- 1Pediatric Department, Latisana-Palmanova Hospital, Azienda Sanitaria Universitaria Friuli Centrale, Udine, Italy
- 2Department of Health Sciences, University of Florence, Florence, Italy
- 3Allergy Unit, Meyer Children's Hospital IRCCS, Florence, Italy
- 4Immunology Laboratory, Meyer Children's Hospital IRCCS, Florence, Italy
- 5Immunology Unit, Meyer Children's Hospital IRCCS, Florence, Italy
Introduction
Anaphylaxis from insect stings is one of the main causes of anaphylaxis in adults (1) and in children (2). Venom immunotherapy (VIT) is, to date, the only available long-term, natural history-modifying treatment for Hymenoptera venom allergy (HVA), and it is currently recommended mostly for sensitized patients with a history of systemic reactions (SR) after insect stings (1–3).
This recommendation is also indicated in the pediatric age (4), in which the risk of future reactions following a previous moderate-to-severe field sting is approximately 32% in untreated children (p = 0.007) (5). As for adults, in some specific pediatric cases, VIT can be suggested even more in the presence of risk factors (e.g., increased risk of a sting as in beekeepers' children, impaired quality of life, such as in the case of anxiety, remoteness from the emergency department) (4). In children with reported large local reactions (LLR, a swelling >10 cm and lasting >24 h), VIT is generally not recommended (5, 6).
Over the decades, VIT has proved to be safe and effective for all ages and for different HVA, providing 77%–84% effectiveness in the case of honeybee allergy and up to 91%–96% in vespid allergy (3).
In 1925, Braun et al. (7) described the first attempt of VIT with honeybee venom obtained from the insect's whole body. This type of therapy was used until the 1970s, when the first report with whole venom (8) and the first randomized controlled study (9) were published by Busse et al. and Hunt and al., respectively. In 1974, Lichtenstein et al. (10) performed the first desensitization with honeybee venom in pediatrics, in a four-year-old boy who was previously treated with whole-body extract without efficacy. In a period of two months, after showing repeated SR, the child reached the maintenance dose of 100 mcg, and he showed no SR at an in-hospital sting challenge.
One of the first studies on pediatric ages was conducted by Chipps et al. (11) on a group of 44 children (4–14 years) with a conventional protocol. The maintenance dose of 100 mcg was reached in six weeks, with a “modified-rush” build-up on day one (from 0.01 to 1 mcg). In this cohort, three (6.8%) children presented a SR, and only one was severe enough to require adrenaline treatment. An in-hospital sting challenge was conducted 15 weeks after starting VIT, showing only one reaction in a child with low IgG titers, demonstrating the efficacy and safety of this procedure in children.
In 2017, an extensive systematic review and meta-analysis was published on VIT by Dhami et al. (12). The authors concluded that VIT reduced the risk of SR (odds ratio, OR = 0.08, 95% confidence interval, CI 0.03–0.26) and improved quality of life (risk difference = 1.41, 95% CI 1.04–1.79). Adverse effects were reported in both the build-up and maintenance phases, but most of them were mild, and no fatalities were recorded. Among these studies, there were only three specifically evaluating VIT in childhood (5, 13, 14) and they were all considered with a low-moderate quality assessment.
Similar results were observed in a Cochrane review published by Boyle et al. in 2012 (15). VIT improved quality of life (mean difference in favor of VIT 1.21 points on a 7-point scale, 95% CI 0.75–1.67) and prevented both LLR (relative risk, RR = 0.41, 95% CI 0.24–0.69) and SR (RR = 0.10, 95% CI 0.03–0.28). VIT was generally well tolerated, with a risk of SR of 9.3% in treated patients vs. 0.7% in the placebo group, higher for honeybee venom (14.2%) than for wasp venom (2.8%).
In clinical practice, tests becoming negative happens only for a small percentage of patients (16). Studies recommend performing VIT for at least 3–5 years and maintaining a follow-up after suspending it (17). In specific cases, it may be suggested to continue VIT for more than 5 years, such as in the presence of specific risk factors like SR from field sting during VIT (4).
