- 1Dipartimento Scienze Della Salute Della Donna, Del Bambino e di Sanità Pubblica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 2Arthritis Center, Reumatologia, Dipartimento di Scienze Cliniche Internistiche, Anestesiologiche e Cardiovascolari, Sapienza Università di Roma, Rome, Italy
Several data have suggested that pregnant women have an increased risk of severe COVID-19 compared to those who are not pregnant. Moreover, different studies have showed that severe COVID-19 is limited mostly to unvaccinated women. The aim of the present study was to ascertain the different maternal and fetal outcomes in pregnant women with COVID-19 according to their vaccination status. A retrospective cohort study was carried out including all women admitted to the high-risk pregnancy unit of our center with COVID-19 between December 2021 and February 2022. Among the 163 women included in the study, 60 were vaccinated with an mRNA vaccine and 103 were unvaccinated. Pregnancy outcome and obstetrical and neonatal complications were encountered. Vaccinated women showed higher educational levels and lower prevalence of cases, with BMI >25 compared to unvaccinated women. Moreover, vaccinated women were admitted mostly for obstetrical indications rather than for COVID-related symptoms. In addition, the risk of developing COVID-19 pneumonia was significantly higher in unvaccinated women (p = 0.01) compared with vaccinated ones. Furthermore, pregnancy and neonatal outcomes showed some differences in the two cohorts. In unvaccinated women, the rate of C-section was higher (p = 0.03), and the mean birthweight percentile in their infants was impaired by COVID-19 infection (p = 0.01) when compared to those born to vaccinated women. Based on these results, we suggest that women who received a full course of vaccination were protected from the severity of the disease, having milder symptoms of SARS-Cov2 infection, while also presenting a more favorable pregnancy outcome.
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV2, was declared a global pandemic in March 2020. Observational data and meta-analysis have suggested that pregnant women have an increased risk of severe COVID-19 compared to those who are not pregnant. In fact, several countries have considered pregnant and postpartum women as an “at risk” priority group for COVID vaccination (1, 2).
As shown in a recent article, there is an increased risk of SARS-CoV-2 infection, hospitalization, and admission in intensive care units in unvaccinated patients, and vaccination is effective in reducing the risk of stillbirth, preterm birth, and neonatal ICU admission (3). Vaccination could be important for pregnant women and the general population to prevent the long-term sequelae of sarsCOV2 infection, known as “long COVID”. This includes neurodegenerative complications, dementia, and Parkinson's disease (4); neurological, physical, and psychological sequelae such as fatigue, sleep difficulties, impaired diffusion capacity for carbon monoxide, hair loss, dyspnea, and anxiety (5); and cardiac impairment in patients with a history of ICU admission (6).
Vaccination represents the most effective prevention strategy to contain the spread of infection and decrease the probability of severe disease. At the beginning of the vaccination period, pregnant women were excluded from clinical trials of COVID-19 vaccines and medication. Subsequently, observational data about the safety of mRNA-based vaccines for COVID-19 in pregnant women were reassuring, and their benefits have been documented by large studies (7–11).
The passage of anti-SARS-CoV-2 immunoglobulins through the placenta and breastmilk after COVID vaccination has been demonstrated in several studies (12–15).
Many obstetric societies recommend the adherence of all pregnant women to vaccination, but vaccine hesitancy is a widely spread phenomenon among pregnant women. Meanwhile, recent data have shown that severe COVID-19 is almost limited to unvaccinated women (16) and that unvaccinated pregnant women have a higher risk of contracting the COVID-19 virus (17).
The rate of COVID-19-associated ICU admissions among pregnant women increased threefold in Italy between February 2021 and June 2021 compared to February 2020 and January 2021 (18). Based on these findings and the mounting evidence of vaccine safety, Italy extended its vaccine recommendation to all pregnant women in September 2021 (circular letter from the Ministry of Health, 24 September 2021).
The aim of the present study is to verify whether different maternal and fetal outcomes occurred between vaccinated and unvaccinated pregnant women who were admitted to our high-risk pregnancy unit after the vaccine recommendation (December 1, 2021 to February 15, 2022) and resulted positive for COVID-19 infection.
