- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Republic of Korea
- 2Department of Radiology, Seoul National University Hospital, Seoul, Republic of Korea
- 3Department of Pediatrics, Chungnam National University Sejong Hospital, Sejong, Republic of Korea
- 4Department of Pediatrics, Seoul National University College of Medicine, Seoul, Republic of Korea
- 5Kidney Research Institute, Seoul National University Medical Research Center, Seoul, Republic of Korea
- 6Department of Pediatrics, Kangwon National University Children's Hospital, Chuncheon, Republic of Korea
Background: We evaluated the long-term clinical outcomes of nephrocalcinosis (NC) according to etiology and grade in preschool-age children with NC.
Methods: We retrospectively analyzed the clinical outcomes and disease grade of children with NC classified into three groups according to etiology: prematurity, tubular disorders, and others.
Results: Overall, 67 children were diagnosed with NC [median age, 0.76 years; interquartile range (IQR) 0.46–2.14 years]. The etiologies of NC included prematurity (28.4%), tubular disorders (25.4%), and others (46.3%). Moreover, 56 (83.6%) children were asymptomatic and diagnosed accidentally through kidney ultrasonography. Newly diagnosed underlying diseases were greater in the tubular disorders group than in the other two groups (P = 0.001). Significantly more newly diagnosed NCs were grade 3 than grade 1 (P = 0.003). The median estimated glomerular filtration rate (eGFR) changed from 96.1 (IQR 68.8–119.2) ml/min/1.72 m2 at diagnosis to 90.9 (IQR 76.4–106.4) ml/min/1.72 m2 at the last follow-up, without a significant difference (P = 0.096). Changes in the kidney function did not differ according to etiology. However, patients without improvement in NC grade showed a decrease in eGFR from 98.1 (IQR 71.1–132.9) to 87.4 (IQR 74.0–104.1) ml/min/1.73 m2 (P = 0.023), while patients with improved NC grade did not show any change in the kidney function.
Conclusions: Early recognition, especially in NC grade 3, can help uncover further diagnoses, such as tubular disorders. Long-term kidney function depends on whether the NC grade improves.
Introduction
Nephrocalcinosis (NC), which is characterized by the deposition of calcium content within the kidney parenchyma or tubular structures, has a multigenic etiology. Various conditions, including hereditary and acquired diseases, can cause increased urinary calcium excretion, which can lead to NC. The etiology of NC differs between adults and children. The most common etiologies of NC in adults include medullary sponge kidney, distal renal tubular acidosis, or primary hyperparathyroidism. In children, however, idiopathic hypercalciuria or hereditary tubulopathies, as well as prematurity, are common causes of NC.
Moreover, children with NC have shown increased incidence of disease progression and stone recurrence. The prognosis of NC largely depends on the underlying causes. In particular, Dent disease, primary hyperoxaluria, or hypomagnesemia with hypercalciuria can progress to kidney failure if not treated effectively (1). As such, it is imperative that the underlying diseases be identified once children are diagnosed with NC.
A few retrospective studies on pediatric NC have been conducted in several centers over the years (2–7). Their results recommended the early diagnosis of underlying diseases for improving kidney outcomes. However, there was insufficient evidence to suggest that the degree of NC was responsible for kidney function or stunted growth, with data suggesting that underlying diseases only mainly affected kidney outcomes. Hence, the current study attempted to identify whether changes in clinical outcomes were present according to NC grade and etiologies. We analyzed the long-term effects of NC grade to better understand the nature of NC in children and its clinical impact on kidney function, growth, and other clinical features.