Venom immunotherapy (VIT) is, to date, the only available long-term treatment for HVA. Recommendations provided by scientific societies may also be applied to childhood, though no international pediatric-specific documents have been published for the management of HVA. In the last years, despite the huge amount of literature on VIT being produced, most of the studies evaluate only adult populations. Some of these also include children, but often, pediatric data are not separately analyzed. In the most recent years, some scientific groups have specifically studied the indications, safety, and efficacy of VIT in childhood. Nonetheless, it is still difficult to compare those studies since they analyze different VIT regimens in heterogeneous populations. In this review, we aim to dissert and evaluate relevant articles with specific data on VIT in pediatric ages.
Materials and methods
We performed a literature search in Medline through PubMed using default keywords related to pediatric Hymenoptera Venom Allergy and Allergen Immunotherapy. Original studies and review articles, with a focus on meta-analyses and randomized controlled trials in English, were identified up to February 1st, 2023.
Results
VIT can be carried out with different protocols, all of which have already been proven safe and effective in pediatric ages. VIT involves a build-up phase with progressively increasing doses (starting from 0.001 mcg to 0.1 mcg) of the chosen venom until the maintenance dose is reached, which is usually 100 mcg (18, 19). A starting dose of 1 mcg may be considered safe as well (20).
As underlined by Golden (21), there is no consensus on the actual duration of the build-up phase, according to existing literature, but the main build-up phase protocols are usually defined (1) conventional: months, (2) clustered or semi-rush: weeks, (3) rush or ultra-rush: days.
During maintenance, the achieved dose should be injected every four weeks in the first year and then every six to eight weeks in the following years, although some authors considered 12-week or even longer intervals between doses safe (22–24). A higher maintenance dose at 150–200 mcg may be prescribed in high-risk patients, e.g., if a patient had a SR at re-sting while on VIT (3, 25), whereas a lower dose (50 mcg) has been suggested sufficient for children (26–28).
Especially in some countries, as highlighted in an editorial by Golden (29), the protocol choice may rely more on healthcare system organization than on scientific or clinical reasons. Rush/ultra-rush protocols are usually preferred to concentrate evaluations and therapies in a few days, in countries where healthcare is provided in specialized centers, and upon the patient's request or need (e.g., rapid achievement of maintenance dose, low compliance with conventional protocol). Even though literature has already proven the safety and efficacy of rush and ultra-rush protocols (30), conventional and clustered protocols seem to be generally preferred in clinical practice. In a survey conducted in the United States (29, 31), most allergists preferred conventional (64%) compared to eight-week (31%) and rush protocols (5%). In the United Kingdom, allergists prefer conventional protocols (92%) while only 25% of respondents have ever used clustered or rush/ultra-rush protocols (32). In another survey, Martínez-Cañavate et al. (33) analyzed one of the largest groups of children treated with VIT in Spain. In most cases, a conventional protocol was chosen (68.2%), followed by rush (18.5%), clustered (11.8%), and ultra-rush (1.5%).
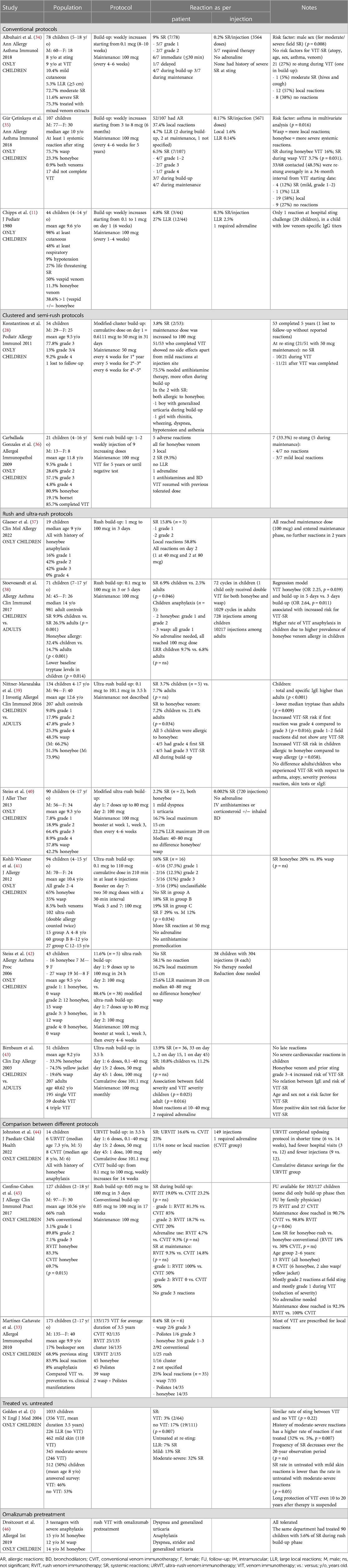
A summary of pediatric studies reporting VIT protocols is shown in Table 1, and some examples are discussed below.