2. Materials and methods
2.1. Study design and patients
We conducted a retrospective cohort study including all women who were admitted in the high-risk pregnancy unit of IRCSS, Fondazione Policlinico Gemelli of Rome, between December 1, 2021 and February 15, 2022. Patients with twin pregnancies or patients with unknown vaccination status were excluded from enrolment. Twin pregnancies were excluded because multiple gestations “per se” are more prone to obstetrical complications, and the indicators of pregnancy outcomes (week of gestation, birthweight, and birth percentile) are not comparable to those of singleton pregnancies. Patients' medical records of maternal and neonatal characteristics along with vaccination status were recorded.
2.2. Variables definitions
The clinical variables included in the analysis were maternal age, level of education (tertiary education), maternal weight, pre-gestational body mass index, presence of known thrombophilia, presence of pregnancy-related complications, length of hospitalization, route of delivery, gestational age at delivery, birth weight, birth weight percentile according to Ferrazzani et al. (19), number of infants small for gestational age (defined as birthweight <10th percentile), and APGAR score at 1 min and at 5 min.
2.3. Indicators of adverse outcomes
The indicators of outcomes were live births, spontaneous abortion, fetal loss, intrauterine death, neonatal death, small for gestational age (SGA), preterm delivery, and rate of caesarean sections. Moreover, the week of delivery, birth weight, and birth weight percentile were encountered.
2.4. Pathology
Histological changes in the placenta were evaluated through pathological examination. Placental findings were reported according to the synoptic framework proposed by Benton et al. (20).
2.5. Statistical analysis
Descriptive statistics included the observed number of cases for categorical variables, while quantitative variables were expressed as mean and standard deviation. Categorical variables were compared using the χ2 test and Fisher's exact test. T-test was used to compare the distribution of continuous data between the two groups. The statistical analysis was conducted at a 95% level of confidence and a 5% level of statistical significance.
3. Results
3.1. Population characteristics
Out of the total of 175 women admitted during the study period, 163 were eligible and enrolled in the study (60 vaccinated and 103 unvaccinated women). Patients suffered from the wild-type, Omicron, and Delta variants of COVID-19. Omicron was the most represented variant at the time of the study in Europe (21, 22) and in Italy (according to report no. 17 of 18th February 2022 of the Italian National Institute of Health) (23).
In regard to the number of doses received, it was observed that 47 (78,3%) received two doses of the mRNA-based vaccine in the second and third trimesters, while 13 patients received only one dose during the second and third trimesters because they suffered from previous COVID-19 infection before pregnancy (n = 5) or received two doses before pregnancy (n = 8).
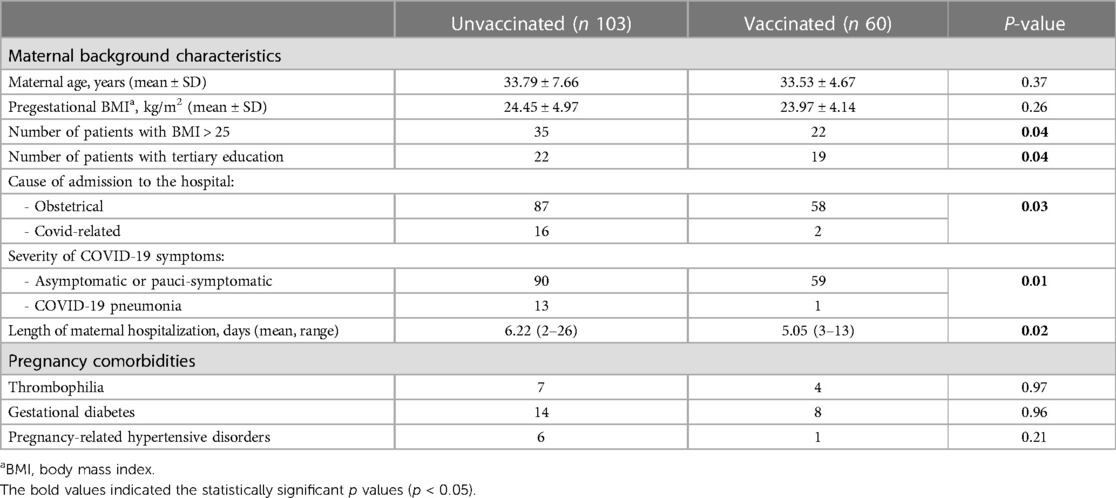
The background characteristics of the two cohorts are shown in Table 1. Compared to unvaccinated women, vaccinated ones presented a significantly lower rate of overweight/obesity. Patients with tertiary education were more diffuse in the cohort of vaccinated women.
