
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 06 November 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1183388
Background: Wave In, which refers to the negativity between waves I and II in auditory brainstem response (ABR), is an electrophysiological phenomenon observed in previous studies. The term “high jugular bulb” (HJB) describes a jugular bulb that is located in a high position in the posterior aspect of the internal acoustic canal. The present study aimed to explore the correlation between wave In and the possibility of a HJB.
Methods: This retrospective study included a cohort of pediatric patients diagnosed with profound hearing loss who were enrolled in a government-sponsored cochlear implantation program at an academic medical center between January 2019 and December 2022. The analysis involved examining the results obtained from the ABR test and high-resolution computed tomography (HRCT) of the temporal bone in the patients. The position of the jugular bulb was classified according to the Manjila and Semaan classification.
Results: A total of 221 pediatric patients were included in the study. Twenty-four patients, with a median age of 3 years and a range of 1–7 years, showed significant bilateral (n = 21) or unilateral (n = 3) wave In (mean latency: right ear, 2.16 ms ± 0.22 ms; left ear, 2.20 ms ± 0.22 ms). The remaining 197 patients showed an absence of ABR. The HRCT images revealed that 18 of the 24 patients (75%) had HJB, but only 41 of the 197 patients who lacked ABR (20.8%) showed signs of HJB. The ratio difference was considered statistically significant based on the chi-squared test (χ2 = 32.10, p < 0.01). More than 50% of the HJBs were categorized as type 4 jugular bulbs, which are located above the inferior margin of the internal auditory canal.
Conclusion: ABR wave In in pediatric patients with profound hearing loss suggests a high possibility of HJB. The physiological mechanism underlying this correlation needs further investigation.
Auditory brainstem response (ABR) refers to the short-term neural electrical activity that is recorded from the scalp and originates from the inner ear, auditory nerve, and auditory brainstem under air- or bone-conducted acoustic stimulation (1). ABR has been commonly used to evaluate the integrity of the auditory pathway. A typical click-evoked ABR shows five primary waves labeled using Roman numerals Ⅰ–Ⅴ in sequence. Each ABR wave comprises a positive peak followed by a negative one, termed, e.g., P1 (or peak I) and N1 (or In) (2, 3). The threshold, amplitude, and latency of ABR waves are key parameters for clinical interpretation (4). In a previous study, Martin et al. (5) noted a negative correlation between waves I and II (wave In) in ABR recorded from human patients undergoing neurosurgical procedures and considered that wave In could be stationary potentials originating from conductivity boundaries existing in the posterior fossa. In a subsequent independent study, Rattay and Danner (6) proposed that peaks In are stationary potentials when volleys of spikes cross the external electrical conductivity barrier at the interface between the bone and dura/cerebrospinal fluid, supporting Martin’s hypothesis.
A jugular bulb (JB) refers to a bulbous enlargement at the junction of the intracranial sigmoid sinus and internal jugular vein (7). The JB is located in the jugular fossa, and the position of the jugular fossa varies among different individuals. The term “high jugular bulb” (HJB) describes anatomical variants of the JB rising to the level of the basal turn of the cochlea, encroaching upon the floor of the internal auditory canal, or protruding into the tympanic cavity or inner ear (8). Reportedly, the incidence of HJB ranges from 6% to 20% in patients undergoing computed tomography (CT) of the temporal bone for any reason (8, 9). A recent retrospective study shows a prevailing rate of 42% for HJB (predominantly unilateral) among 194 children who underwent cranial CT primarily due to head trauma (10). Awareness of vascular abnormalities is beneficial in minimizing clinical complications during otologic surgery (11). Of note, a recent case–control study has demonstrated that HJB is associated with hearing loss in patients diagnosed with bilateral large vestibular aqueduct syndrome (LVAS) (14).
Based on the previous findings, we assumed that the presence of ABR wave In could be related to certain anatomical variants in the posterior fossa. Thus, we explored the correlation between wave In and HJB in a cohort of pediatric patients with profound hearing loss who were enrolled for cochlear implantation.
