- Department of Pediatrics, Division of Neurology and Developmental Neuroscience, Baylor College of Medicine and Jan and Dan Duncan Neurological Research Institute, Texas Children’s Hospital, Houston, TX, United States
Introduction: Developmental synaptopathies are neurodevelopmental disorders caused by genetic mutations disrupting the development and function of neuronal synapses.
Methods: We administered the validated Social Responsiveness Scale, Second Edition (SRS-2) to investigate the phenotypic presentation of social-behavioral impairments for the developmental synaptopathy—SYNGAP1-related Intellectual Disability (SYNGAP1-ID) (n = 32) compared with a phenotypically similar disorder Phelan-McDermid syndrome (PMD) (n = 27) and healthy controls (n = 43). A short form SRS-2 analysis (n = 85) was also conducted.
Results: Both SYNGAP1-ID and PMD had significantly elevated total and subcategory T-scores, with no significant score differences between SYNGAP1-ID and PMD, consistent between the full and short form. Mild to severe deficiencies in reciprocal social behavior were found in 100% of PMD individuals and 87.1% of SYNGAP1-ID individuals. Surprisingly, a positive correlation between age and total score was discovered for SYNGAP1-ID participants and not found in individuals with PMD or healthy controls.
Discussion: The short form demonstrated greater utility for SYNGAP1-ID participants due to lower item-omission rates. In conclusion, significant impairment in reciprocal social behaviors is highly prevalent in SYNGAP1-ID.
Introduction
Genetic mutations affecting the development and function of the neuronal synapse lead to developmental synaptopathies, which frequently include developmental delays or intellectual disability, behavioral dysregulations, epilepsy and social impairments. There are a growing number of genes which encode proteins found at the neuronal synapse that when mutated result in neurodevelopmental or neuropsychiatric disorders. Indeed, large genomic sequencing studies have determined that synaptic genes are one of the more common classes of genes where mutations are a significant risk factor for the development of autism spectrum disorders (1). Despite a broad understanding of possible neurologic and psychiatric phenotypes associated with mutations of synaptic genes, detailed phenotypic analyses of these disorders are largely lacking. Here, we investigated the social responsiveness for the developmental synaptopathy—SYNGAP1-related Intellectual Disability (SYNGAP1-ID) in comparison with another better characterized developmental synaptopathy, Phelan McDermid syndrome (PMD), and healthy controls (HC).
SYNGAP1-ID is a genetic disorder caused primarily by de novo loss-of-function single nucleotide variants in SYNGAP1 or, less commonly, a hemizygous deletion of chromosome 6p21.3, the cytogenic location of SYNGAP1 (2–5). This gene encodes for the SYNGAP1 protein, which functions as a GTPase that is essential for synaptic development, structure, function and plasticity (4, 6). Mutations of SYNGAP1 increase neurotransmission at excitatory glutamatergic synapses resulting in imbalance of excitatory/inhibitory neurotransmission (2, 4, 5, 7).
PMD is another genetic developmental synaptopathy caused primarily by the deletion of the distal long arm of chromosome 22 (encompassing 22q13), with clinical manifestations remarkably similar to SYNGAP1-ID (8). The neurological deficits associated with PMD have been linked to haploinsufficiency of SHANK3, a gene encoding a protein enriched in the postsynaptic density of excitatory synapses (8). Reduction in the SHANK3 protein due to haploinsufficiency of its gene reduces excitatory glutamatergic neurotransmission also resulting in an imbalance of excitatory/inhibitory neurotransmission, although thought to be in the opposite direction due to mutations in SYNGAP1 (9, 10).
Despite mutations in SYNGAP1 and SHANK3 resulting in opposite effects on E/I ratio, the phenotypic presentations of both SYNGAP1-ID and PMD are similar in that they can include epilepsy, intellectual disability (ID), autism spectrum disorder (ASD), severe expressive and receptive speech delay, hypotonia, sleep abnormalities, global developmental delays, and behavioral issues (3, 5, 8, 11–14). Autism-related social impairments are commonly reported symptoms in both patient populations. In the largest cohort reported to date, 30/57 (52.6%) of patients with pathogenic SYNGAP1 mutations were diagnosed with autism spectrum disorder (5). Similarly, in a cohort of 27 participants with SYNGAP1-ID, 52% were found to have an ASD diagnosis (15). In a study analyzing 32 patients with PMD, 27 (84%) met the criteria for autism spectrum disorder, as assessed by the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule-G (14). However, the prevalence and severity of social impairments for children with SYNGAP1-ID has yet to be assessed systematically. Moreover, no study to date has compared the severity of social impairment between developmental synaptopathies or with neurotypical children.
