
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 30 March 2023
Sec. Pediatric Surgery
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1174357
Objective: There are subclinical cardiac abnormalities (SCA) in children with biliary atresia (BA). However, data on the consequences of these cardiac changes after liver transplantation (LT) remain controversial in the pediatric field. We aimed to determine the relationship between outcomes and the subclinical cardiac abnormalities in pediatric patients with BA based on two-dimensional echocardiography (2DE) parameters.
Methods: A total of 205 children with BA were enrolled in this study. The relationship between 2DE parameters and outcomes, including death and serious adverse events (SAE) after LT, was analyzed by regression analysis. Using receiver operator characteristic (ROC) curves to determine the optimal cut-off values of 2DE parameters for outcomes. Differences in the AUCs were compared using DeLong's test. The Kaplan -Meier method with log-rank testing was used to evaluate survival outcomes between groups.
Results: Left ventricular mass index (LVMI) and relative wall thickness (RWT) were found to be independently associated with SAE (OR: 1.112, 95% CI: 1.061 − 1.165, P < 0.001 and OR: 1.193, 95% CI: 1.078 − 1.320, P = 0.001, respectively). The cutoff value of LVMI for predicting the SAE was 68 g/m2.7 (AUC = 0.833, 95% CI 0.727-0.940, P < 0.001), and the cutoff value of RWT for predicting the SAE was 0.41 (AUC = 0.732, 95% CI 0.641-0.823, P < 0.001). The presence of subclinical cardiac abnormalities (LVMI > 68 g/m2.7, and/or RWT > 0.41) was associated with lower patient survival (1-year, 90.5% vs 100.0%; 3-year, 89.7% vs 100.0, log-rank P = 0.001). and higher incidence of SAE events.
Conclusions: Subclinical cardiac abnormalities were correlated with post-LT mortality and morbidity in children with BA. LVMI can predict the occurrence of death and serious adverse events after liver transplantation.
Biliary atresia (BA) is an obstructive biliary disease, which is the main cause of neonatal cholestasis. It develops quickly to secondary biliary cirrhosis and results in mortality within the first two years of life if not treated (1). Currently, in most pediatric centers BA is the most common indication for liver transplantation (LT) (2).
It is believed that children with end-stage liver disease display cardiac structural changes associated with cirrhotic cardiomyopathy (CCM) (3). CCM is often a subclinical cardiac dysfunction in patients with liver cirrhosis, characterized by blunted contractile stress response, attenuated diastolic relaxation, and electrophysiological abnormalities without known cardiovascular disease (4). At the 2005 World Gastroenterology Congress, a group of experts proposed the diagnostic criteria for adult CCM based on the Doppler echocardiogram for the first time, including lower ejection fraction (<55%), an E/A ratio < 1, isovolumetric relaxation time > 80 ms, E-wave deceleration time > 200 ms, a left atrial volume increase, and increased myocardial mass (5). The existence of CCM leads to an increase in mortality and morbidity after liver transplantation (6).
Unlike in adults, the diagnostic criteria for pediatric CCM have not been established or validated, CCM in children is still relatively unrecognized and understudied (7). At present, there are few studies in the pediatric field that associated post-LT outcomes with cardiac changes associated with CCM. Among the first studies in children, Desai et al. found that up to 70% of patients with BA awaiting LT had identifiable functional and/or structural abnormalities detected on two-dimensional echocardiography (2DE) (8). In their follow-up study published by Gorgis et al. eight years later, they found that the specific 2DE parameters of pediatric CCM were correlated with peri-transplant outcomes, with a high incidence rate of morbidity and mortality (9). This finding is in contrast to the study by Junge et al. who documented a prolonged hospital stay but no increased morbidity and mortality in children with CCM (10). So, further evaluation of the influence of CCM on LT in children is required.