Methods
Study design
We enrolled children who were diagnosed with medullary NC between January 2004 and December 2021 at Seoul National University Children’s Hospital. The inclusion criteria were as follows: children diagnosed under the age of 5 years and having a follow-up period of more than 1 year. All patients were diagnosed with medullary NC based on kidney sonographic findings and classified into the following three groups according to etiology: prematurity group, tubular disorders group, and others group. Prematurity was defined as birth before 37 weeks of gestation age. Tubular disorders group included distal and proximal renal tubular acidosis, Dent diseases, Lowe syndrome, Bartter syndrome, and renal Fanconi syndrome. The others group included those with idiopathic hypercalciuria, Alagille syndrome, neonatal intrahepatic cholestasis caused by citrin deficiency, primary hyperoxaluria, Williams syndrome, congenital hypothyroidism, and immobilization. Only patients who received confirmation through genetic tests such as Sanger sequencing, targeted exome sequencing, or whole exome sequencing were considered as cases of genetic diseases. Patients’ medical records were reviewed to access data on sex, gestational age at birth, onset age, growth, clinical presentation, medication history, kidney sonography, and laboratory findings. These data were obtained, if available, at the time of diagnosis and last follow-up. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB no. 2208-144-1353).
Definitions and clinical outcomes
Medullary NC was graded as follows based on kidney sonography: grade 0, no echogenicity; grade 1, mild echogenicity around the medullary pyramid borders; grade 2, moderate echogenicity around and inside the pyramids; grade 3, severe echogenicity of all pyramids (8). Serial NC sonographic grading of individual patients was reviewed by a pediatric radiologist. Glomerular function was estimated as estimated glomerular filtration rate (eGFR), calculated using the Schwartz formula (9). Calciuria was calculated using the spot urine calcium to creatinine (Ca/Cr) ratio (mg/mg). Hypercalciuria was defined per the reference range for each age group. At 0–1 year, urine (Ca/Cr) <0.8 mg/mg of creatinine was considered normal, <0.56 for 1–2 year, <0.5 for 2–3 year, <0.41 for 3–5 year, <0.3 for 5–7 years, <0.25 for 7–10 years, and age 10-17 years <0.24 for 10–17 years of age (10). The height Z score was calculated using the 2017 growth reference for Korean children and adolescents (11). Growth impairment was defined as a height Z score < −1.88. We evaluated long-term clinical outcomes, including changes in eGFR, hypercalciuria, NC grade, and height growth.