Here, we assess the severity of social behavioral impairments resulting from the developmental synaptopathy, SYNGAP1-related intellectual disability (SYNGAP1-ID) using a validated instrument, the Social Responsiveness Scale Second Edition (SRS-2) (16). We utilized the SRS-2 to quantify the social behavioral phenotypes of participants diagnosed with SYNGAP1-ID because it is well-validated in multiple populations and easy and quick to administer. The SRS-2 is a 65-item, Likert-scale, objective measure of autism-associated reciprocal social interactions, typically completed by a caregiver or teacher of a child. It is an extensively cited measure within ASD literature, and is used in practice to screen for ASD, or in clinical settings to detect subtle variations in the severity of symptoms over time, across individuals, or as a treatment outcome measure (16). The raw scores are normalized by gender, age group (preschool, school-age, and adult), and rater identity (parent or teacher). Besides the total severity score, the survey also creates sub-scores for the following categories: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behaviors.
Previous studies assessing the psychometric properties of the full SRS-2 in children with severe ID and language impairments suggests that the full SRS-2 is limited in its ability to assess behavioral phenotypes in these individuals (17, 18). A shortened version of the SRS-2 was created in an effort to limit the influence of age, expressive language, behavior problems and nonverbal IQ on survey scores, while maintaining a unidimensional factor structure (18). This version of the SRS-2 consists of 16 items taken from the full form (18). A study analyzing the psychometric properties of both the full and shortened SRS-2 forms in PMD patients (n = 91) found that the shortened SRS-2 showed significant improvement in validity and reliability as compared to the full form for this patient population (17). For this reason, we conducted analysis with both the full and shortened versions of the SRS-2 form for participants that completed the School-Age form, also known as the original SRS form.
Methods
Recruitment
Participants for this study were recruited from the Bluebird Circle Clinic for Pediatric Neurology at Texas Children's Hospital with assistance from the following family advocacy foundations: Phelan McDermid Syndrome Foundation, SYNGAP1 Foundation and the SynGAP Research Fund, Inc. Family advocacy foundations either contacted families that previously indicated they were interested in research by emailing a recruitment letter or by posting information on social media.
Adult caregivers of children younger than 21 years of age with a diagnosis of SYNGAP1-ID or PMD were eligible to participate. Participant's IQ or presence of ASD diagnosis was not ascertained. Exclusionary criteria included individuals greater than 21 years old. Those interested in participating in the study were given information about the study over the phone. Consent forms were reviewed in the same call, and then mailed to the family for signature with a return envelope. Once consent was obtained, the questionnaire was completed over the phone and answers were recorded by a member of the research staff. Questions were read to participants as they were not local. Participants were asked 65 total questions from the relevant SRS-2 questionnaire. For children between the age of 1.5 and 4.5 years old, a Preschool form was completed, and all other subjects (4.5–21 y/o) completed the School Age form. Completing the questionnaire took 15–20 min. Caregivers were given the option to leave questions unanswered if not applicable or unknown. However, in accordance with the assessment instructions, if 7 or more items were left blank the survey was deemed unusable.
Once raw scores were collected, the accompanying scoring worksheet was completed by a member of the research staff to obtain treatment subscales and total raw scores. The raw scores were then used to determine T-scores, normalized for age-group. School Age forms also normalized scores for gender and rater identity (which was a parent in all cases). T-scores below 59 were considered within normal limits, and any score above was further subcategorized into the mild (60–65), moderate (66–75), or severe range (≥76). T-scores were obtained for the total score as well as each subscale.
Participating caregivers were also asked to complete the survey for neurotypical siblings to participate as healthy control individuals. The surveys were completed between December 2020 and January 2023.
Ethical approval was obtained from the Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals (H-48239).