Patients who received a full course of vaccination had a lower risk of admission for COVID-related symptoms; in fact, they were admitted mostly for obstetrical reasons (p = 0.01). Moreover, the risk of developing COVID pneumonia with the necessity of oxygen supplementation was higher in unvaccinated patients [OR 8.52 95% CI (1.08–66.8864), p = 0.04]. In our cohort, none of the vaccinated women were admitted in the ICU or needed high-flow oxygen therapy, while 7 of the 103 unvaccinated patients required such support. In addition, patients who did not receive the course of vaccination had a significantly longer period of hospitalization, with the longest hospitalization reaching 26 days.
3.2. Delivery and postpartum outcomes
Of the 163 patients admitted in our unit, 146 delivered during the period of admission. In Table 2, we report the pregnancy outcome, mode of delivery, and neonatal characteristics of the unvaccinated and vaccinated women. We observed a higher rate of C-sections (p = 0.03) in unvaccinated patients who delivered during hospitalization.
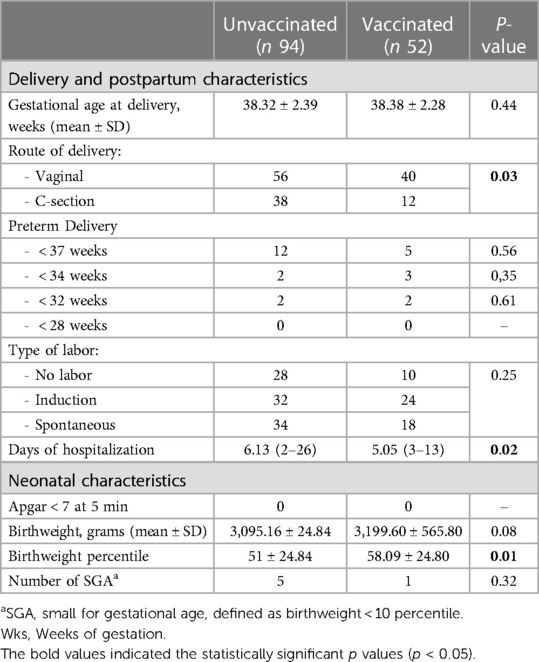
Table 2. Comparison of delivery data and neonatal characteristics in the two groups of pregnant patients.
The infants of the unvaccinated women showed a trend of lower birthweight when compared to those of the unvaccinated women (p = 0.08), and the birthweight percentile was significantly reduced in infants born to the unvaccinated patients (p = 0.01). No difference was found in the two groups for the following features: preterm delivery (at <37 weeks, <34 weeks, <32 weeks, and <28 weeks), APGAR at 5 min, and gestational age at delivery.
Linear regression was used to determine the association of maternal characteristics (age, pregestational BMI, vaccination status) and pregnancy characteristics (preeclampsia, gestational diabetes) with the birthweight percentile. The analysis showed the correlation of neonatal sex (R = −8,737, p = 0.04) and preeclampsia (R = −41.18, p = 0.016). Moreover, the independent association between vaccination and the birthweight percentile was confirmed (R = 9.08, p = 0,04). No significant correlations were found between maternal age and pregestational BMI.
3.3. Pathological characteristics of placentas
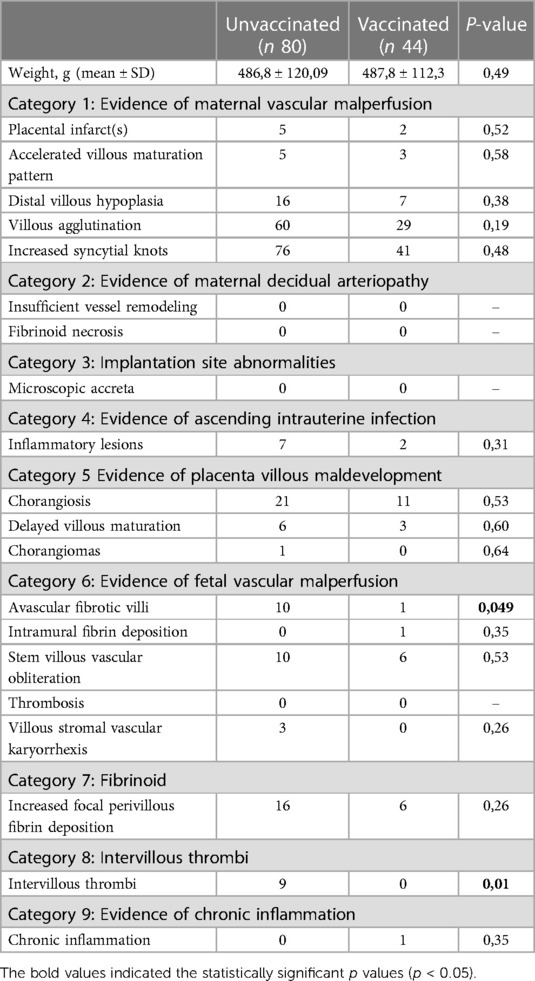
Of the 146 patients who delivered during the period of admission, it was possible to retrieve 124 histological reports. Pathological lesions were reported according to the framework proposed by Benton et al. (20). In Table 3, we report the main pathological characteristics of placentas from the vaccinated and unvaccinated women. Placentas from the newborns of vaccinated mothers showed a lower rate of avascular fibrotic villi (p = 0.049) and intervillous thrombi (p = 0.01). No statistically significant differences were found in the two groups for the other pathological outcomes.