This retrospective study was approved by the Institutional Review Board (IRB) of the Second Xiangya Hospital of Central South University, and written informed consent was obtained.
The patients included in the study were children with profound deafness who were enrolled for government-sponsored cochlear implantation at the Department of Otolaryngology of the Second Xiangya Hospital of Central South University between January 2019 and December 2022. The patients underwent ABR testing and high-resolution computed tomography (HRCT) of the temporal bone as part of their presurgical evaluation.
ABR testing was performed with a Neuro-Audio system (Neurosoft Ltd., Ivanovo, Russia) in an acoustically and electrically shielded booth. The active, reference, and ground electrodes were placed in the middle of the forehead at the hairline, the bilateral mastoids, and the nose root, respectively. The resistance between electrodes was ≤4 kΩ. An ER-3A plug-in earphone (Etymotic Research, Inc., Elk Grove Village, IL, USA) was used to deliver the click stimulus of 1,024 sweeps at a rate of 21.1 /s, with a bandpass filter setting from 100 Hz to 3,000 Hz and a recording time window of 15 ms. The stimulus intensity started from 80 dB nHL and declined/increased in 10 dB increments. The measurement was repeated at least three times for each stimulus level. A threshold of ≤30 dB nHL for click-ABR wave V was considered to be within the normal range (0 dB nHL = 28.7 dBSPL). The latency of wave In is in reference to the previously reported value (mean, 2.06 ms; standard deviation, 0.11 ms) (3).
HRCT scans were performed on a Somaton Plus 4A CT scanner (Siemens AG). The acquisition parameters are as follows: 120 kVs, 100 mAs, 0.75 mm collimation, 1 mm reconstruction increment, a pitch factor of 1, and a field of view of 100 mm. All radiographs regarding the position of JB were reviewed by two radiologists unaware of the study design. Referencing the Manjila and Semaan classification, JB was classified as follows: type 1: no bulb; type 2: below the inferior margin of the posterior semicircular canal (PSCC); type 3: between the inferior margin of the PSCC and the inferior/margin of the internal auditory canal (IAC); and type 4: above the inferior margin of the IAC (11).
Descriptive statistical analysis of the numerical data (including age and wave latency) and the chi-squared test on frequencies were processed by software Origin 8.0 (OriginLab Corporation, Northampton, MA, USA).
A total of 221 pediatric patients were included in the study. There were 24 patients, comprising 14 boys and 10 girls, with a median age of 3 years and a range of 1–7 years, who had significant bilateral (n = 21) or unilateral (n = 3) wave In (mean latency: right ear, 2.16 ms ± 0.22 ms; left ear, 2.20 ms ± 0.22 ms). The other 197 patients showed an absence of ABR at the maximum stimulation intensity of 100 dB nHL (Figures 1 and 2 and Table 1). The HRCT images revealed that 18 of the 24 patients (75%) exhibited bilateral (n = 8) or unilateral (n = 10) HJB, compared with 41 of the 197 patients who lacked ABR (20.8%) and showed bilateral or unilateral HJB. The difference in ratios was considered statistically significant based on the chi-squared test (χ2 = 32.10, p < 0.01). According to the Manjila and Semaan classification of the JB location, 15 of the 26 (58%) HJBs in the patients with wave In belong to type 4 JBs and six (23%) and five (19%) HJBs belong to type 3 and type 2 JBs, respectively (Table 1).