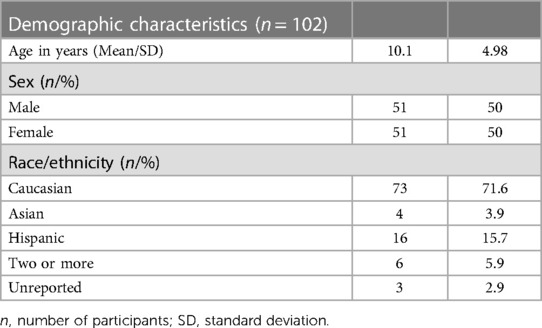
Demographics
Participants ranged from age 2 to 21 years. Table 1 describes the general demographic characteristics of all participants for which data was obtained.
Full SRS-2 analysis
Participants included in the full SRS-2 analysis were those who satisfactorily completed a version of the full SRS form (School Age or Preschool versions). A Kruskal–Wallis test was used to compare medians for age in the three groups. A Chi-squared test was used to determine statistical significance in sex distribution among the three groups. No statistically significant difference was found between age or sex distribution amongst all three groups (Table 2).
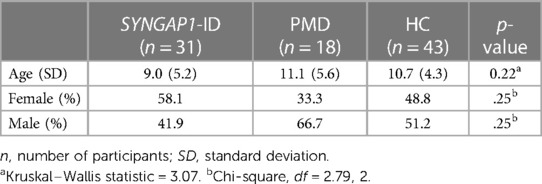
Table 2. Mean age and gender distributions amongst all participant groups used for full SRS-2 analysis.
Short SRS-2 analysis
Only subjects who filled out the School Age form were included in the shortened SRS analysis, because the short form was originally derived from the SRS-2 School Age form (18). A total score and item-level analysis was conducted.
No statistically significant difference was found between age or sex distribution amongst all three groups for this analysis group (Table 3). In the short form analysis, participants that failed to answer 2 or more of the 16 items (12.5%) were omitted (n = 5). This decision was made based on the SRS-2 manual's guidelines to omit participants who failed to answer 7/65 (10.7%) of the items on the full form (16).
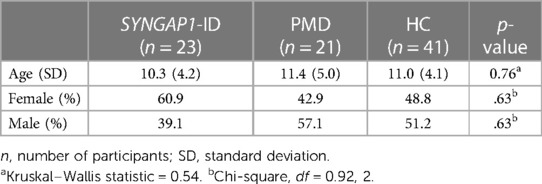
Table 3. Mean age and sex distributions amongst all participant groups used for short SRS survey analysis.
Statistical analysis
Raw scores and T scores from surveys were recorded in Microsoft Excel. Descriptive statistics for total scores and subscale scores (social awareness, social cognition, social communication, social motivation, restricted interests and repetitive behavior), both raw and T scores, were performed using GraphPad Prism version 9. Descriptive statistics for item-level responses were also obtained for the short SRS form. Complete analysis of full and short form scores was conducted. Spearman correlation tests were performed to determine the correlation between full and short form scores and correlation with age. For all analyses, to compare the three population groups, the Kruskal–Wallis non-parametric test was used because data did not pass D’Agostino & Pearson normality test. For pairwise comparisons, Dunn's multiple comparison test was used. All statistical tests were performed using GraphPad Prism version 9.
Results
Statistical analysis
Full form analysis
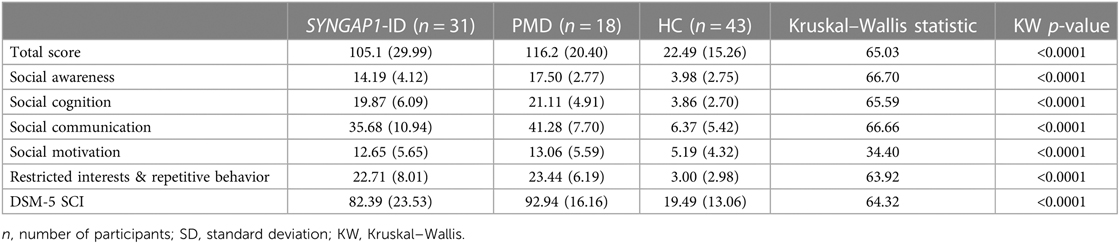
The SRS total scores, both raw and T scores, were significantly increased (p < 0.0001) for both SYNGAP1-ID and PMD populations as compared to healthy controls (Figure 1 and Table 4). No significant difference was found between the total scores of SYNGAP1-ID and PMD patients. Additionally, for all SRS-2 subscale scores (raw and T scores), significant difference (p < 0.0001) was found between PMD and SYNGAP1-ID participants and healthy controls, with no significant difference between PMD and SYNGAP1-ID scores. A total of 10 participants were omitted from the full form analysis due to incomplete data sets caused by a number of inapplicable questions for parents. (SYNGAP1-ID, n = 1; PMD, n = 9). Analyses were performed to determine whether total scores were impacted by sex, but results demonstrated no significant difference between total scores based on sex (Supplementary Figure S1).