Conventional protocols
In a group of 78 children with a history of SR (mild 10.4%, moderate 72.7%, severe 11.6%) studied by Albuhairi et al., treatment with a conventional protocol resulted in 9% of SR during VIT (34). None of those were severe, with a 0.2% SR rate per injection. Twenty-one children were re-stung during VIT, and 12 (57%) showed a local reaction, one (5%) showed a SR, and eight (38%) showed no reactions at all. No statistically significant risk factor for SR prediction has been identified (e.g., age, atopy, gender, asthma, or type of venom).
A similar result was obtained in another study by Gür Çetinkaya et al. (35) which analyzed 107 children with at least one SR after a Hymenoptera sting, treated with a conventional protocol. Overall, 52 (48.5%) children showed an allergic reaction during VIT: 40 (37.4%) local reactions, seven (6.5%) SR, and five (4.7%) LLR. Most local reactions occurred during the build-up phase (p < 0.001) and were more frequently observed with wasp VIT (p = 0.047). Regarding SR, these were mostly mild-moderate (grades 1–3 according to Mueller classification) and occurred more frequently for honeybee VIT (p = 0.031). Of the 68 children whose parents completed a follow-up survey, a re-sting during or after VIT occurred in 33 (48.5%) subjects, and 24 (72.7%) showed reactions that were more frequently local. Pre-existing asthma was the only risk factor for LLR and SR in a multivariate analysis (p = 0.016).
Clustered and semi-rush protocols
In a study by Konstantinou et al., 54 children with at least one anaphylactic reaction to Hymenoptera venom were treated with a modified clustered protocol, consisting of a build-up phase lasting roughly 5 weeks and reaching a maintenance dose of 50 mcg (28). The maintenance dose was given every 4 weeks for the first year, then every 5 weeks for the second and third years, and every 6 weeks for the last two years. One child was lost to follow-up. Almost all remaining children (52/53) tolerated the protocol without side effects except for mild injection site reactions. Two children (3.8%) showed SR during VIT, and in these cases, the maintenance dose was increased to 100 mcg. Twenty-one (41.2%) of the 51 children who completed the modified-clustered protocol have been re-stung at least once with no SR reported, thus demonstrating the safety of clustered protocol and the efficacy even with a lower maintenance dose.
In another study by Carballada González et al., 21 children who were mostly allergic to honeybees (80.9%) and treated with a semi-rush protocol with one or two weekly injections of nine increasing doses of venom, an SR rate of 9.5% was reported (2/21 children) (36). Seven of 21 patients (33%) were re-stung after VIT, and none showed an SR, confirming the efficacy of the intervention.
Rush and ultra-rush protocols
In a small study by Glaeser et al., three of the included 19 patients (15.8%) showed an anaphylactic reaction to rush honeybee venom immunotherapy (37). Nonetheless, all children could continue VIT with a modified up-dosing protocol and did not experience further side effects, but no conclusions can be drawn due to the small sample size of the study.
Another study by Stoevesandt et al. analyzed the safety of a 3- or 5-days rush protocol in a cohort of 71 children/adolescents and 981 adult controls (38). In this work, 5-day build-up protocols (p = 0.011; OR 2.64; CI 1.25–5.57) and honeybee VIT (p = 0.039; OR 2.25; CI 1.04–4.87) were associated with an increased risk of SR during VIT. While children usually may show a lower rate of SR than adults, in this study, SR were reported in 6.9% of children compared to 2.5% of adults (p = 0.046). However, all pediatric SR cases were mild/moderate (grades 1–2), and this discrepancy was attributed by the authors to the higher frequency of honeybee allergy in children (32.4%) than in adults (14.7%) (p < 0.001).