Statistical analysis
Continuous values were expressed as medians with interquartile ranges (IQR). Percentages were expressed as effective percentages in patients with available data. Statistical analyses were performed using the Wilcoxon rank-sum test and the McNemar test for paired samples. Comparisons between the three groups were performed using the Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables. Post-hoc analysis to determine which groups differed was conducted using the Mann–Whitney test. For post-hoc analysis, Bonferroni’s method was used to determine statistical significance. P < 0.05 indicated statistical significance. All data were analyzed using SPSS version 27.0 (IBM, Armonk, NY, USA).
Results
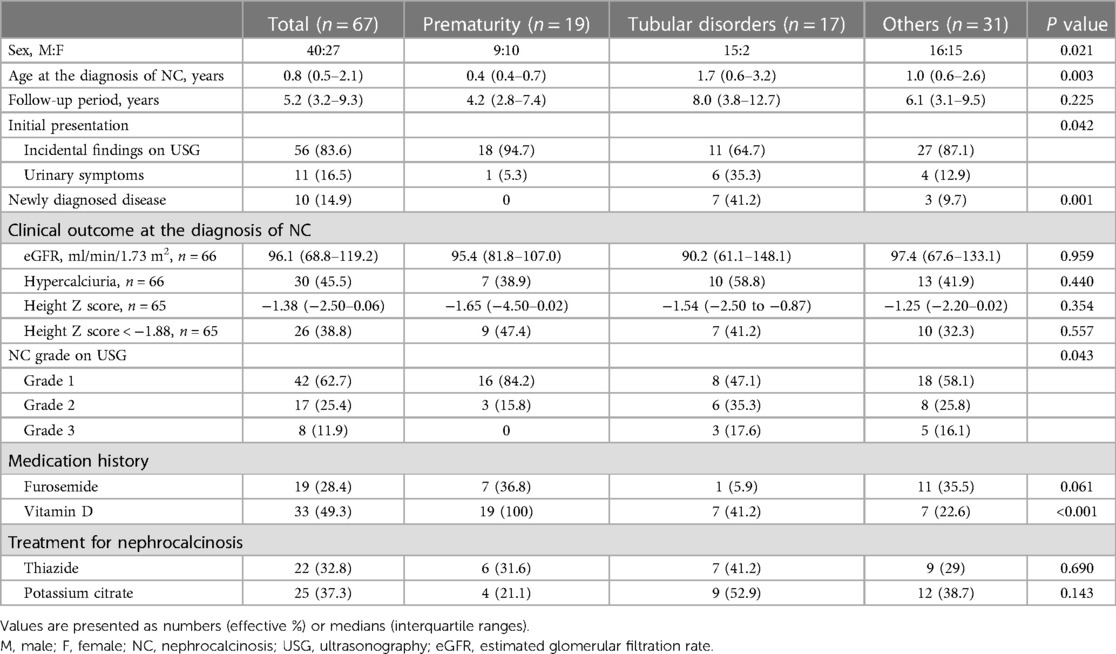
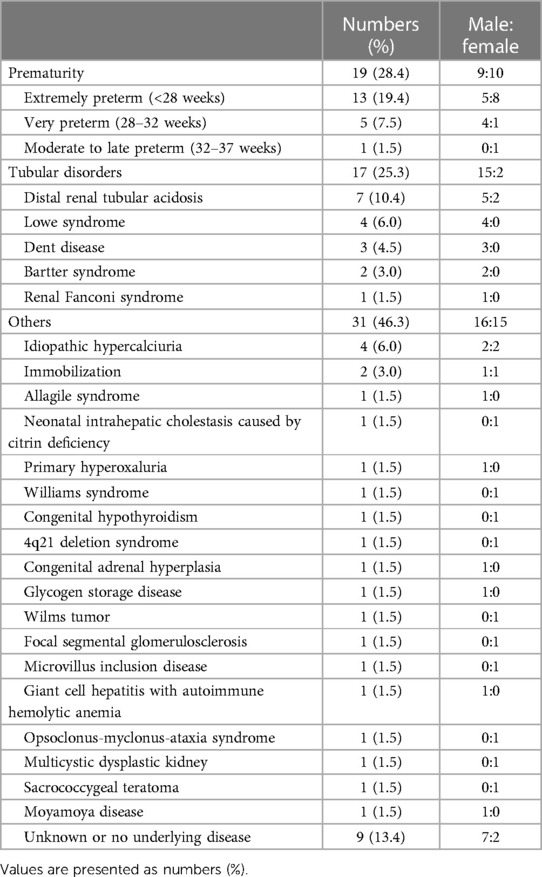
A total of 67 children (male:female = 40:27) were diagnosed with medullary NC at the median age of 0.76 (IQR 0.46–2.14) years (Table 1), among whom 40 patients were younger than 1 year at the initial diagnosis. The median age at the last follow-up was 7.03 (4.35–11.5) years. The most common cause of NC was a prematurity (n = 19). Tubular disorders (n = 17) included distal renal tubular acidosis (RTA) (n = 7), Lowe syndrome (n = 4), Dent disease (n = 3), Bartter syndrome (n = 2), and renal Fanconi syndrome (n = 1). Specific causes of NC are shown in Table 2. Two patients born prematurely who had tubular disorders were included in the tubular disorder category. Additionally, 56 (83.6%) children were asymptomatic and had been diagnosed incidentally through kidney sonography at the median age of 0.73 (IQR 0.44–1.81) years. Only 7 (10.4%) patients had hematuria at the initial diagnosis of NC. In 10 (14.9%) children, the causative diseases leading to NC were newly diagnosed, which included distal RTA (n = 3), Dent disease (n = 3), Bartter syndrome (n = 1), primary hyperoxaluria (n = 1), congenital hypothyroidism (n = 1), and glycogen storage disease (n = 1). Upon diagnosis of NC, 62.7%, 25.4%, and 11.9% of the patients showed grades 1, 2, and 3 on kidney sonography, respectively.