4. Discussion
We have confirmed that infection with coronavirus SARS-CoV2 in pregnant women confers an increased risk of ICU admission compared to non-pregnant women, as also demonstrated in recent literature (24, 25). Furthermore, we confirm the presence of COVID-19 vaccination barriers in our population despite Italy having extended its vaccine recommendation to all pregnant women, considering that only one-third of the enrolled population had the vaccination. A difference in educational status between the unvaccinated and vaccinated patients was also observed, in agreement with previous studies (26, 27).
Although our cohort represents only a small subset of patients from a single-center experience, including different variants of sarsCov2 (wild type, Omicron, and Delta variant), we observed a significant influence of COVID-19 in infants born to unvaccinated mothers, particularly for the mean birthweight percentile. In the literature, patients with COVID-19 are reported to present a higher risk of preeclampsia, stillbirth, and preterm birth (28). It is probable that the mechanisms underlying lower birthweight are the same as those that lead to preeclampsia and stillbirth. A pathogenic role of SARS-CoV-2 has been hypothesized through the binding to angiotensin convertin enzyme 2 receptors causing a renin–angiotensin system dysfunction and vasoconstriction (29), thus determining vascular damage of the placenta. Other studies suggest the possibility of an endothelial dysfunction induced by the proinflammatory state caused by SARS-CoV2 infection (30, 31).
Recently, it was reported that maternal SARS-Cov2 infection during pregnancy may increase the risk of an exacerbated thrombophilic status (32–35). In our series, we did not observe any case of clinically relevant maternal or neonatal thrombosis, probably because of the small size of the sample or because the omicron variant that was more diffuse at the time of the study has less aggressive behavior (22). In the present study, however, we confirmed the fact that the infection with COVID-19 led to a thrombophilic status, as evident from the histological examination of the placentas that showed a higher risk of histological thrombosis.
To our knowledge, different studies have shown differing pregnancy outcomes and maternal and fetal complications between vaccinated and unvaccinated patients (10). Based on the results of the present study, we can suggest that women who received a full course of vaccination were protected from the severity of the disease, experiencing decreased effects of coronavirus SARS-Cov2 infection, and had a more favorable pregnancy outcome.
More data are needed to explore the long-term safety of COVID-19 vaccination during pregnancy in respect to maternal and neonatal health.
