducted the subgroup analysis. All authors contributed to the article and approved the submitted version.
This study was supported by the Jilin Province Science and Technology Development Plan Project (No. 20200404043YY and 20220401062YY).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1226933/full#supplementary-material
1. Smith JD, Fu E, Kobayashi MA. Prevention and management of childhood obesity and its psychological and health comorbidities. Annu Rev Clin Psychol. (2020) 16:351–78. doi: 10.1146/annurev-clinpsy-100219-060201
2. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362
3. Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388(10053):1659–724. doi: 10.1016/s0140-6736(16)31679-8
4. Pervanidou P, Akalestos A, Bastaki D, Apostolakou F, Papassotiriou I, Chrousos G. Increased circulating high-sensitivity troponin T concentrations in children and adolescents with obesity and the metabolic syndrome: a marker for early cardiac damage? Metab Clin Exp. (2013) 62(4):527–31. doi: 10.1016/j.metabol.2012.09.012
5. Blüher S, Molz E, Wiegand S, Otto KP, Sergeyev E, Tuschy S, et al. Body mass index, waist circumference, and waist-to-height ratio as predictors of cardiometabolic risk in childhood obesity depending on pubertal development. J Clin Endocrinol Metab. (2013) 98(8):3384–93. doi: 10.1210/jc.2013-1389
6. DeBoer MD. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients. (2019) 11(8):1788. doi: 10.3390/nu11081788
7. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. (2015) 373(14):1307–17. doi: 10.1056/NEJMoa1502821
8. Han XX, Zhao FY, Gu KR, Wang GP, Zhang J, Tao R, et al. Development of precocious puberty in children: surmised medicinal plant treatment. Biomed Pharmacother. (2022) 156:113907. doi: 10.1016/j.biopha.2022.113907
9. Bräuner EV, Busch AS, Eckert-Lind C, Koch T, Hickey M, Juul A. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998–2017. JAMA Netw Open. (2020) 3(10):e2015665. doi: 10.1001/jamanetworkopen.2020.15665
10. Kim YJ, Kwon A, Jung MK, Kim KE, Suh J, Chae HW, et al. Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. J Pediatr. (2019) 208:221–8. doi: 10.1016/j.jpeds.2018.12.022
11. Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. (2007) 36(3):263–74. doi: 10.1111/J.1552-6909.2007.00145.x
12. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. (2017) 92(2):251–65. doi: 10.1016/j.mayocp.2016.09.017
13. Sisson SB, Krampe M, Anundson K, Castle S. Obesity prevention and obesogenic behavior interventions in child care: a systematic review. Prev Med. (2016) 87:57–69. doi: 10.1016/j.ypmed.2016.02.016
14. Zhou X, Hu Y, Yang Z, Gong Z, Zhang S, Liu X, et al. Overweight/obesity in childhood and the risk of early puberty: a systematic review and meta-analysis. Front Pediatr. (2022) 10:795596. doi: 10.3389/fped.2022.795596
15. Mamun AA, Hayatbakhsh MR, O'Callaghan M, Williams G, Najman J. Early overweight and pubertal maturation–pathways of association with young adults’ overweight: a longitudinal study. Int J Obes (Lond). (2009) 33(1):14–20. doi: 10.1038/ijo.2008.220
16. Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. (2001) 108(2):347–53. doi: 10.1542/peds.108.2.347
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1136/bmj.n71
18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
19. Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. (2015) 61:78–89. doi: 10.1016/j.comppsych.2015.05.001
20. Bratberg GH, Nilsen TIL, Holmen TL, Vatten LJ. Early sexual maturation, central adiposity and subsequent overweight in late adolescence. A four-year follow-up of 1,605 adolescent Norwegian boys and girls: the young hunt study. BMC Public Health (2007) 7:54. doi: 10.1186/1471-2458-7-54
21. Himes JH, Obarzanek E, Baranowski T, Wilson DM, Rochon J, McClanahan BS. Early sexual maturation, body composition, and obesity in African-American girls. Obes Res. (2004) 12(Suppl(S9)):64s–72s. doi: 10.1038/oby.2004.270
22. Majun T, Li YH, Chen MM, Ma Y, Gao D, Chen L, et al. Associations between early onset of puberty and obesity types in children: based on both the cross-sectional study and cohort study. Beijing Da Xue Xue Bao Yi Xue Ban. (2022) 54(5):961–70. doi: 10.19723/j.issn.1671-167X.2022.05.025
23. Ribeiro J, Santos P, Duarte J, Mota J. Association between overweight and early sexual maturation in Portuguese boys and girls. Ann Hum Biol. (2006) 33(1):55–63. doi: 10.1080/00207390500434135