Two studies on different populations used the same ultra-rush protocol. Birnbaum et al. (43) showed a 10.8% rate of SR in 51 children (74.5% with yellow jacket allergy), while Nittner-Marszalska et al. (39) reported a 3.7% rate in 134 children (51.5% with honeybee allergy). In both studies, the risk of severe SR during VIT significantly increased with the severity of the first reaction. Besides honeybee allergy, no other risk factors were found to be statistically significant.
Kohli-Weisner et al. (41) also used an ultra-rush protocol in 94 children, mostly with an allergy to honeybees (65%). Among 102 desensitization procedures, 16 (16%) showed adverse effects. Most of these reactions were, however, grades 1–2 (50%), and none required adrenaline administration. Interestingly, younger children (4–8 years) had no SR, compared to older children and adolescents, who reported 18% and 19% SR, respectively.
In another modified ultra-rush protocol study in a cohort of 38 children by Steiss et al. (42) no SR was reported, and most children (58.1%) showed no reactions at all. In a more recent retrospective study by the same group, performed on a larger cohort, the same modified ultra-rush protocol was demonstrated to be safe as well, despite a 2.2% rate of mild SR (40).
Analyzing the various published studies, it can be seen that some defined ultra-rush protocols are really similar compared to others labeled as rush.
Comparison between different protocols
In a study by Johnston et al., ultra-rush and conventional protocols were compared in a small cohort of 14 pediatric honeybee-allergic children. Six received ultra-rush honeybee VIT and showed a lower rate of SR (16%) compared with the conventional group (25%) while requiring fewer injections, hospital visits, and saving travel distance (44).
Similarly, in a study by Confino-Cohen et al., rush and conventional protocols were compared in children (45). Eighty-four out of 127 children were treated with rush VIT and compared to those treated with conventional VIT. Slightly more SR (23.2% vs. 19.0%) were reported during the build-up phase in the conventional group, but this difference was not statistically significant. No severe SR was reported, and the need for adrenaline was more frequent in the conventional group (9.3% vs. 4.7%, not statistically significant). Nonetheless, a significantly higher proportion of children in the rush group reached the maintenance dose compared to the conventional protocol (98.8% vs. 90.7%). Authors conclude that rush protocols are safe in childhood and more efficient than conventional protocols in terms of compliance, despite being less frequently prescribed in the United States.
Omalizumab pretreatment
Droitcourt et al. (46) have pretreated three male teenagers undergoing VIT with omalizumab. All had severe anaphylaxis with the Hymenoptera sting (two by honeybee and one by wasp) and reported SR during the build-up phase of a rush VIT protocol. Pretreatment with omalizumab 2 and 4 weeks before VIT onset allowed to complete build-up and reach the maintenance dose without SR.
Conclusion
In children, the occurrence of SR with conventional VIT may be similar to other protocols, although conventional VIT appears to be more frequently chosen in clinical practice than other protocols. However, greater severity of reported allergic reactions may not be excluded with faster protocols. It is also important to underline the difficulty in comparing published studies on different regimens, where there seems to be high variability among different experiences.
To date, it is still difficult to recommend one VIT protocol over another in children. The choice of protocol must always be discussed with caregivers, even though sometimes, they may be dependent on local expertise. Future extensive data coming from high-quality studies, including, e.g., the potential role of component-resolved diagnostics, of the molecular identification of allergens in the extracts used for VIT or of specific biomarkers may help tailor the choice of protocol on patients in a precision medicine perspective.