We are aware that this study has some limitations due to its retrospective nature and the small group of patients enrolled in a single-center experience. However, the present results confirm the efficacy and safety of COVID-19 vaccination in pregnancy and suggest a potential role in protecting maternal health and fetal growth from the consequences of COVID-19 infection during pregnancy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because of a retrospective study design. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements because of a retrospective study design. A written consent was routinely administered from the hospital to the patients to use clinical data for research. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization, RT, CG, and SD; Methodology, RT and SD; Data curation, RT, CG, TP, and FR; Writing, RT, CG, and SD; Formal Analysis, FR and RT; Supervision, AL and SD. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. (2021) 193(16):E540–8. PMID: 33741725; PMCID: PMC8084555. doi: 10.1503/cmaj.202604
2. Kuriloff M, Patel E, Mueller A, Dada T, Duncan C, Arnolds D, et al. COVID-19 and obstetric outcomes: a single-center retrospective experience in a predominantly black population. J Matern Fetal Neonatal Med. (2023) 36(1):2196364. PMID: 37005011. doi: 10.1080/14767058.2023.2196364
3. Rahmati M, Yon DK, Lee SW, Butler L, Koyanagi A, Jacob L, et al. Effects of COVID-19 vaccination during pregnancy on SARS-CoV-2 infection and maternal and neonatal outcomes: a systematic review and meta-analysis. Rev Med Virol. (2023) 33(3):e2434. PMID: 36896895. doi: 10.1002/rmv.2434
4. Rahmati M, Yon DK, Lee SW, Soysal P, Koyanagi A, Jacob L, et al. New-onset neurodegenerative diseases as long-term sequelae of SARS-CoV-2 infection: a systematic review and meta-analysis. J Med Virol. (2023) 95(7):e28909. PMID: 37394783. doi: 10.1002/jmv.28909
5. Rahmati M, Udeh R, Yon DK, Lee SW, Dolja-Gore X, McEVoy M, et al. A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: a call to action for neurological, physical, and psychological sciences. J Med Virol. (2023) 95(6):e28852. PMID: 37288652. doi: 10.1002/jmv.28852
6. Rahmati M, Koyanagi A, Banitalebi E, Yon DK, Lee SW, Il Shin J, et al. The effect of SARS-CoV-2 infection on cardiac function in post-COVID-19 survivors: a systematic review and meta-analysis. J Med Virol. (2023) 95(1):e28325. PMID: 36401352. doi: 10.1002/jmv.28325
7. Falsaperla R, Leone G, Familiari M, Ruggieri M. COVID-19 vaccination in pregnant and lactating women: a systematic review. Expert Rev Vaccines. (2021) 20:1619–28. Taylor and Francis Ltd. doi: 10.1080/14760584.2021.1986390
8. Kalafat E, O’Brien P, Heath PT, le Doare K, von Dadelszen P, Magee L, et al. Benefits and potential harms of COVID-19 vaccination during pregnancy: evidence summary for patient counseling. Ultrasound in Obstetrics and Gynecology. (2021) 57:681–6. John Wiley and Sons Ltd. doi: 10.1002/uog.23631
9. Male V. Are COVID-19 vaccines safe in pregnancy? Nature Reviews Immunology. Nature Research. (2021) 21:200–1. doi: 10.1038/s41577-021-00525-y
10. Prasad S, Kalafat E, Blakeway H, Townsend R, O’Brien P, Morris E, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. (2022) 13(1):2414. doi: 10.1038/s41467-022-30052-w
11. Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. (2022) 226(2):236–e1-236.e14. doi: 10.1016/j.ajog.2021.08.007
12. Prahl M, Golan Y, Cassidy AG, Matsui Y, Li L, Alvarenga B, et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy. Nat Commun. (2022) 13(1):4422. PMID: 35908075; PMCID: PMC9338928. doi: 10.1038/s41467-022-32188-1
13. Nir O, Schwartz A, Toussia-Cohen S, Leibovitch L, Strauss T, Asraf K, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM. (2022) 4(1):100492. PMID: 34547533; PMCID: PMC8451978. doi: 10.1016/j.ajogmf.2021.100492
14. Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa-Cohen Y, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. (2021) 131(13):e150319. Erratum in: J Clin Invest. (2021) 131(19). PMID: 34014840; PMCID: PMC8245182. doi: 10.1172/JCI150319
15. Mithal MSCI LB, Otero SB, Shanes ED, Goldstein JA, Miller MPH ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. (2021) 225(2):192–94. PMID: 33812808; PMCID: PMC8012273. doi: 10.1016/j.ajog.2021.03.035
16. Engjom H, van den Akker T, Aabakke A, Ayras O, Bloemenkamp K, Donati S, et al. Severe COVID-19 in pregnancy is almost exclusively limited to unvaccinated women−time for policies to change. Lancet Reg Health Eur. (2022) 13:100313. doi: 10.1016/j