At present, the definition for pediatric CCM is proposed using 2DE parameters, principally including left ventricular mass index (LVMI) and relative wall thickness (RWT). In previous medical literature, no study has been reported about cardiac change associated with cirrhosis and its post-LT outcomes in Chinese pediatric patients. Therefore, we conducted this study to identify the influence of subclinical cardiac abnormalities (SCA) on LT and ascertain the prognostic value of LVMI and RWT for the outcomes after LT in children with BA at a large pediatric LT center.
Clinical and demographic characteristics, as well as the outcomes, were extracted from a prospectively maintained database. In this retrospective cohort study, we screened 272 consecutive pediatric patients with BA who underwent LT from January 2019 to December 2020 at the Tianjin First Central Hospital, China. The exclusion criteria included the following: (i) Patients with congenital heart disease, including patent ductus arteriosus, ventricular septal defect, atrial septal defect and pulmonary stenosis;(ii) Having history of LT; (iii) lack of the data of 2DE before LT. A total of 205 patients were enrolled in this study. The study plan of enrollees is shown in Figure 1. The study adhered to the Helsinki Declaration and was approved by the institutional Ethics Committee of Tianjin First Central Hospital. (2021N168KY).
Data regarding age at LT, sex, history of a Kasai portoenterostomy (KPE), Pediatric End-Stage Liver Disease score (PELD) at time of LT, laboratory data including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), albumin, total bilirubin levels (TB), international normalized ratio (INR), prothrombin time (PT), platelet count (PLT), and C-reactive protein (CRP) were recorded. Body mass index (BMI) was indexed as the weight divided by the square of the height (kg/m2).
To evaluate the structural and functional state of the heart, a complete transthoracic echocardiography including 2D, M-Mode, and Doppler echocardiography was performed in all patients using Philips iE33 ultrasound equipment. The key parameters that determined the LV geometry in the 2DE report were measured: LV end-diastolic diameter (LVIDd), LV posterior wall thickness during diastole (LVPWTd), and interventricular septum thickness during diastole (IVSd). The LV mass (LVM) was calculated based on the Devereux formula according to the American Society of Echocardiography guidelines (11). LVMI was indexed using height2.7 (g/m2.7). RWT was calculated as follows: (IVSd+LVPWd)/LVIDd. Furthermore, the E/A ratio (a ratio of early and late ventricular filling) was evaluated.
Outcomes were measured using patient survival rate and the occurrence of any component of the SAE (9) after LT. The SAE comprised the following: (i) loss of graft due to re-transplantation; (ii) hepatic artery and portal vein thrombosis; (iii) organ support treatment is required, including mechanical ventilation due to respiratory failure, vasoactive drug support or continuous renal replacement therapy (CRRT); (iv) Spontaneous hemorrhage (including gastrointestinal or intracranial hemorrhage) causes hemorrhagic shock. These parameters were chosen because they may seriously affect the patient's survival and postoperative outcomes after LT.
All patients were followed up from the time of transplant to death or the date of November 11, 2021.The median follow-up time after LT was 24 months (range, 0–34 months).
The normal distributed continuous variables are shown as ± S and were analyzed using Student's t-test. The non-normally distributed continuous variables are expressed as median (quartile 25, quartile 75) and were analyzed using the Mann–Whitney U test. The categorical variables are presented as number (n) and proportion (%) and were compared using Chi-square or Fisher's exact test. Univariate analysis was used to compare the baseline characteristics of patients with and without SAE, Statistically significant variables (P < 0.05) were included in multivariate logistic regression analysis to analyze the independent association between variables and SAE. The laboratory values such as bilirubin, INR, and albumin, which could reflect liver function, were included in the PELD score, so we did not include other laboratory values. The optimal cut-off values for each parameter and the diagnostic criteria's clinical efficacy were evaluated using receiver operator characteristic (ROC) curves. Differences in the AUCs were compared using DeLong's test. Survival outcomes between groups were compared using the Kaplan-Meier method and log-rank test. Data analysis was performed using SPSS (version 26.0, IBM Corp, Armonk, NY, USA) and MedCalc (version 19.1, MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019). A two-sided P value < 0.05 was considered to be statistically significant.