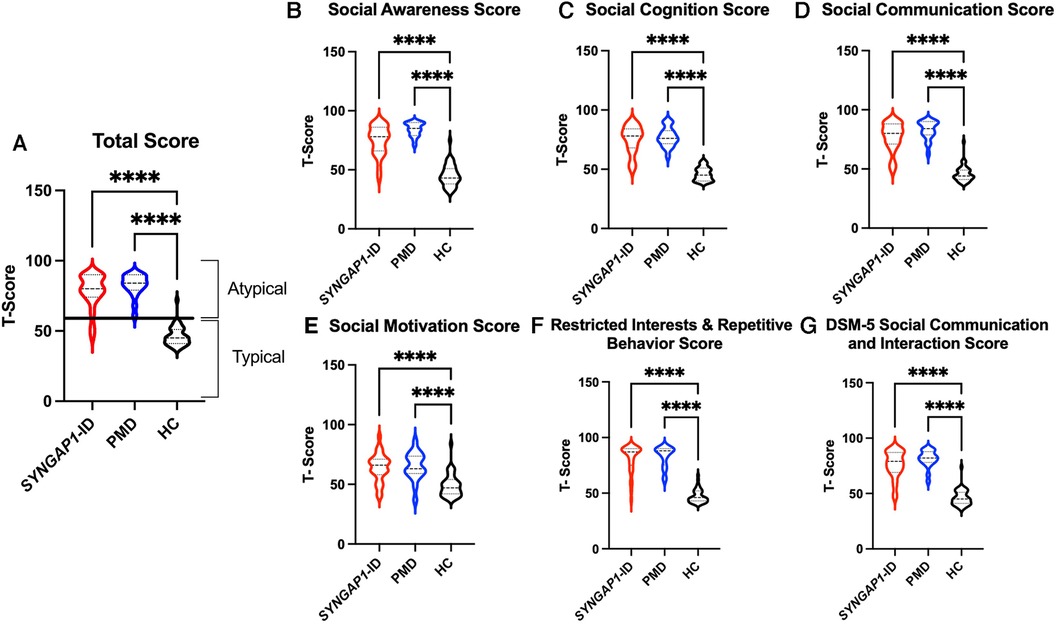
Figure 1. Total T-scores (A) and subscale T-scores for SYNGAP1-related intellectual disability (SYNGAP1-ID), Phelan-McDermid syndrome (PMD), and healthy controls (HC). Scores in typical and atypical range are labelled. Subscales: (B) Social Awareness, (C) Social Cognition, (D) Social Communication, (E) Social Motivation, (F) Restricted Interests and Repetitive Behavior, (G) DSM-5 Social Communication and Interaction Score. Dunn's multiple comparison test ****p < 0.0001. Thick dash lines represent medians and thin dashed lines represent quartiles.
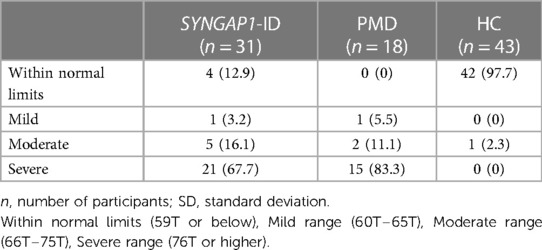
Based on the SRS-2 full form cut-offs, 100% of PMD individuals and 87.1% of SYNGAP1-ID individuals met the criteria for mild to severe deficiencies in reciprocal social behavior, as defined by the SRS scale (Table 5). The most common category was “Severe” deficiencies with 83.3% of PMD individuals and 67.7% of SYNGAP1-ID individuals falling into this category. A small percentage of SYNGAP1-ID individuals (12.9%) fell in the “Within normal limits” category. These data suggest that impairments in reciprocal social behavior is very common for individuals with these developmental synaptopathies, with a majority presenting with severe impairments.