Differences in baseline characteristics according to etiology
Sex differences in etiology were observed with the tubular disorders group having significantly more males compared to the other two groups (Table 1, P = 0.021). This is because hereditary tubular disorders, such as Dent disease and Lowe syndrome, are X linked. Patients in the prematurity group were diagnosed with NC earlier than those in the other two groups (P = 0.003). Although 35.3% of those in the tubular disorders group had urinary symptoms, only a minority of those in the other two groups presented urinary symptoms (P = 0.042). The tubular disorders group had significantly more newly diagnosed diseases (41.2%) compared to the other two groups (prematurity group, 0%; others group, 9.7%; P = 0.001). NC grade 1 was more prevalent in the prematurity group than in the tubular disorders group (P = 0.043). No significant differences in eGFR, hypercalciuria, and height growth were observed between the three groups. No specific differences in furosemide use were noted between the groups (P = 0.061). However, vitamin D medication history was significantly higher in the prematurity group than in other two groups (P < 0.001).
Clinical outcomes according to etiology
The median eGFR changed from 96.1 (IQR 68.8–119.2) ml/min/1.73 m2 at diagnosis of NC to 90.9 (IQR 76.4–106.4) ml/min/1.73 m2 at the last follow-up, without a significant difference (P = 0.096). No significant differences in eGFR were observed in all three groups (Figure 1A). To account for potential variations in follow-up duration, we employed a time-normalized change in eGFR to assess the differences between the three groups. The median time-normalized change in eGFR revealed no statistically significant differences among the three groups (P = 0.443); −1.086 ml/min/1.73 m2 per year (IQR −5.236 to 1.217) in the prematurity group, −3.728 ml/min/1.73 m2 per year (IQR −13.350 to 0.465) in the tubular disorders group, and −1.070 ml/min/1.73 m2 per year (IQR −9.161 to 4.964) in the others group. At the last follow-up, the tubular disorders group showed significantly worse eGFR the others group (P = 0.013). The proportion of hypercalciuria decreased from 45.6% to 28.1% (P = 0.041). There were no significant decreases in the proportion of hypercalciuria within each group (Figure 1B). Significant differences in the distribution of NC grade based on kidney ultrasonography were observed between the time of diagnosis and the last follow-up appointment (P < 0.001). NC grade changes between the initial and last follow-up were as follows: improved in 27.7%, sustained in 63.1%, and aggravated in 9.2%. Significant differences in NC grade changes were observed according to etiology (P = 0.003) (Figure 1C). Notably, the tubular disorder group had worse NC grade outcomes compared to the other two groups (post-hoc analysis, tubular disorders group vs. prematurity group, P = 0.014; tubular disorders vs. others group, P < 0.001). No improvement in NC grade was observed in the tubular disorders group, whereas 36.8% of the prematurity group and 36.7% of the others group showed improvement in NC grade.
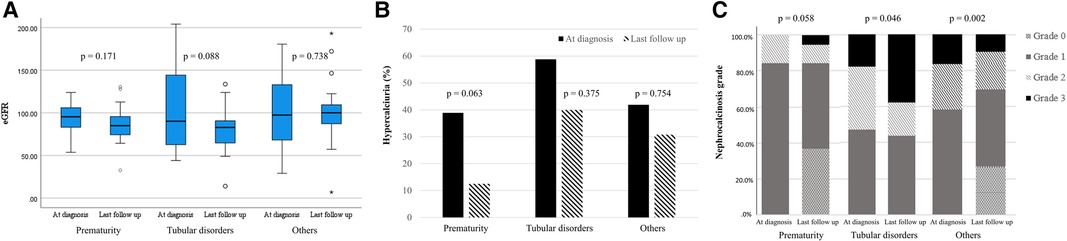
Figure 1. Changes in clinical outcomes according to etiology. (A) estimated glomerular filtration rate (ml/min/1.73 m2); (B) hypercalciuria (%); (C) nephrocalcinosis grade.
Growth impairment defined as a height Z score < −1.88 were found in 26 (38.8%) and 19 (29.2%) patients at diagnosis and the last follow-up, respectively (Supplementary Table S1). No etiological differences in height Z scores and percentage of height Z score < −1.88 were observed between the time of diagnosis and last follow-up appointment (P = 0.557 and 0.336, respectively). Half of the 26 children with growth impairment at diagnosis maintained their stunted growth at the last follow-up, whereas only 12 (46.2%) children with initially stunted growth showed catch-up growth. Among the 12 children who successfully achieved catch-up growth, 5 were preterm, 3 had tubular disorders, and 4 had other etiologies. Among the 41 children who did not have growth impairment at diagnosis, 6 developed growth impairment at the last follow-up. Among the six children, one was preterm, three had tubular disorders, and two had other etiologies.