A total of 205 pediatric BA patients with a median age level of 7.80 months at transplant participated in the study. 153 (75.0%) patients had a history of KPE. The median level of the PELD score was 16. Among the 205 patients, 25 (12.2%) had suffered SAE after LT, and 180 (87.8%) did not. Patients in the SAE group were younger at the time of LT than those in the No-SAE group (P = 0.045). Compared with the No-SAE group, the SAE group had higher LVMI, RWT, and PELD score (P < 0.001). The incidence of death was more common in the SAE group than in the No-SAE group (32.0% vs. 0.6%, P < 0.001). While EF and FS for systolic function and E/A ratio for diastolic function did not differ significantly between the two groups (P > 0.05). Other parameters showed no statistical difference between the two groups (P > 0.05). As shown in Table 1.
After adjusting for potential confounding factors using a multivariate logistic regression model, LVMI and RWT were found to be independently associated with SAE (odds ratio [OR]: 1.112, 95% confidential interval [CI]: 1.061–1.165, P < 0.001 and OR: 1.193, 95% CI: 1.078–1.320, P = 0.001, respectively). Only LVMI was independently associated with death (OR: 1.045, 95% CI: 1.013–1.078, P = 0.005). As shown in Table 2.
The cutoff value of LVMI for predicting the SAE was 68 g/m2.7, with a sensitivity of 76.0%, specificity of 87.8% [area under the curve (AUC) = 0.833, 95% CI 0.727–0.940, P < 0.001], and the cutoff value of RWT for predicting the SAE was 0.41, with a sensitivity of 64.0%, specificity of 73.3% (AUC = 0.732, 95% CI 0.641–0.823, P < 0.001). Compared with PELD, LVMI showed improved AUCs in the prediction of SAE. (LVMI AUC: 0.833, 95% CI 0.727–0.940, P < 0.001 vs. PELD AUC 0.690, 95% CI 0.578–0.803, P = 0.002, respectively). Compared with LVMI, the model 1 constructed by LVMI, RWT, and PELD and the model 2 constructed by LVMI and PELD didn't significantly improve the prediction ability of SAE (Model 1 AUC: 0.899, 95% CI 0.836–0.962, P < 0.001 and Model 2 AUC: 0.853, 95% CI 0.760–0.946, P < 0.001). (As shown in Figure 2 and Table 3).
Our results showed that LVMI and RWT are predictive of the outcomes after LT in children with BA. These two parameters are an important part of the CCM diagnostic criteria. We used the appeal optimal cut-off value as the diagnostic threshold (LVMI > 68 g/m2.7, and/or RWT > 0.41) of subclinical cardiac abnormalities for the next study. The presence of subclinical cardiac abnormalities can predict the occurrence of SAE after LT, with a sensitivity of 100.0%, specificity of 63.9% [AUC = 0.819 (95% CI, 0.760–0.879), P < 0.001]. Similarly, the presence of subclinical cardiac abnormalities can also predict the occurrence of death after LT, with a sensitivity of 100.0%, specificity of 58.7% [AUC = 0.793 (95% CI, 0.704–0.883), P = 0.003]. As shown in Figure 3.