Short form analysis
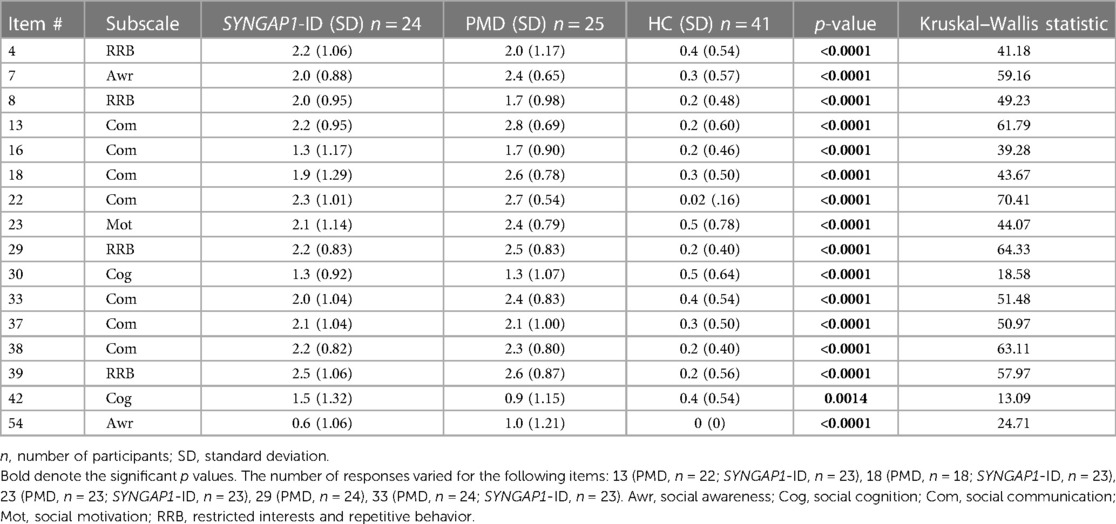
An analysis of participants who satisfactorily completed a School Age form (n = 85) showed that both SYNGAP1-ID (n = 23) and PMD (n = 21) individuals had significantly elevated (p < 0.0001) total scores compared to healthy controls (n = 41) (Figure 2). A total of 5 participants were omitted from the total score short form analysis due to missing data. Additionally, both SYNGAP1-ID and PMD individuals had significantly elevated (p < 0.0001) scores as compared to healthy controls in every item, except item 42 (Table 6). No significant differences were found between scores of SYNGAP1-ID and PMD individuals in any of the items or in total score. Average scores from all items on the short form were obtained and are displayed in Table 6. Item 42 scores showed a statistically significant difference between SYNGAP1-ID and HC, but a nonsignificant difference between PMD and HC. Item 42 stated: “Seems overly sensitive to sounds, textures, or smells.” T-scores could not be calculated for the short form, so all analyses were performed with raw scores.
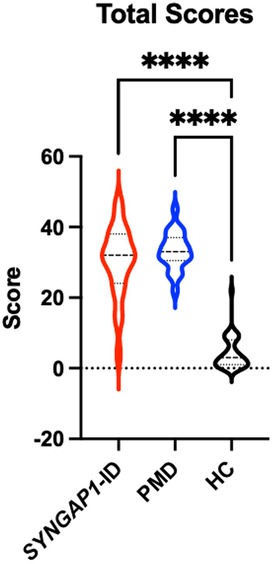
Figure 2. Short SRS form total raw scores for SYNGAP1-related intellectual disability (SYNGAP1-ID), Phelan-McDermid syndrome (PMD), and healthy controls (HC). Dunn's multiple comparison tests ****p < 0.0001. Thick dash lines represent medians and thin dashed lines represent quartiles.
A Spearman correlation test demonstrated a strong correlation of full form total scores to short form total scores (r = 0.96, Supplementary Figure S2).
Correlation with age
Finally, we sought to determine if there was a correlation with scores of the full or short forms of the SRS-2 with age in any of our populations (Figure 3). Using Spearman's correlation, we discovered a moderate but significant correlation between age and total T-score for individuals with SYNGAP1-ID (r = 0.49, p < 0.01) (Figure 3A). There was also a moderate correlation between age and total score of the short form of the SRS-2 that did not reach statistical significance for individuals with SYNGAP1-ID (r = 0.41, p = 0.0528) (Figure 3B). No correlation between age and total score of either the full form or short form was present for either Phelan-McDermid syndrome or healthy controls (Figure 3C–F).