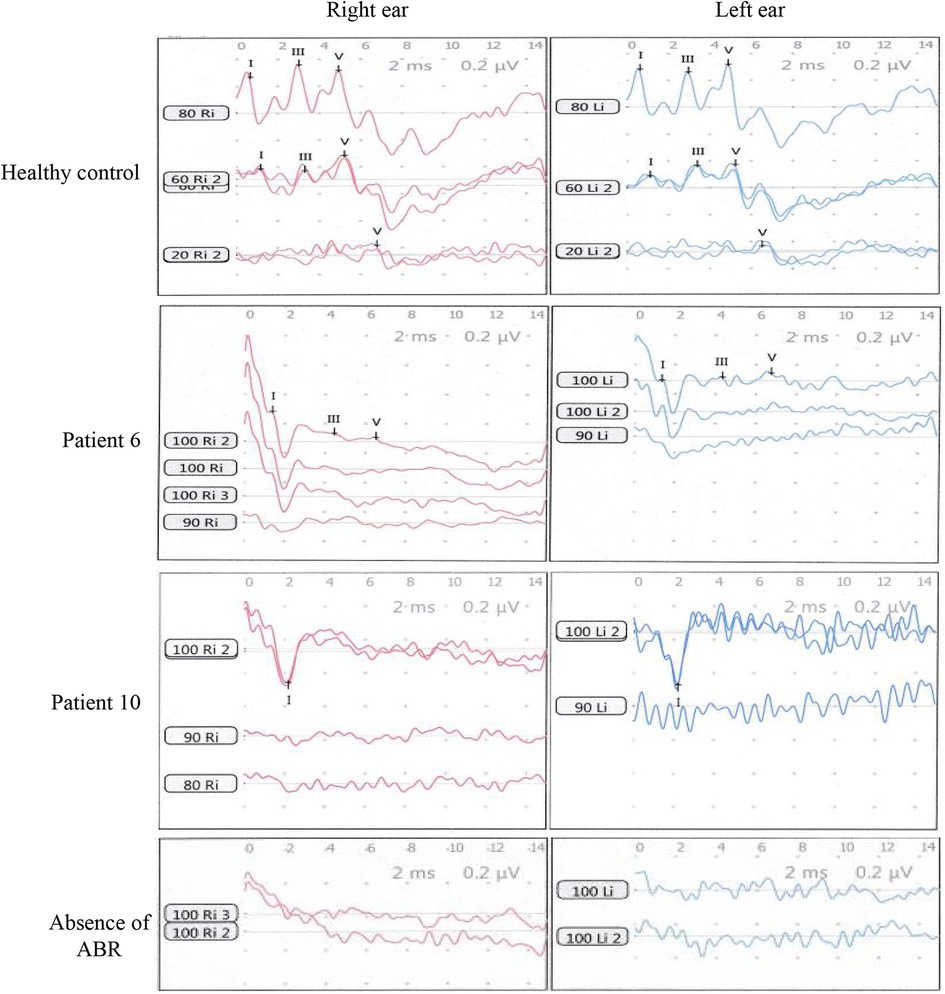
Figure 2. ABR waves In were recorded from the pediatric patients with profound hearing loss. The healthy control was a boy of age 2.5 years with normal hearing. ABR waves of patient 6 and patient 10 in Table 1 are representatively shown. A control of the absence of ABR was shown at the bottom panel.
ABR wave In, the negative peak after peak I, is an electrophysiological phenomenon observed in earlier studies (5, 6). This study identified 24 patients with wave In by reviewing the ABR results of 221 pediatric patients who were enrolled for cochlear implantation. Interestingly, the HRCT of the temporal bone reveals that 18 of the 24 patients with wave In demonstrated the presence of HJB (bilateral or unilateral), displaying a high rate of 75% for HJB compared with a rate of 20.8% (41/197) observed in patients without wave In (ABR). The rate (75%) is also much higher than a rate of 6%–20% for HJB in patients undergoing HRCT of the temporal bone for any reason (8) and a rate of 42% for HJB among 194 children who underwent cranial CT mainly due to head trauma in a recent study (10). By further looking at the JB location, we observed that more than half of the HJBs from patients with wave In belong to the type 4 JB according to the Manjila and Semaan classification. In a recent study by Hu et al. (12) to screen causative HJB in patients with Meniere’s disease, type 4 JB had a prevalence rate of 8.7% (8/92) in patients with hydropic ears and a prevalence rate of 1.1% (1/90) in patients with non-hydropic ears. Despite the fact that a high rate of type 4 JB was accompanied by wave In in pediatric patients with profound hearing loss in our study, the physiological correlation between wave In and HJB remains unclear.