Author contributions
MG and EN: conceptualized the work. FS, MG, BP and EN: collected the data and drafted the manuscript. FS, MG, BP, SB, GL, LS, LT, CF, FP, CV, SR, CA, EN and FM: analyzed the data. FS, MG, BP, SB, GL, LS, LT, CF, FP, CV, SR, CA, EN and FM: critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wood RA, Camargo CAJ, Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. (2014) 133(2):461–7. doi: 10.1016/j.jaci.2013.08.016
2. Alvarez-Perea A, Ameiro B, Morales C, Zambrano G, Rodríguez A, Guzmán M, et al. Anaphylaxis in the pediatric emergency department: analysis of 133 cases after an allergy workup. J Allergy Clin Immunol Pract. (2017) 5(5):1256–63. doi: 10.1016/j.jaip.2017.02.011
3. Sturm GJ, Varga E-M, Roberts G, Mosbech H, Bilò MB, Akdis CA, et al. EAACI Guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. (2018) 73(4):744–64. doi: 10.1111/all.13262
4. Bilò MB, Pravettoni V, Bignardi D, Bonadonna P, Mauro M, Novembre E, et al. Hymenoptera venom allergy: management of children and adults in clinical practice. J Investig Allergol Clin Immunol. (2019) 29(3):180–205. doi: 10.18176/jiaci.0310
5. Golden DBK, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. N Engl J Med. (2004) 351(7):668–74. doi: 10.1056/NEJMoa022952
6. Lange J, Cichocka-Jarosz E, Marczak H, Lis G, Brzyski P, Nowak-Węgrzyn A. Natural history of hymenoptera venom allergy in children not treated with immunotherapy. Ann Allergy Asthma Immunol. (2016) 116(3):225–9. doi: 10.1016/j.anai.2015.12.032
7. Braun. Notes on desensitisation of a patient hypersensitive to bee stings. Bee World. (1926) 8(10):159–159. doi: 10.1080/0005772x.1926.11096103
8. Busse W, Reed C, Lichtenstein LM. Protection following honey-bee venom immunotherapy in a case of bee sting anaphylaxis. J Allergy Clin Immunol. (1974) 53:104.
9. Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med. (1978) 299(4):157–61. doi: 10.1056/NEJM197807272990401
10. Lichtenstein LM, Valentine MD, Sobotka AK. A case for venom treatment in anaphylactic sensitivity to hymenoptera sting. N Engl J Med. (1974) 290(22):1223–7. doi: 10.1056/NEJM197405302902204
11. Chipps BE, Valentine MD, Kagey-Sobotka A, Schuberth KC, Lichtenstein LM. Diagnosis and treatment of anaphylactic reactions to hymenoptera stings in children. J Pediatr. (1980) 97(2):177–84. doi: 10.1016/s0022-3476(80)80470-7
12. Dhami S, Zaman H, Varga E-M, Sturm GJ, Muraro A, Akdis CA, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. (2017) 72(3):342–65. doi: 10.1111/all.13077
13. Schuberth KC, Lichtenstein LM, Kagey-Sobatka A, Szklo M, Kwiterovich KA, Valentine MD. Epidemiologic study of insect allergy in children. II. Effect of accidental stings in allergic children. J Pediatr. (1983) 102(3):361–5. doi: 10.1016/S0022-3476(83)80649-0
14. Valentine MD, Schuberth KC, Kagey-Sobotka A, Graft DF, Kwiterovich KA, Szklo M, et al. The value of immunotherapy with venom in children with allergy to insect stings. N Engl J Med. (1990) 323(23):1601–3. doi: 10.1056/NEJM199012063232305
15. Boyle RJ, Oude Elberink J. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. (2012) 10:10. doi: 10.1002/14651858.CD008838.pub2
16. Müller UR, Ring J. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol Pract. (2015) 3(3):324–8. doi: 10.1016/j.jaip.2014.11.018
17. Fiedler C, Miehe U, Treudler R, Kiess W, Prenzel F. Long-Term follow-up of children after venom immunotherapy: low adherence to anaphylaxis guidelines. Int Arch Allergy Immunol. (2017) 172(3):167–72. doi: 10.1159/000458707