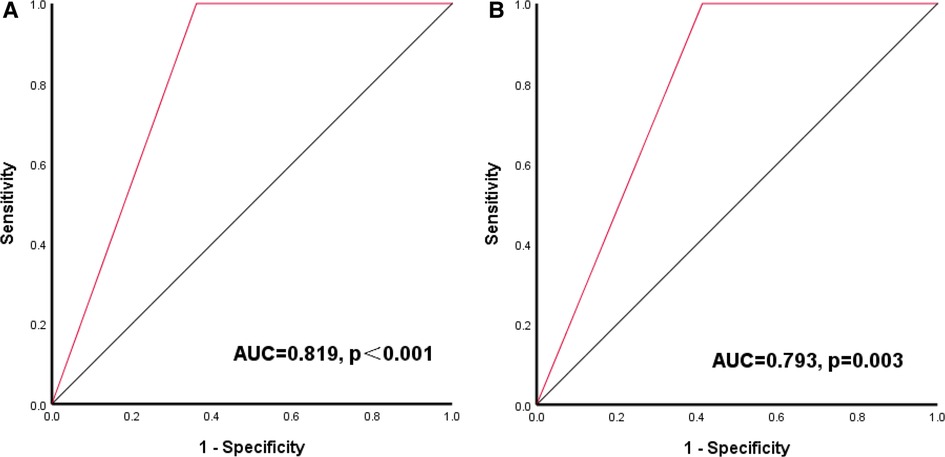
Figure 3. Receiver-operating characteristic (ROC) curve of LVMI combined with RWT for outcomes after LT (A) ROC curve of LVMI combined with RWT for SAE; (B) ROC curve of LVMI combined with RWT for death.
According to the optimal cut-off values of LVMI and RWT, 90 of 205 (43.9%) patients had subclinical cardiac abnormalities (SCA). Patients in the SCA group were younger at the time of LT than those in the No- SCA group (P = 0.027) and had a higher PELD score (P = 0.015). E/A ratio for the diastolic function was significantly lower in the SCA group than in the No-SCA group (P < 0.001).While EF and FS for systolic function did not differ significantly between the two groups (P > 0.05). There were no statistically significant differences in other baseline characteristics (P > 0.05). There were a total of 9 deaths, all of which occurred in the SCA group. The survival rate in SCA group was significantly lower than that of No SCA group (P < 0.001). Compared with the No-SCA group, patients in the SCA group were more likely to suffer SAE after LT (P < 0.001). 25 suffered SAE after LT, including 1 retransplant, 16 requiring organ support therapy, 7 hemorrhages, and 5 thromboses (hepatic artery or portal vein), all coming from the SCA group. Patients in the SCA group also had longer ICU and total hospital stays than those in the non-SCA group (P < 0.05). As shown in Table 4.
As depicted in Figure 4, we compared patient survival by the Kaplan–Meier method in the SCA and No SCA group. The SCA group had a lower patient survival rate than the No-SCA group (log-rank P = 0.001). The 1-year and 3-year patient survival of the SCA group vs. the No-SCA group were 90.5% vs. 100.0% and 89.7% vs. 100.0%, respectively. Notably, the survival difference between the No-CHD group and the CHD group mainly happened in the first 12 months after liver transplantation, and after that, the survival curves are parallel. Although the 3-year survival rate was lower in the SCA group vs. the No-SCA group, the cluster of deaths within the first 12 months is noteworthy, and further research is needed. We should concentrate on the 1-year survival rate of SCA group clinically.
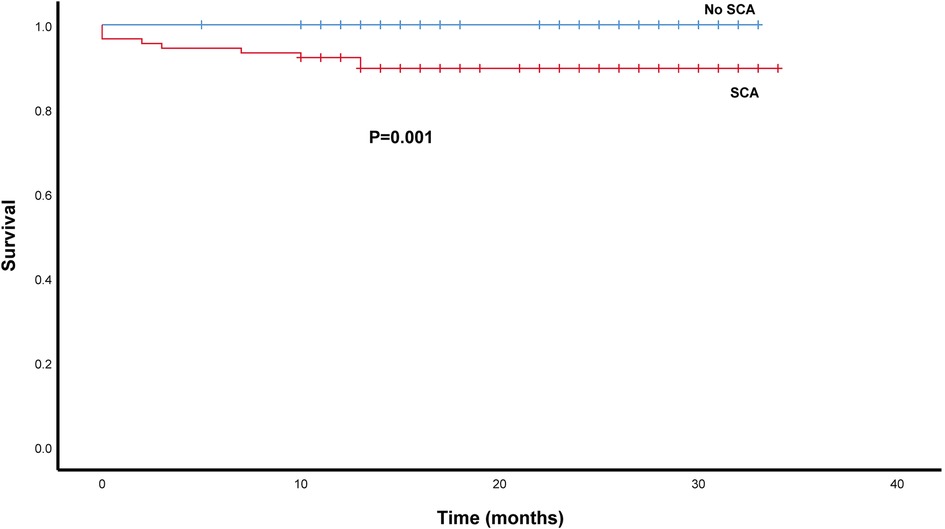
Figure 4. Survival analysis of SCA patients after LT using the kaplan–meier method. The patients survival rate was lower in the SCA group than in the non -SCA group (log-rank P = 0.001).