24. Wang Y. Is obesity associated with early sexual maturation? a comparison of the association in American boys versus girls. Pediatrics. (2002) 110(5):903–10. doi: 10.1542/peds.110.5.903
25. Chen X, Wang Y. The influence of sexual maturation on blood pressure and body fatness in African-American adolescent girls and boys. Am J Hum Biol. (2009) 21(1):105–12. doi: 10.1002/ajhb.20832
26. Adair LS, Gordon-Larsen P. Maturational timing and overweight prevalence in US adolescent girls. Am J Public Health. (2001) 91(4):642–4. doi: 10.2105/ajph.91.4.642
27. Papadimitriou A, Nicolaidou P, Fretzayas A, Chrousos GP. Clinical review: constitutional advancement of growth, a.k.a. early growth acceleration, predicts early puberty and childhood obesity. J Clin Endocrinol Metab. (2010) 95(10):4535–41. doi: 10.1210/jc.2010-0895
28. Aguirre RS, Eugster EA. Central precocious puberty: from genetics to treatment. Best Pract Res Clin Endocrinol Metab. (2018) 32(4):343–54. doi: 10.1016/j.beem.2018.05.008
29. Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. (2009) 150(7):3214–20. doi: 10.1210/en.2008-1733
30. Shi L, Jiang Z, Zhang L. Childhood obesity and central precocious puberty. Front Endocrinol (Lausanne). (2022) 13:1056871. doi: 10.3389/fendo.2022.1056871
31. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of kiss1/nkb neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. (2009) 297(5):E1212–21. doi: 10.1152/ajpendo.00461.2009
32. Deng Y, Liang J, Zong Y, Yu P, Xie R, Guo Y, et al. Timing of spermarche and menarche among urban students in Guangzhou, China: trends from 2005 to 2012 and association with obesity. Sci Rep. (2018) 8(1):263. doi: 10.1038/s41598-017-18423-6
33. Gill D, Brewer CF, Del Greco MF, Sivakumaran P, Bowden J, Sheehan NA, et al. Age at menarche and adult body mass index: a Mendelian randomization study. Int J Obes (Lond). (2018) 42(9):1574–81. doi: 10.1038/s41366-018-0048-7
34. Chen C, Zhang Y, Sun W, Chen Y, Jiang Y, Song Y, et al. Corrections: investigating the relationship between precocious puberty and obesity: a cross-sectional study in Shanghai, China. BMJ Open. (2017) 7(8):e014004corr1. doi: 10.1136/bmjopen-2016-014004corr1
35. Malik IA, Durairajanayagam D, Singh HJ. Leptin and its actions on reproduction in males. Asian J Androl. (2019) 21(3):296–9. doi: 10.4103/aja.aja_98_18
36. Liu Y, Yu T, Li X, Pan D, Lai X, Chen Y, et al. Prevalence of precocious puberty among Chinese children: a school population-based study. Endocrine. (2021) 72(2):573–81. doi: 10.1007/s12020-021-02630-3
37. Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass Index. J Clin Endocrinol Metab. (2010) 95(1):263–70. doi: 10.1210/jc.2009-1478
38. Liu M, Cao B, Luo Q, Wang Q, Liu M, Liang X, et al. The critical bmi hypothesis for puberty initiation and the gender prevalence difference: evidence from an epidemiological survey in Beijing, China. Front Endocrinol (Lausanne). (2022) 13:1009133. doi: 10.3389/fendo.2022.1009133
39. Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, et al. Sexual dimorphisms in the associations of bmi and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. (2014) 99(8):E1519–29. doi: 10.1210/jc.2014-1384
40. World Health Organization. Physical status: The use of and interpretation of anthropometry, report of a who expert committee. Geneva: World Health Organization (1995). p. 1–452.
41. Wang Y, Adair L. How does maturity adjustment influence the estimates of overweight prevalence in adolescents from different countries using an international reference? Int J Obes Relat Metab Disord. (2001) 25(4):550–8. doi: 10.1038/sj.ijo.0801580
Keywords: precocious puberty, general obesity, central obesity, overweight, meta-analysis
Citation: Song Y, Kong Y, Xie X, Wang Y and Wang N (2023) Association between precocious puberty and obesity risk in children: a systematic review and meta-analysis. Front. Pediatr. 11:1226933. doi: 10.3389/fped.2023.1226933
Received: 22 May 2023; Accepted: 31 July 2023;
Published: 11 August 2023.
Edited by:
Andrea Cassidy-Bushrow, Henry Ford Health System, United StatesReviewed by:
Elpis Vlachopapadopoulou, Panagiotis & Aglaia Kyriakou Children's Hospital, Greece© 2023 Song, Kong, Xie, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongji Wang d2FuZ3lqY2N6eXlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.