18. Hoffman DR, Jacobson RS. Allergens in hymenoptera venom XII: how much protein is in a sting? Ann Allergy. (1984) 52(4):276–8. PMID: 6711914
19. Schumacher M, Tveten M, Egen N. Rate and quantity of delivery of venom from honeybee stings. J Allergy Clin Immunol. (1994) 93(5):831–5. doi: 10.1016/0091-6749(94)90373-5
20. Roumana A, Pitsios C, Vartholomaios S, Kompoti E, Kontou-Fili K. The safety of initiating hymenoptera immunotherapy at 1 μg of venom extract. J Allergy Clin Immunol. (2009) 124(2):379–81. doi: 10.1016/j.jaci.2009.05.026
21. Golden DBK. Venom immunotherapy. Immunol Allergy Clin North Am. (2020) 40(1):59–68. doi: 10.1016/j.iac.2019.09.002
22. Goldberg A, Confino-Cohen R. Maintenance venom immunotherapy administered at 3-month intervals is both safe and efficacious. J Allergy Clin Immunol. (2001) 107(5):902–6. doi: 10.1067/mai.2001.114986
23. Goldberg A, Confino-Cohen R. Effectiveness of maintenance bee venom immunotherapy administered at 6-month intervals. Ann Allergy Asthma Immunol. (2007) 99(4):352–7. doi: 10.1016/S1081-1206(10)60552-2
24. Simioni L, Vianello A, Bonadonna P, Marcer G, Severino M, Pagani M, et al. Efficacy of venom immunotherapy given every 3 or 4 months: a prospective comparison with the conventional regimen. Ann Allergy Asthma Immunol. (2013) 110(1):51–4. doi: 10.1016/j.anai.2012.09.014
25. Ruëff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. (2001) 108(6):1027–32. doi: 10.1067/mai.2001.119154
26. Reisman R, Livingston A. Venom immunotherapy: 10 years of experience with administration of single venoms and 50 μg maintenance doses. J Allergy Clin Immunol. (1992) 89(6):1189–95. doi: 10.1016/0091-6749(92)90304-K
27. Houliston L, Nolan R, Noble V, Pascoe E, Hobday J, Loh R, et al. Honeybee venom immunotherapy in children using a 50-μg maintenance dose. J Allergy Clin Immunol. (2011) 127(1):98–9. doi: 10.1016/j.jaci.2010.07.031
28. Konstantinou GN, Manoussakis E, Douladiris N, et al. A 5-year venom immunotherapy protocol with 50 μg maintenance dose: safety and efficacy in school children. Pediatr Allergy Immunol. (2011) 22(4):393–7. doi: 10.1111/j.1399-3038.2010.01137.x
29. Golden DBK. Rush venom immunotherapy: ready for prime time? J Allergy Clin Immunol Pract. (2017) 5(3):804–5. doi: 10.1016/j.jaip.2016.12.031
30. van der Zwan JC, Flinterman J, Jankowski IG, Kerckhaert JA. Hyposensitisation to wasp venom in six hours. Br Med J. (1983) 287(6402):1329–31. doi: 10.1136/bmj.287.6402.1329
31. Golden DBK, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. (2017) 118(1):28–54. doi: 10.1016/j.anai.2016.10.031
32. Diwakar L, Noorani S, Huissoon AP, Frew AJ, Krishna MT. Practice of venom immunotherapy in the United Kingdom: a national audit and review of literature. Clin Exp Allergy. (2008) 38(10):1651–8. doi: 10.1111/j.1365-2222.2008.03044.x
33. Martínez-Cañavate A, Tabar AI, Eseverri JL, Martín F, Pedemonte-Marco C. An epidemiological survey of hymenoptera venom allergy in the spanish paediatric population. Allergol Immunopathol. (2010) 38(5):259–62. doi: 10.1016/j.aller.2010.02.004
34. Albuhairi S, El Khoury K, Yee C, Schneider L, Rachid R. A twenty-two-year experience with hymenoptera venom immunotherapy in a US pediatric tertiary care center 1996–2018. Ann Allergy Asthma Immunol. (2018) 121(6):722–728.e1. doi: 10.1016/j.anai.2018.08.002
35. Gür Çetinkaya P, Esenboğa S, Uysal Soyer Ö, Tuncer A, Şekerel BE, Şahiner ÜM. Subcutaneous venom immunotherapy in children. Ann Allergy Asthma Immunol. (2018) 120(4):424–8. doi: 10.1016/j.anai.2018.01.015