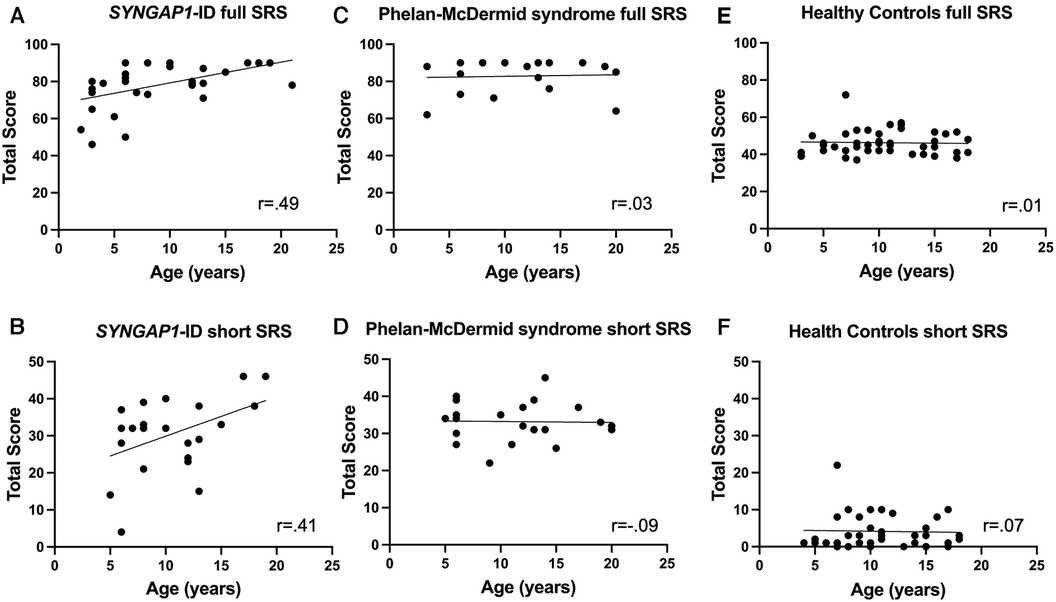
Figure 3. Correlation of total T-score with age. Correlation between age of total T-score for (A,B) SYNGAP1-ID, (C,D) Phelan-McDermid syndrome (PMD) and Healthy Controls (E,F) for SRS-2 full form (A,C,E) and short form (B,D,F).
Discussion
Social behavioral abnormalities are commonly reported in individuals with SYNGAP1-ID; however, they have not previously been investigated systematically. Here, we administered the validated Social Responsiveness Scale-2 (SRS-2) to determine whether this assessment could appropriately characterize social behavioral phenotypes of individuals with this disorder. Individuals with SYNGAP1-ID have elevated total and sub-section scores as compared to healthy controls and indistinguishable impairment compared with those diagnosed a similar monogenic developmental synaptopathy, PMD. Moreover, the vast majority (>85%) of individuals with SYNGAP1-ID had a total score greater than the standard cut-off for abnormal social interactions with more than the majority falling into the severe category. Thus, we demonstrate for the first time that reciprocal social interactions are abnormal based upon a standardized measurement.
While the results of the survey demonstrated scores with detectable differences from healthy controls, some participants were omitted from analysis due to incomplete surveys (10.9%). A significant portion (90%) of individuals omitted were individuals with PMD.
The shortened form was created by Sturm et al. to better assess individuals with severe intellectual disability. The three most commonly (n > 15) omitted items from our populations (10, 15, 62) on the full form were all removed in the short form, demonstrating its utility in these populations. However, a portion of individuals (5.75%) were still omitted from the short form analysis because they failed to answer 2 or more of the 16 items. Commonly omitted items from the shortened form were items 18 and 13 (n = 8.5). Table 7 overviews the item content of commonly omitted survey items.