Kidney outcome according to the nephrocalcinosis grade
Significant differences in initial eGFR were observed according to initial NC grade (P = 0.008) (Table 3). Post-hoc analysis results showed that those with NC grade 3 had lower eGFR compared to those with NC grade 1 (P = 0.002). No significant changes in eGFR were observed according to initial NC grade. However, the median eGFR decreased from 98.1 (IQR 71.14–132.9) to 87.4 (IQR 74.0–104.1) ml/min/1.73 m2 (P = 0.023) in patients without improvement in NC grade while no change in kidney function was observed in patients with improved NC grade (Figure 2A). Additionally, the median time-normalized change in eGFR revealed a significant difference between patients with or without improved NC grade (1.020 ml/min/1.73 m2 per year (IQR −3.120 to 4.960) vs. −2.050 ml/min/1.73 m2 per year (IQR −11.570 to 1.280), P = 0.031). However, there were no significant changes observed for hypercalciuria in patients with or without improved NC grade (Figure 2B).
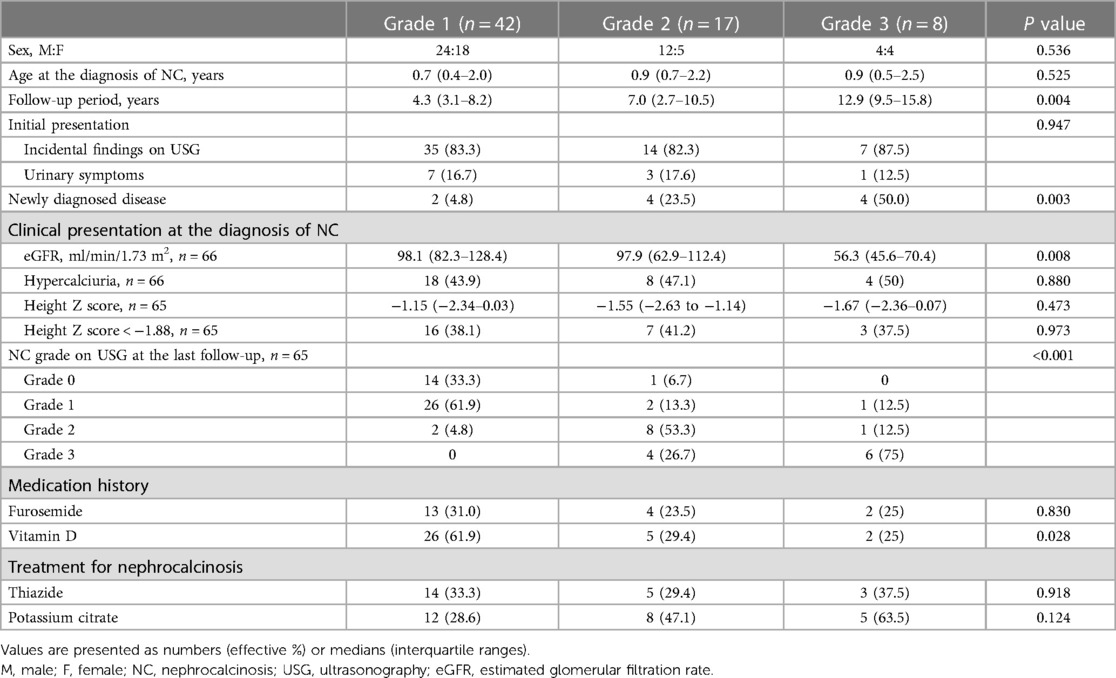
Table 3. Baseline characteristics and last nephrocalcinosis grade distribution according to initial nephrocalcinosis grade.