As far as we know, this is the first study to describe subclinical cardiac abnormalities associated with pediatric BA from China. We found that specific 2DE parameters including LVMI and RWT can predict the poor outcomes after liver transplantation. Subclinical cardiac abnormalities were correlated with post-LT mortality and morbidity in children with BA. Patients in the SCA group had a lower survival rate and a higher incidence of SAE events, and it was also related to prolonged ICU and hospital stays. Notably, the survival difference between the SCA and No-SCA groups occurred primarily in the first 12 months after transplantation, so we should focus on the 1-year survival rate of SCA group clinically.
The median age at transplantation was 7.8 months in our study. It is known that in the mouse heart the development of cardiac alterations including hypertrophy and hyperdynamic contractility appears even within a few weeks of biliary tract injuries (12). And if so, the inclusion of very young patients with BA, a disease of biliary cirrhosis that occurs soon after birth made the results even more influential. This made it possible to infer that even patients with short-term cirrhosis may already have myocardial involvement, even subclinical.
In patients with cholestatic BA, cardiac remodeling can occur in three ways. First, there is hemodynamic stress and a chronic inflammatory state in pediatric patients with BA, which contribute to pathological cardiac hypertrophy, cardiomyocytes respond to a number of pathological stimuli by releasing chemokines, inflammatory cytokines, and damage-associated molecular patterns, further increasing the inflammatory response. This immune response can subsequently activate pro-hypertrophic and profibrotic pathways, resulting in heart hypertrophy and remodeling (13, 14). Second, increased circulating bile acids have been linked to cardiac dysfunction in preclinical models of cirrhosis, possibly by activating the bile acid receptors farnesoid X receptor and TGR5 (15). Third, other factors that may contribute to the cardiovascular abnormalities found in infants with cholestasis include nitric oxide, endogenous opioids, and endocannabinoids. These factors may work together to produce vasodilation and impair cardiovascular responses to sympathetic stimulation (16). The effects of cirrhosis on cardiac functions and two-dimensional echocardiography parameters have mostly been published in adult studies (17, 18). Although the number of studies about the cardiac effects of cirrhosis has increased recently, especially in children, the number of studies conducted in children is still lacking.
We have to emphasize the importance of identifying LVMI among children with BA. In our study, we found that LVMI can predict the occurrence of death and serious adverse events after liver transplantation. LVMI has a stronger predictive effect than RWT and PELD. And compared with LVMI, the model constructed by LVMI, RWT, and PELD and the model constructed by LVMI and PELD have no obvious advantages, this has not been reported in previous studies. There is no doubt that changes in cardiac structure and function remodeling have been observed in patients with biliary atresia. In the 2011 report of Desai et al., children with BA had significant increases in multiple 2DE parameters, notably left ventricle mass index and left ventricle wall thickness, similar to our findings (8). As far as we know, in the pediatric field only a few studies correlated post-LT consequences to cardiac changes associated with cirrhosis (9, 10). While, the existing data is controversial. Gorgis et al. have shown that the specific 2DE parameters of abnormal cardiac findings are related to peri-transplant outcomes, while Junge et al. did not find this. Our results are consistent with Gorgis. Therefore, this study is of prime value because it is one of the few studies in the literature indicating that the left ventricular mass index correlates with peri-transplant outcomes in children with BA. Further verification is needed in more prospective, multi-center studies in the future.