No previous study has recorded the use of the SRS survey in SYNGAP1-ID patients, however one previous study has assessed the psychometric properties of the full and short SRS survey in PMD patients. Gergoudis et al. found that the full SRS-2 has limited efficacy in the PMD population, and that the shortened form is a more reliable and valid alternative (17). This previous study found that many items on the full form have limited relevance and utility for the PMD population, which is resolved in the shortened form. Our data similarly supports the use of the shortened SRS form as compared to the full form in this patient population. Additionally, the strong correlation between the full form and short form total scores adds confidence for the use of the shortened form. However, the omission of 4/25 (16%) of PMD individuals as compared to 1/24 (4.2%) of SYNGAP1-ID individuals suggests that even the short form has limitations for the PMD population. Nevertheless, the short form of the Social Responsiveness Scale might have more general utility across neurodevelopmental disorders.
Although no significant differences were found between the scores of PMD and SYNGAP1-ID individuals in either the long or short SRS form, the high rate of item-omission in the PMD population differs drastically from the SYNGAP1-ID population. Reasons for this observation may relate to the level of non-social behavioral symptom severity in PMD populations as compared to those with SYNGAP1-ID, making it difficult for caregivers of these individuals to provide answers to specific items. The omission-rate discrepancies between PMD and SYNGAP1-ID individuals warrants further investigation.
Surprisingly, the total T-scores for individuals with SYNGAP1-ID were positively correlated with age. This differed from our data of individuals with Phelan-McDermid syndrome in which there was no hint of a correlation. The previously published study regarding the psychometric properties of the SRS-2 in Phelan-McDermid syndrome did not report correlation of scores with age at time of administration. Moreover, previously published work in idiopathic autism found a negative correlation between SRS-2 T-score and age with adults having significantly lower total T-scores than children (19). It is unclear why social responsiveness would worsen with age in this population and warrants further investigation.
This study was limited by a relatively small sample size that resulted in using non-parametric statistical tests to analyze data, yielding less power than their parametric counterparts. Moreover, as the SRS utilizes data provided by caregivers' impressions of their child's social interactions, and not directly observed interactions, the conclusions of this study could be impacted by their bias. Because recruitment was performed largely through patient advocacy foundations, information regarding autism or intellectual disability diagnoses was not available and warrants further investigation in future studies.
Conclusion
By utilizing the Social Responsiveness Scale assessment, we discovered the majority of individuals with SYNGAP1-related intellectual disability (SYNGAP1-ID) have severe impairments in reciprocal social behavior similar to individuals with a related developmental synaptopathy—Phelan McDermid Syndrome (PMD). We also found that the shortened SRS survey by Sturm et al. is a more feasible assessment for use in children with these developmental synaptopathies because of lesser influence of confounding factors, such as NVIQ, on scores. However, differences in omission rate between SYNGAP1-ID and PMD individuals point to lower utility of the assessment for individuals with PMD. We propose that the shortened SRS form can be utilized to characterize social behavioral phenotypes in patients with SYNGAP1-ID and has less utility for those with PMD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Baylor College of Medicine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HN collected data, performed data analysis, wrote the first draft of the manuscript and revised the manuscript. MM helped conceive the study, collected the data. JH conceived the study, supervised data collection, analyzed the data and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AS declared a past co-authorship with the author JH.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1188117/full#supplementary-material
References