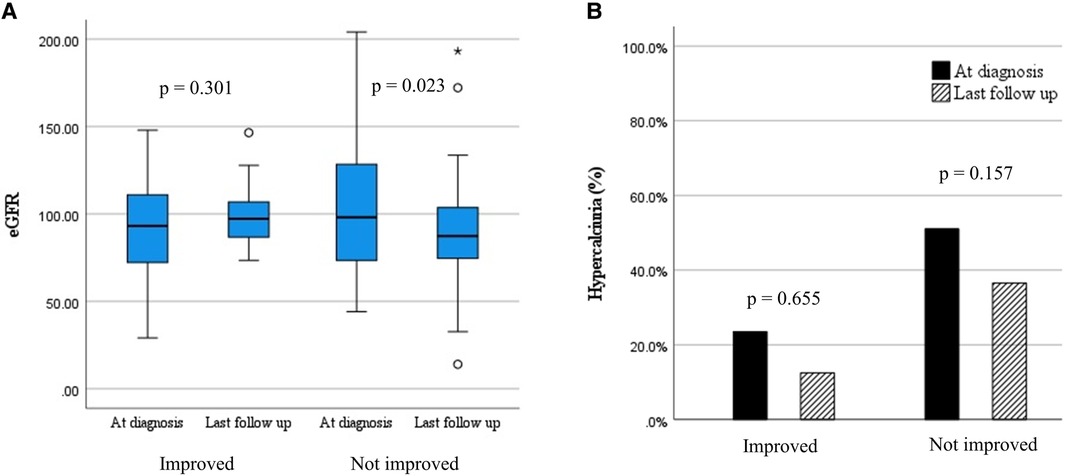
Figure 2. Changes in clinical outcomes according to the change in nephrocalcinosis grade. (A) estimated glomerular filtration rate (ml/min/1.73 m2); (B) hypercalciuria (%).
Discussion
To the best of our knowledge, this has been the first study on preschool-age children with NC who had been followed for a long period of time, allowing us to thoroughly determine long-term effects of NC on various clinical aspects. Most patients with NC were asymptomatic, and in some cases, the underlying disorders were newly diagnosed, especially tubular disorders. A significant decrease in kidney function occurred in the absence of NC grade improvement. These findings suggest the necessity of monitoring long-term kidney function and NC grade in preschool-age children with NC.
NC is mostly asymptomatic and often diagnosed when sonography is performed for other reasons, especially during early childhood. In the current study, 56 (83.6%) patients were diagnosed with NC incidentally on sonography without any urinary symptoms. The proportion of hypercalciuria, a known risk factor for NC, was similar between patients with incidentally found NC (47.3%) and the whole study population (45.5%). A seventh of the children were newly diagnosed with underlying disorders after being diagnosed with NC. Among them, 80% had genetic kidney disorders, such as distal RTA, Dent disease, Batter syndrome, and primary oxaluria. Considerable evidence has shown that children with NC have several underlying genetic disorders (12). However, no specific genetic tests are available to determine when children have NC. Hence, clinicians should consider the possibility of an underlying disease in children with NC incidentally discovered on sonography.
Preterm infants could develop NC due to an immature kidney and various injuries after birth (13, 14). The current study showed that no significant change in eGFR was observed among preterm infants with nephrocalcinosis. However, it remains uncertain whether NC is associated with decreased kidney function in preterm infants. A prospective observational study revealed that up to 60% of preterm infants with NC had long-term sequelae for glomerular and tubular function with low plasma bicarbonate and high urine calcium/citrate ratios (15). In contrast, two case–control studies showed no differences in long-term kidney outcomes between preterm infants with NC and without NC (16, 17). Fayard et al. reported that NC resolved in 61% of preterm infants within 12–18 months after diagnosis (13).
NC is common in patients with kidney tubular disorders that cause hypercalciuria, tubular injury, or crystal nucleation (18, 19). The tubular disorders group had the worst kidney function outcomes and NC grade among the studies groups at last the follow-up and showed sustained or aggravated NC grade, a finding consisted with the results of previous retrospective survey (2). Previous studies have shown that kidney function depended on the underlying disease in children with NC (2, 3, 6, 7). NC is a risk factor for chronic kidney disease (CKD) progression in kidney tubular disorders, especially Lowe syndrome and Dent disease (20). Given that most patients with Dent disease develop kidney failure in their forties, determining the relationship between NC and eGFR is imperative (20). Evidence has shown that treatment of underlying distal RTA prevents progression of NC and deterioration of kidney function (21). Hence, early diagnosis and appropriate management are needed to slow the progression of CKD in children with NC caused by tubular disorders.