36. Carballada González FJ, Crehuet Almirall M, Manjón Herrero A, De la Torre F, Boquete París M. Hymenoptera venom allergy: characteristics, tolerance and efficacy of immunotherapy in the paediatric population. Allergol Immunopathol. (2009) 37(3):111–5. doi: 10.1016/S0301-0546(09)71721-5
37. Glaeser A, Müller C, Bode S. Anaphylactic reactions in the build-up phase of rush immunotherapy for bee venom allergy in pediatric patients: a single-center experience. Clin Mol Allergy. (2022) 20(1):4. doi: 10.1186/s12948-022-00170-3
38. Stoevesandt J, Hosp C, Kerstan A, Trautmann A. Safety of 100 µg venom immunotherapy rush protocols in children compared to adults. Allergy Asthma Clin Immunol. (2017) 13(1):32. doi: 10.1186/s13223-017-0204-y
39. Nittner-Marszalska M, Cichocka-Jarosz E, Małaczyńska T, Kraluk B, Rosiek-Biegus M, Kosińska M, et al. Safety of ultrarush venom immunotherapy: comparison between children and adults. J Investig Allergol Clin Immunol. (2016) 26(1):20–47. doi: 10.18176/jiaci.0006
40. Steiss JO, Lindemann H. Safety of modified ultra-rush venom immunotherapy in children. J Allergy Ther. (2013) 04(02):134. doi: 10.4172/2155-6121.1000134
41. Köhli-Wiesner A, Stahlberger L, Bieli C, Stricker T, Lauener R. Induction of specific immunotherapy with hymenoptera venoms using ultrarush regimen in children: safety and tolerance. J Allergy. (2012) 2012:1–5. doi: 10.1155/2012/790910
42. Steiss JO, Jödicke B, Lindemann H. A modified ultrarush insect venom immunotherapy protocol for children. Allergy Asthma Proc. (2006) 27(2):148–50. PMID: 16724635
43. Birnbaum J, Ramadour M, Magnan A, Vervloet D. Hymenoptera ultra-rush venom immunotherapy (210 min): a safety study and risk factors. Clin Exp Allergy. (2003) 33(1):58–64. doi: 10.1046/j.1365-2222.2003.01564.x
44. Johnston N, Belcher J, Preece K, Bhatia R. Review of venom immunotherapy at a regional tertiary paediatric centre. J Paediatr Child Health. (2022) 58(7):1228–32. doi: 10.1111/jpc.15964
45. Confino-Cohen R, Rosman Y, Goldberg A. Rush venom immunotherapy in children. J Allergy Clin Immunol Pract. (2017) 5(3):799–803. doi: 10.1016/j.jaip.2016.10.011
Keywords: precision medicine, hymenoptera venom allergy, protocols, pediatrics, venom immunotherapy
Citation: Saretta F, Giovannini M, Pessina B, Barni S, Liccioli G, Sarti L, Tomei L, Fazi C, Pegoraro F, Valleriani C, Ricci S, Azzari C, Novembre E and Mori F (2023) Venom immunotherapy protocols in the pediatric population: how to choose?. Front. Pediatr. 11:1192081. doi: 10.3389/fped.2023.1192081
Received: 22 March 2023; Accepted: 5 June 2023;
Published: 7 September 2023.
Edited by:
Marzia Duse, Sapienza University of Rome, ItalyReviewed by:
Jernej Zavrsnik, Health Center dr Adolf Drolc, SloveniaLinda Cox, Consultant, United States
© 2023 Saretta, Giovannini, Pessina, Barni, Liccioli, Sarti, Tomei, Fazi, Pegoraro, Valleriani, Ricci, Azzari, Novembre and Mori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benedetta Pessina YmVuZWRldHRhLnBlc3NpbmFAdW5pZmkuaXQ=
†These authors share first authorship
‡ORCID Benedetta Pessina orcid.org/0000-0002-1387-3463
 Francesca Saretta
Francesca Saretta Mattia Giovannini
Mattia Giovannini Benedetta Pessina
Benedetta Pessina Simona Barni
Simona Barni Giulia Liccioli
Giulia Liccioli Lucrezia Sarti3
Lucrezia Sarti3 Leonardo Tomei
Leonardo Tomei Francesco Pegoraro
Francesco Pegoraro Silvia Ricci
Silvia Ricci Elio Novembre
Elio Novembre Francesca Mori
Francesca Mori