1. Satterstrom FK, Breen MS, Grove J, Klei L, Mulhern MS, Xu X, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. (2020) 180(3):568–584.e23. doi: 10.1016/j.cell.2019.12.036
2. Agarwal M, Johnston MV, Stafstrom CE. SYNGAP1 mutations: clinical, genetic, and pathophysiological features. Int J Dev Neurosci. (2019) 78(1):65–76. doi: 10.1016/j.ijdevneu.2019.08.003
3. Jimenez-Gomez A, Niu S, Andujar-Perez F, McQuade EA, Balasa A, Huss D, et al. Phenotypic characterization of individuals with SYNGAP1 pathogenic variants reveals a potential correlation between posterior dominant rhythm and developmental progression. J Neurodev Disord. (2019) 11(1):18. doi: 10.1186/s11689-019-9276-y
4. Keller R, Basta R, Salerno L, Elia M. Autism, epilepsy, and synaptopathies: a not rare association. Neurol Sci. (2017) 38(8):1353–61. doi: 10.1007/s10072-017-2974-x
5. Vlaskamp DRM, Shaw BJ, Burgess R, Mei D, Montomoli M, Xie H, et al. SYNGAP1 encephalopathy: a distinctive generalized developmental and epileptic encephalopathy. Neurology. (2019) 92(2):E96–E107. doi: 10.1212/WNL.0000000000006729
6. Gamache T, Araki Y, Huganir RL. Twenty years of SynGAP research: from synapses to cognition. J Neurosci. (2020) 40(8):1596–605. doi: 10.1523/JNEUROSCI.0420-19.2020
7. Kozol RA, Cukier HN, Zou B, Mayo V, De Rubeis S, Cai G, et al. Two knockdown models of the autism genes SYNGAP1 and SHANK3 in zebrafish produce similar behavioral phenotypes associated with embryonic disruptions of brain morphogenesis. Hum Mol Genet. (2015) 24(14):4006–23. doi: 10.1093/hmg/ddv138
8. Phelan K, McDermid H. The 22q13.3 deletion syndrome (Phelan-McDermid syndrome). Mol Syndromol. (2012) 2(3-5):186–201. doi: 10.1159/000334260
9. Shcheglovitov A, Shcheglovitova O, Dolmetsch RE, Yazawa M, Portmann T, Rui Shu S, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature (London). (2013) 503(7475):267–71. doi: 10.1038/nature12618
10. Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature (London). (2011) 472(7344):437–42. doi: 10.1038/nature09965
11. Berryer MH, Hamdan FF, Klitten LL, Møller RS, Carmant L, Schwartzentruber J, et al. Mutations in SYNGAP1 cause intellectual disability, autism, and a specific form of epilepsy by inducing haploinsufficiency. Hum Mutat. (2013) 34(2):385–94. doi: 10.1002/humu.22248
12. Mignot C, von Stülpnagel C, Nava C, Ville D, Sanlaville D, Lesca G, et al. Genetic and neurodevelopmental spectrum of SYNGAP1-associated intellectual disability and epilepsy. J Med Genet. (2016) 53(8):511–22. doi: 10.1136/jmedgenet-2015-103451
13. Smith-Hicks C, Wright D, Kenny A, Stowe RC, McCormack M, Stanfield AC, et al. Sleep abnormalities in the synaptopathies—SYNGAP1-related intellectual disability and Phelan–McDermid syndrome. Brain Sci. (2021) 11(9):1229. doi: 10.3390/brainsci11091229
14. Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, et al. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism. (2013) 4(1):18. doi: 10.1186/2040-2392-4-18
15. Wright D, Kenny A, Eley S, McKechanie AG, Stanfield AC. Clinical and behavioural features of SYNGAP1-related intellectual disability: a parent and caregiver description. J Neurodev Disord. (2022) 14(1):34. doi: 10.1186/s11689-022-09437-x
16. Constantino JN, Gruber CP. Social responsiveness scale, second edition (SRS-2) [manual]. Torrance, CA: Western Psychological Services (2012).
17. Gergoudis K, Weinberg A, Templin J, Farmer C, Durkin A, Weissman J, et al. Psychometric study of the social responsiveness scale in Phelan–McDermid syndrome. Autism Res. (2020) 13(8):1383–96. doi: 10.1002/aur.2299
18. Sturm A, Kuhfeld M, Kasari C, McCracken JT. Development and validation of an item response theory-based social responsiveness scale short form. J Child Psychol Psychiatry. (2017) 58(9):1053–61. doi: 10.1111/jcpp.12731
Keywords: SYNGAP1 gene, Phelan McDermid syndrome, SRS, autism, intellectual disability
Citation: Naveed H, McCormack M and Holder JL Jr (2023) Social behavioral impairments in SYNGAP1-related intellectual disability. Front. Pediatr. 11:1188117. doi: 10.3389/fped.2023.1188117
Received: 16 March 2023; Accepted: 8 November 2023;
Published: 29 November 2023.
Edited by:
Marco Carotenuto, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Francesca Felicia Operto, University of Salerno, ItalyAndrew Stanfield, University of Edinburgh, United Kingdom
© 2023 Naveed, McCormack and Holder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Lloyd Holder Jr aG9sZGVyQGJjbS5lZHU=
 Hajer Naveed
Hajer Naveed J. Lloyd Holder Jr
J. Lloyd Holder Jr