The current study analyzed clinical outcomes according to initial NC grade and changes therein. Notably, no differences in kidney function changes were observed according to the initial NC grade at diagnosis. Although patients with improved NC grade maintained their kidney function, those with sustained or aggravated NC grade showed a significant decrease in kidney function. Our findings showed that the change in NC grade rather than the initial NC grade affected long-term kidney function. To date, there have been few reports evaluating changes in kidney function according to changes in NC grade. Among such studies, Ronnefarth et al. showed that children with NC grade 3 presented a significantly lower GFR and urinary osmolality (2). Most patients (72.3%) with NC included herein showed no improvement in NC grade. None of the patients with initial grade 3 NC achieved disease clearance, with 75% maintaining their original NC grade 3. These findings suggest that early diagnosis and treatment of NC before it becomes severe is important in preventing the severity of NC and deterioration of kidney function in children with NC. However, the underlying disease, especially tubular disorders, has the potential to contribute to CKD. The highest persistence in NC grade was observed in the tubular disorders group. Therefore, it remains unclear whether this effect is directly related to NC or attributed to the underlying disease.
Conclusion
Our analysis of available data allowed us to determine the long-term outcomes of NC in preschool-age children. This study focused on differences in clinical manifestation according to etiology and change in NC grade kidney ultrasonography. Overall, our findings suggest the need for careful monitoring of kidney function especially in those who show no improvement in NC grade given the decrease in long-term kidney function observed in such populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Seoul National University Hospital (IRB no. 2208-144-1353). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it was a retrospective observational study.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HAW, YHC, YHA, and HKL. The first draft of the manuscript was written by HAW and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the New Faculty Startup Fund from Seoul National University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1214704/full#supplementary-material
References
2. Ronnefarth G, Misselwitz J. Nephrocalcinosis in children: a retrospective survey. Members of the arbeitsgemeinschaft fur padiatrische nephrologie. Pediatr Nephrol. (2000) 14(10-11):1016–21. doi: 10.1007/s004670050065
3. Mantan M, Bagga A, Virdi VS, Menon S, Hari P. Etiology of nephrocalcinosis in northern Indian children. Pediatr Nephrol. (2007) 22(6):829–33. doi: 10.1007/s00467-006-0425-7
4. Ammenti A, Pelizzoni A, Cecconi M, Molinari PP, Montini G. Nephrocalcinosis in children: a retrospective multi-centre study. Acta Paediatr. (2009) 98(10):1628–31. doi: 10.1111/j.1651-2227.2009.01401.x
5. Dogan CS, Uslu-Gokceoglu A, Comak E, Alimoglu E, Koyun M, Akman S. Renal function and linear growth of children with nephrocalcinosis: a retrospective single-center study. Turk J Pediatr. (2013) 55(1):58–62.23692833
6. Al-Bderat JT, Mardinie RI, Salaita GM, Al-Bderat AT, Farrah MK. Nephrocalcinosis among children at king hussein medical center: causes and outcome. Saudi J Kidney Dis Transpl. (2017) 28(5):1064–8. doi: 10.4103/1319-2442.215138
7. Ramya K, Krishnamurthy S, Sivamurukan P. Etiological profile of nephrocalcinosis in children from southern India. Indian Pediatr. (15 2020) 57(5):415–9. doi: 10.1007/s13312-020-1814-x
8. Boyce AM, Shawker TH, Hill SC, Choyke PL, Hill MC, James R, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endorinol Metab. (2013) 98(3):989–94. doi: 10.1210/jc.2012-2747
9. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. (2009) 4(11):1832–43. doi: 10.2215/CJN.01640309
10. Emma F, Goldstein SL, Bagga A, Bates CM. Rukshana shroff: Pediatric nephrology. 8th edn Philadelphia: Springer Cham (2022). 1301.