We also found that the combination of LVMI and RWT is predictive of the outcomes after LT in children with BA. Elevated LVMI and RWT are used to identify left ventricular hypertrophy (LVH), which has been related to poor outcomes, including death, in both adults and pediatric patients with cirrhosis, and has also been related to morbidity in other diseases (8, 18–20). At present, there are no established thresholds for determining LVH in children. In our study, we used the optimal cut-off values (LVMI > 68 g/m2.7, and/or RWT > 0.41) to identify the subclinical cardiac abnormalities (SCA). This is similar to the method used by Gorgis et al. to derive the definition of CCM for children (9). In our cohort, patients with subclinical cardiac abnormalities were at risk of worse post-LT outcomes than those without. Notably, the survival difference between the SCA group and the No-SCA group mainly occurred in the first 12 months after transplantation. Some researchers suggest that abnormal 2DE parameters associated with CCM may cause some problems in the early stage after LT, but the long-term prognosis is better (21, 22). This may be explained by the regression of subclinical cardiac change after LT. Studies have shown that in both adults and children, this cardiac abnormality can recover about one year after LT (9, 23).
It has been reported that changes in diastolic function first appeared in ESLD patients with cardiac abnormalities, while systolic function may be normal (24). Our results were consistent with that. In our study, EF and FS for systolic function did not differ significantly between the SCA and No SCA groups. While, E/A ratio for the diastolic function was significantly lower in the SCA group than in the No SCA group, even though these values didn't exceed the normal range. Systolic function is usually reserved for a very late course of disease, and this dysfunction could become evident clinically if pharmacological or physical stress tests were conducted. Compared with adults, pediatric patients with BA may have had greater cardiac reserves (25).
Before pediatric liver transplantation or at the time of transplantation, there are few parameters with solid evidence to predict outcome. The PELD score, as a recognized parameter for determining organ allocation and priority, has been recognized for its effectiveness in predicting the risk of death on the waiting list before transplantation (26). However, the PELD score has a limited ability to predict the postoperative mortality of pediatric patients (27). With the increase in organ allocation based on the PELD exception score, the fairness of the PELD score has been questioned (28). In our study, we found that elevated LVMI and RWT can predict poor outcomes after liver transplantation. And a recent study suggests that abnormal 2DE features are present early in life in patients with BA and usually occur at the age when BA is diagnosed (29). We surmise that if we compared these 2DE parameters to the whole pool of patients on a liver transplant waiting list, this extrahepatic feature could optimize organ allocation and direct clinical care in the future. Overall, our findings hope to draw attention to the presence of subclinical cardiac abnormalities in children with BA in clinical work. These findings complement the data of pediatric LT and will provide tools for future improvement.
We were aware of the limitations of our study. First, this was a single-center retrospective study, and the results may not be representative of patient populations in other centers. Second, we did not conduct regular monitoring of cardiac ultrasound after liver transplantation, so we could not understand the evolution of cardiac abnormalities in this patient population. Further verification needs more prospective, multi-center studies in the future.