More recently, Kwesi et al. (13) conducted a case–control study to explore the effect of unilateral HJB on hearing loss in 36 patients diagnosed with bilateral LVAS. In addition to the major finding that LVAS with concurrent HJB was associated with higher air conduction thresholds, they also found that the laterality of HJB was mostly in the right ears and that the prevalence of HJB was not correlated with gender and age. In our cohort, significant laterality preference of HJB was not observed (12 right HJBs vs. 14 left HJBs), either the gender difference of patients with HJB (10 males vs. eight females). A proportion (6/24) of the patients in our cohort had a bilateral large vestibular aqueduct; however, this finding was not associated with the presence of HJB.
The present study provokes an interesting discussion regarding the physiological origin of wave In in the patients. Although ABR wave In in our study is similar to the summating potential (SP) in electrocochleography (ECochG) described elsewhere (14), there are three main aspects to differentiate wave In from SP. First, ABR wave I is generated by the distal portion of the auditory nerve (15), whereas SP is a presynapse response, representing direct-current receptor potentials generated by cochlear hair cells. Second, wave In in the study is a far-field measurement, compared with the SP, which is a near-field recording. In addition, the latency of wave In was 2 ms in the present study, but the SP showed a latency of approximately 1 ms in previous studies (16, 17). Therefore, wave In here is unlikely to be a form of SP.
The patients in our study had no residual hearing. However, the possibility of wave I cannot be excluded due to the recorded high thresholds. The recorded wave I could be generated by the residual hair cells in the apical turns of the cochlear triggering the auditory nerves, thus showing a prolonged latency period and a special form. However, the potentials did not yield brainstem neural activation, indicating that the synchronization of potentials is compromised. Alternatively, the recorded waves Ⅰ in the study are synapse potentials, other than neural action potentials.
The strengths of this study include establishing a correlation between ABR wave In and a high jugular bulb in pediatric patients with profound hearing loss. The limitations of this study include the small sample size of patients with wave In identified from the cohort and a lack of data on other clinical audiology assessments (such as ECochG) in the cohort due to the retrospective nature of the analysis. Therefore, including additional patients with wave In in the study group and giving a comprehensive audiology evaluation of the patients would help to further verify the findings in this study.
Collectively, the physiological nature of wave In needs further investigation. As a high jugular bulb has been implicated in conductive or sensorineural hearing loss in certain patients (18–20), it would be interesting to explore a correlation between a high jugular bulb, altered ABR waves, and hearing loss in future studies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Review Board of the Second Xiangya Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JL and GZ designed and performed the experiments, analyzed the data, and wrote the paper. WX analyzed the data and wrote the paper. YD, YH, RL, and PH collected and analyzed the data. All authors contributed to the article and approved the submitted version.
This work was supported by the Changsha Municipal Natural Science Foundation (Grant No. kq2208327).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Young A, Cornejo J, Spinner A. Auditory brainstem response. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2023). PMID: 33231991
2. Brown DJ, Patuzzi RB. Evidence that the compound action potential (CAP) from the auditory nerve is a stationary potential generated across dura mater. Hear Res. (2010) 267(1–2):12–26. doi: 10.1016/j.heares.2010.03.091
3. Ananthanarayan AK, Durrant JD. On the origin of wave II of the auditory brain stem evoked response. Ear Hear. (1991) 12(3):174–9. doi: 10.1097/00003446-199106000-00003