11. Kim JH, Yun S, Hwang S-S, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean national growth charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. (2018) 61(5):135–49. doi: 10.3345/kjp.2018.61.5.135
12. Shavit L, Jaeger P, Unwin RJ. What Is Nephrocalcinosis? Kidney Int. (2015) 88(1):35–43. doi: 10.1038/ki.2015.76
13. Fayard J, Pradat P, Lorthois S, Bacchetta J, Picaud JC. Nephrocalcinosis in very low birth weight infants: incidence, associated factors, and natural course. Pediatr Nephrol. (2022) 37(12):3093–104. doi: 10.1007/s00467-021-05417-w
14. Kim HS, Jeong K, Choi YY, Song ES. Risk factors and outcome of nephrocalcinosis in very low birth weight infants. KoreanJ Perinatol. (2015) 26(1):35–45. doi: 10.14734/kjp.2015.26.1.35
15. Kist-van Holthe JE, van Zwieten PH, Schell-Feith EA, Zonderland HM, Holscher HC, Wolterbeek R, et al. Is nephrocalcinosis in preterm neonates harmful for long-term blood pressure and renal function? Pediatrics. (2007) 119(3):468–75. doi: 10.1542/peds.2006-2639
16. Porter E, McKie A, Beattie TJ, McColl JH, Aladangady N, Watt A, et al. Neonatal nephrocalcinosis: long term follow up. Arch Dis Child Fetal Neonatal Ed. (2006) 91(5): F333–6. doi: 10.1136/adc.2006.094755
17. Jones C, King S, Shaw N, Judd B. Renal calcification in preterm infants: follow up at 4–5 years. Arch Dis Child Fetal Neonatal Ed. (1997) 76(3):F185–9. doi: 10.1136/fn.76.3.F185
18. Oliveira B, Kleta R, Bockenhauer D, Walsh SB. Genetic, pathophysiological, and clinical aspects of nephrocalcinosis. Am J Physiol Renal Physiol. (2016) 311(6):F1243–52. doi: 10.1152/ajprenal.00211.2016
19. Park E, Cho MH, Hyun HS, Shin JI, Lee JH, Park YS, et al. Genotype-phenotype analysis in pediatric patients with distal renal tubular acidosis. Kidney Blood Press Res. (2018) 43(2):513–21. doi: 10.1159/000488698
Keywords: nephrocalcinosis, children, kidney function, tubular disorders, preterm, kidney ultrasonography
Citation: Woo HA, Lee H, Choi YH, Min J, Kang HG, Ahn YH and Lee HK (2023) Clinical outcomes of nephrocalcinosis in preschool-age children: association between nephrocalcinosis improvement and long-term kidney function. Front. Pediatr. 11:1214704. doi: 10.3389/fped.2023.1214704
Received: 30 April 2023; Accepted: 25 September 2023;
Published: 9 October 2023.
Edited by:
Michael L. Moritz, University of Pittsburgh, United StatesReviewed by:
Guenter Klaus, KfH-Kuratorium für Dialyse und Nierentransplantation, GermanyRobert P. Woroniecki, Stony Brook Children’s Hospital, United States
© 2023 Woo, Lee, Choi, Min, Kang, Ahn and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yo Han Ahn eWhhaG5Ac251LmFjLmty Hyun Kyung Lee aGtwZWRAbmF2ZXIuY29t
†These authors have contributed equally to this work
Abbreviations CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile ranges; IRB, institutional review board; NC, nephrocalcinosis.
‡ORCID Hyun Ah Woo orcid.org/0000-0001-5800-2090 Hyeonju Lee orcid.org/0000-0003-0457-635X Young Hun Choi orcid.org/0000-0002-1842-9062 Jeesu Min orcid.org/0000-0003-1535-7769 Hee Gyung Kang orcid.org/0000-0001-8323-5320 Yo Han Ahn orcid.org/0000-0002-8185-4408 Hyun Kyung Lee orcid.org/0000-0001-5625-9079
 Hyun Ah Woo
Hyun Ah Woo Hyeonju Lee
Hyeonju Lee Young Hun Choi2,‡
Young Hun Choi2,‡ Hee Gyung Kang
Hee Gyung Kang Yo Han Ahn
Yo Han Ahn