Subclinical cardiac abnormalities were correlated with post-LT mortality and morbidity in children with BA. LVMI can predict the occurrence of death and serious adverse events after liver transplantation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The study adhered to the Helsinki Declaration and was approved by the institutional Ethics Committee of Tianjin First Central Hospital. (2021N168KY). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Study design: CLu and TL; Data collection: XW, XH, and CLi; Data analysis: TTL and XYY; Manuscript writing: TL, ZL, and QL; Final approval of the manuscript: CLu and WG. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81970303), the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-054B) and the Natural Science Foundation of Tianjin (21JCYBJC00250).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
SCA, subclinical cardiac abnormalities; CCM, cirrhotic cardiomyopathy; BA, biliary atresia; LT, liver transplantation; ESLD, end-stage liver disease; SAE, serious adverse events; KPE, Kasai portoenterostomy; BMI, body mass index; LVMI, left ventricular mass index; RWT relative wall thickness; EF, left ventricular ejection fraction; FS, fraction shortening; PELD, pediatric end-stage liver disease score; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; ICU, intensive care unit; TB, total bilirubin levels; INR, international normalized ratio; PT, prothrombin time; CRP, C-reactive protein; 2DE, two-dimensional echocardiography.
1. Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. (2011) 58(3):223–31. doi: 10.1016/j.jacc.2011.03.026
2. He L, Chung PHY, Lui VCH, Tang CSM, Tam PKH. Current understanding in the clinical characteristics and molecular mechanisms in different subtypes of biliary Atresia. Int J Mol Sci. (2022) 23(9):4841. doi: 10.3390/ijms23094841
3. Cho HS, Kim WJ, Lee BA, Cho J, Shin WJ, Hwang GS. Pretransplant increases in left ventricular volume and mass are associated with QT prolongation during pediatric liver transplantation. Pediatr Transplant. (2018):e13237. doi: 10.1111/petr.13237. [Online ahead of print]29908011
4. Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. (2008) 57(2):268–78. doi: 10.1136/gut.2006.112177
5. Møller S, Lee SS. Cirrhotic cardiomyopathy. J Hepatol. (2018) 69(4):958–60. doi: 10.1016/j.jhep.2018.01.006
6. Rahman S, Mallett SV. Cirrhotic cardiomyopathy: implications for the perioperative management of liver transplant patients. World J Hepatol. (2015) 7(3):507–20. doi: 10.4254/wjh.v7.i3.507
7. Jang DM, Jun IG, Moon YJ, Shin WJ, Song JG, Hwang GS. Pretransplant left ventricular dysfunction adversely affects perioperative outcomes in pediatric liver transplantation: a retrospective observational study. Transplant Proc. (2016) 48(10):3328–35. doi: 10.1016/j.transproceed.2016.08.049
8. Desai MS, Zainuer S, Kennedy C, Kearney D, Goss J, Karpen SJ. Cardiac structural and functional alterations in infants and children with biliary atresia, listed for liver transplantation. Gastroenterology. (2011) 141(4):1264–72; 1272.e1261–1264. doi: 10.1053/j.gastro.2011.06.082
9. Gorgis NM, Kennedy C, Lam F, Thompson K, Coss-Bu J, Akcan Arikan A, et al. Clinical consequences of cardiomyopathy in children with biliary atresia requiring liver transplantation. Hepatology. (2019) 69(3):1206–18. doi: 10.1002/hep.30204
10. Junge N, Junge C, Schröder J, Pfister ED, Leiskau C, Hohmann D, et al. Pediatric cirrhotic cardiomyopathy: impact on liver transplant outcomes. Liver Transpl. (2018) 24(6):820–30. doi: 10.1002/lt.25076
11. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. (1986) 57(6):450–8. doi: 10.1016/0002-9149(86)90771-x
12. Desai MS, Shabier Z, Taylor M, Lam F, Thevananther S, Kosters A, et al. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. (2010) 51(6):2097–107. doi: 10.1002/hep.23585
13. Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, et al. The innate immune system in chronic cardiomyopathy: a European society of cardiology (ESC) scientific statement from the working group on myocardial function of the ESC. Eur J Heart Fail. (2018) 20(3):445–59. doi: 10.1002/ejhf.1138
14. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. (2018) 15(7):387–407. doi: 10.1038/s41569-018-0007-y
15. Voiosu A, Wiese S, Voiosu T, Bendtsen F, Møller S. Bile acids and cardiovascular function in cirrhosis. Liver Int. (2017) 37(10):1420–30. doi: 10.1111/liv.13394
16. Chahal D, Liu H, Shamatutu C, Sidhu H, Lee SS, Marquez V. Review article: comprehensive analysis of cirrhotic cardiomyopathy. Aliment Pharmacol Ther. (2021) 53(9):985–98. doi: 10.1111/apt.16305
17. Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int. (2017) 37(5):700–6. doi: 10.1111/liv.13287
18. Darstein F, König C, Hoppe-Lotichius M, Grimm D, Knapstein J, Mittler J, et al. Preoperative left ventricular hypertrophy is associated with reduced patient survival after liver transplantation. Clin Transplant. (2014) 28(2):236–42. doi: 10.1111/ctr.12304
19. Izzy M, Oh J, Watt KD. Cirrhotic cardiomyopathy after transplantation: neither the transient nor innocent bystander. Hepatology. (2018) 68(5):2008–15. doi: 10.1002/hep.30040
20. Akın A, Unal E, Yildirim R, Ture M, Balik H, Haspolat YK. Left and right ventricular functions may be impaired in children diagnosed with subclinical hypothyroidism. Sci Rep. (2020) 10(1):19711. doi: 10.1038/s41598-020-76327-4
21. Liu H, Lee SS. What happens to cirrhotic cardiomyopathy after liver transplantation? Hepatology. (2005) 42(5):1203–5. doi: 10.1002/hep.20911
22. Celtik C, Durmaz O, Oner N, Yavuz T, Gökce S, Aydogan A, et al. Investigation of cardiomyopathy in children with cirrhotic and noncirrhotic portal hypertension. J Pediatr Gastroenterol Nutr. (2015) 60(2):177–81. doi: 10.1097/mpg.0000000000000580
23. Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. (2005) 42(1):68–74. doi: 10.1016/j.jhep.2004.09.008
24. Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. (2001) 49(2):268–75. doi: 10.1136/gut.49.2.268
25. Brito MM, Miyatani HT, de Alencar Pereira PR, Tannuri ACA, Tannuri U. Evaluation of ventricular systolic function by speckle tracking technique in patients with biliary atresia before and after liver transplantation. Sci Rep. (2021) 11(1):17807. doi: 10.1038/s41598-021-97096-8
26. McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. (2002) 74(2):173–81. doi: 10.1097/00007890-200207270-00006
27. Barshes NR, Lee TC, Udell IW, O'Mahoney CA, Karpen SJ, Carter BA, et al. The pediatric end-stage liver disease (PELD) model as a predictor of survival benefit and posttransplant survival in pediatric liver transplant recipients. Liver Transpl. (2006) 12(3):475–80. doi: 10.1002/lt.20703
28. Thalji L, Thalji NM, Heimbach JK, Ibrahim SH, Kamath PS, Hanson A, et al. Renal function parameters and serum sodium enhance prediction of wait-list outcomes in pediatric liver transplantation. Hepatology. (2021) 73(3):1117–31. doi: 10.1002/hep.31397
Keywords: subclinical cardiac abnormalities, children, biliary atresia, liver transplantation, outcomes
Citation: Li T, Wei X, Hao X, Ye X, Li C, Li Q, Li Z, Gao W and Lu C (2023) Subclinical cardiac abnormalities in children with biliary atresia correlate with outcomes after liver transplantation. Front. Pediatr. 11:1174357. doi: 10.3389/fped.2023.1174357
Received: 26 February 2023; Accepted: 20 March 2023;
Published: 30 March 2023.
Edited by:
Antonino Morabito, University of Florence, ItalyReviewed by:
Maria Giovanna Grella, Santobono Hospital, Italy© 2023 Li, Wei, Hao, Ye, Li, Li, Li, Gao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Gao Z2Fvd2VpX3RqZmNoQDE2My5jb20= Chengzhi Lu NTAyMDIwMDA3MkBuYW5rYWkuZWR1LmNu
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.