4. Spitzer E, White-Schwoch T, Carr KW, Skoe E, Kraus N. Continued maturation of the click-evoked auditory brainstem response in preschoolers. J Am Acad Audiol. (2015) 26(1):30–5. doi: 10.3766/jaaa.26.1.4
5. Martin WH, Pratt H, Schwegler JW. The origin of the human auditory brain-stem response wave II. Electroencephalogr Clin Neurophysiol. (1995) 96(4):357–70. doi: 10.1016/0168-5597(94)00326-A
6. Rattay F, Danner SM. Peak I of the human auditory brainstem response results from the somatic regions of type I spiral ganglion cells: evidence from computer modeling. Hear Res. (2014) 315:67–79. doi: 10.1016/j.heares.2014.07.001
7. Friedmann DR, Eubig J, McGill M, Babb JS, Pramanik BK, Lalwani AK. Development of the jugular bulb: a radiologic study. Otol Neurotol. (2011) 32(8):1389–95. doi: 10.1097/MAO.0b013e31822e5b8d
8. Friedmann DR, Le BT, Pramanik BK, Lalwani AK. Clinical spectrum of patients with erosion of the inner ear by jugular bulb abnormalities. Laryngoscope. (2010) 120(2):365–72. doi: 10.1002/lary.20699
9. Atilla S, Akpek S, Uslu S, Ilgit ET, Işik S. Computed tomographic evaluation of surgically significant vascular variations related with the temporal bone. Eur J Radiol. (1995) 20(1):52–6. doi: 10.1016/0720-048X(95)00619-2
10. Aksoy SH, Yurdaisik I. High riding jugular bulb: prevalence and significance in asymptomatic children. Acta Radiol. (2023) 64(2):792–7. doi: 10.1177/02841851221085674
11. Amorosa L, Molinari G, Botti C, Presutti L. Management of jugular bulb injury during transcanal endoscopic tympanoplasty. Otol Neurotol. (2021) 42(8):e1186–7. doi: 10.1097/MAO.0000000000003214
12. Hu J, Peng A, Deng K, Huang C, Wang Q, Pan X, et al. Value of CT and three-dimensional reconstruction revealing specific radiological signs for screening causative high jugular bulb in patients with Meniere’s disease. BMC Med Imaging. (2020) 20(1):103. doi: 10.1186/s12880-020-00504-0
13. Kwesi AB, Yu J, Wang C, Wang Y, Chuang F, Yan X, et al. Effect of high jugular bulb on the hearing loss characteristics in patients with LVAS: a pilot study. Front Cell Dev Biol. (2021) 9:743463. doi: 10.3389/fcell.2021.743463
14. Santarelli R, Arslan E. Electrocochleography in auditory neuropathy. Hear Res. (2002) 170(1–2):32–47. doi: 10.1016/S0378-5955(02)00450-1
15. Yasuhara A, Hori A. A comparison of the three-dimensional auditory brainstem response and the conventional auditory brainstem response in children. Brain Dev. (2002) 24(8):750–7. doi: 10.1016/S0387-7604(02)00098-0
16. Marangos N. Hearing loss in multiple sclerosis: localization of the auditory pathway lesion according to electrocochleographic findings. J Laryngol Otol. (1996) 110(3):252–7. doi: 10.1017/S002221510013333X
17. Huang T, Santarelli R, Starr A. Mutation of OPA1 gene causes deafness by affecting function of auditory nerve terminals. Brain Res. (2009) 1300:97–104. doi: 10.1016/j.brainres.2009.08.083
18. Sayit AT, Gunbey HP, Fethallah B, Gunbey E, Karabulut E. Radiological and audiometric evaluation of high jugular bulb and dehiscent high jugular bulb. J Laryngol Otol. (2016) 130(11):1059–63. doi: 10.1017/S0022215116009166
19. Totten DJ, Manzoor NF, Aulino J, Santapuram P, Rivas A. Persistent conductive hearing loss after tympanostomy tube placement due to high-riding jugular bulb. Laryngoscope. (2021) 131(4):E1272–4. doi: 10.1002/lary.28920
Keywords: auditory brainstem response, high jugular bulb, hearing loss, cochlear implant, wave I
Citation: Liu J, Xie W, Ding Y, Hu Y, Lai R, Hu P and Zhu G (2023) Wave In in auditory brainstem response suggests a high possibility of a high jugular bulb. Front. Pediatr. 11:1183388. doi: 10.3389/fped.2023.1183388
Received: 22 May 2023; Accepted: 18 October 2023;
Published: 6 November 2023.
Edited by:
Jason Hauptman, Seattle Children’s Hospital, United StatesReviewed by:
Wei Sun, University at Buffalo, United States© 2023 Liu, Xie, Ding, Hu, Lai, Hu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ganghua Zhu Z2FuZ2h1YXpodUBjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.