17. Magon N, Prasad S, Mahato C, Sharma JB. COVID-19 vaccine and pregnancy: a safety weapon against pandemic. Taiwan J Obstet Gynecol. (2022) 61(2):201–9. doi: 10.1016/j.tjog.2022.02.005
18. Donati S, Corsi E, Maraschini A, Salvatore M, Arena MG, Boldrini R, et al. SARS-Cov-2 infection among hospitalised pregnant women and impact of different viral strains on COVID-19 severity in Italy: a national prospective population-based cohort study. BJOG. (2022) 129(2):221–31. doi: 10.1111/1471-0528.16980
19. Ferrazzani S, Degennaro VA, Di Stasio E, Poppa G, Moresi S, Salvi S, et al. Development of a new fetal growth curve from a large sample of Italian population. Minerva Pediatr. (2017) 69(4):245–50. PMID: 26365747. doi: 10.23736/S0026-4946.16.04258-4
20. Benton SJ, Lafreniere AJ, Grynspan D, Bainbridge SA. A synoptic framework and future directions for placental pathology reporting. Placenta. (2019) 77:46–57. doi: 10.1016/j.placenta.2019.01.009
21. Markov P V, Ghafari M, Beer M, Lythgoe K, Simmonds P, Stilianakis NI, et al. The evolution of SARS-CoV-2. Nat Rev Microbiol. (2023) 21(6):361–79. doi: 10.1038/s41579-023-00878-2
22. Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. (2022) 399(10332):1303–12. doi: 10.1016/S0140-6736(22)00462-7
23. Prevalence and distribusion of SARS-CoV-2 varians in Italy—Report no. 17 of 18 February 2022. Available at: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-18-febbraio-2022.pdf
24. Thornton JG. SARS-CoV-2 infection among hospitalised pregnant women and impact of different viral strains on COVID-19 disease severity in Italy. BJOG. (2022) 129:232. John Wiley and Sons Inc; doi: 10.1111/1471-0528.16981
25. Örtqvist AK, Magnus MC, Aabakke AJM, Urhoj SK, Vinkel Hansen A, Nybo Andersen A, et al. Severe COVID -19 during pregnancy in Sweden, Norway, and Denmark. Acta Obstet Gynecol Scand. (2023) 102(6):681–89. PMID: 36928990; PMCID: PMC10201957. doi: 10.1111/aogs.14552
26. Riad A, Jouzová A, Üstün B, Lagová E, Hruban L, Janků P, et al. COVID-19 vaccine acceptance of pregnant and lactating women (plw) in czechia: an analytical cross-sectional study. Int J Environ Res Public Health. (2021) 18(24):13373. PMID: 34948987; PMCID: PMC8708407. doi: 10.3390/ijerph182413373
27. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Pub Health. (2021) 6(4):e210–21. doi: 10.1016/S2468-2667(21)00012-8
28. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. (2021) 193:E540–8. doi: 10.1503/cmaj.202604
29. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-Converting enzyme 2: sARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. (2020) 126(10):1456–74. PMID: 32264791; PMCID: PMC7188049. doi: 10.1161/CIRCRESAHA.120.317015
30. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
31. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. (2020) 20(7):389–91. doi: 10.1038/s41577-020-0343-0
32. Beslow LA, Linds AB, Fox CK, Kossorotoff M, Zuñiga Zambrano YC, Hernández-Chávez M, et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. (2021) 89(4):657–65. doi: 10.1002/ana.25991
33. Campi F, Longo D, Bersani I, Savarese I, Lucignani G, Haass C, et al. Neonatal cerebral venous thrombosis following maternal SARS-CoV-2 infection in pregnancy. Neonatology. (2022) 119(2):268–72. doi: 10.1159/000520537
34. Fletcher-Sandersjöö A, Bellander BM. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. (2020) 194:36–41. doi: 10.1016/j.thromres.2020.06.027
Keywords: COVID-19 vaccination, pregnancy outcome, COVID-19 infection, COVID-19 pneumonia, birthweight percentile
Citation: Tudisco R, Garufi C, Rizzo F, Polimeno T, Lanzone A and De Carolis S (2023) Impact of mRNA-based vaccines in the prevention of adverse outcomes of COVID-19 infection in pregnancy: a single-center cohort study. Front. Pediatr. 11:1214768. doi: 10.3389/fped.2023.1214768
Received: 30 April 2023; Accepted: 2 October 2023;
Published: 24 October 2023.
Edited by:
Fiammetta Piersigilli, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Iliana Bersani, Bambino Gesù Children’s Hospital (IRCCS), ItalyMasoud Rahmati, Lorestan University, Iran
© 2023 Tudisco, Garufi, Rizzo, Polimeno, Lanzone and De Carolis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Rizzo ZnJhcml6em85MkBnbWFpbC5jb20=
†These authors share co-authorship
 Riccardo Tudisco
Riccardo Tudisco Cristina Garufi
Cristina Garufi Francesca Rizzo
Francesca Rizzo Teresa Polimeno
Teresa Polimeno Antonio Lanzone
Antonio Lanzone Sara De Carolis
Sara De Carolis