
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 14 June 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1171223
This article is part of the Research Topic Neonatal Sepsis: Current Insights and Challenges View all 11 articles
 Alemayehu Mekonnen Gezmu1
Alemayehu Mekonnen Gezmu1 Endale Tefera1
Endale Tefera1 Kagiso Mochankana1
Kagiso Mochankana1 Fizzah Imran1
Fizzah Imran1 Dipesalema Joel1
Dipesalema Joel1 Irene Pelaelo2
Irene Pelaelo2 Britt Nakstad1,3*
Britt Nakstad1,3*
Introduction: Pulmonary hemorrhage (PH) is a life-threatening complication seen in very sick newborns with high morbidity and mortality. There is little data on the incidence, risk factors, and ultimate survival of newborns with pulmonary hemorrhage in sub-Saharan countries, where the healthcare provision and facility differ in many ways compared to high-income countries. Hence, this study aimed to determine the incidence, identify the risk factors, and describe the outcome of pulmonary hemorrhage in newborns in a low middle income country setting.
Methods and materials: A cohort study with prospective data collection was conducted in a public, tertiary-level hospital in Botswana, the Princess Marina Hospital (PMH). All newborns admitted to the neonatal unit from 1 January 2020 to 31 December 2021 were included in the study. Data were collected using a checklist developed on the RedCap database (https//:ehealth.ub.ac.bw/redcap). The incidence rate of pulmonary hemorrhage was calculated as the number of newborns who had pulmonary hemorrhage per 1,000 newborns in the 2-year period. Group comparisons were made using X2 and Student’s t-tests. Multivariate logistic regression was used to identify risk factors independently associated with pulmonary hemorrhage.
Result: There were 1,350 newborns enrolled during the study period, of which 729 were male newborns (54%). The mean (SD) birth weight was 2,154(±997.5) g, and the gestational age was 34.3 (±4.7) weeks. In addition, 80% of the newborns were delivered in the same facility. The incidence of pulmonary hemorrhage was 54/1,350 {4% [95% CI (3%–5.2%)]} among the newborns admitted to the unit. The mortality rate in those diagnosed with pulmonary hemorrhage was 29/54 (53.7%). Multivariate logistic regression identified birth weight, anemia, sepsis, shock, disseminated intravascular coagulopathy (DIC), apnea of prematurity, neonatal encephalopathy, intraventricular hemorrhage, mechanical ventilation, and blood transfusion as risk factors independently associated with pulmonary hemorrhage.
Conclusion: This cohort study identified a high incidence and mortality rate of pulmonary hemorrhage in newborns in PMH. Multiple risk factors, such as low birth weight, anemia, blood transfusion, apnea of prematurity, neonatal encephalopathy, intraventricular hemorrhage, sepsis, shock, DIC, and mechanical ventilation, were identified as independently associated risk factors for PH.
Pulmonary hemorrhage (PH) is diagnosed when a discharge of large amounts of bloody fluid from the endotracheal tube or respiratory tract is identified in very sick newborns who have shown acute clinical deterioration (1, 2). PH is one of the most acute and feared causes of mortality in newborns. The mortality from PH ranges from 50% to 68%, and the PH incidence rate is reported to be 1–2 per 1,000 live births occurring most commonly within the first few days of life (2, 3). PH results in an acute deterioration reflected by respiratory compromise, hemodynamic instability, and the need for ventilatory support (4).
Numerous studies have identified multiple risk factors for PH. A higher incidence of PH was observed in premature newborns with extreme prematurity and very low birth weight, as compared to newborns with higher birth weights and gestational ages (GA) (1). The literature identifies risk factors for PH as intrauterine growth restriction, respiratory distress syndrome (RDS), lung hypoplasia, sepsis, coagulopathy, thrombocytopenia, intraventricular hemorrhage (IVH), patent ductus arteriosus (PDA), ventilator treatment, and surfactant treatment (3, 5, 6).
Understanding the risk factors and identifying high-risk patients is critical for devising effective preventive and treatment measures as this would help to mitigate the high mortality and morbidity associated with PH in newborns. In a systematic review, ventilatory support, epinephrine administration, management of coagulopathy, and administration of tolazoline were all found to be effective primary treatments for PH (2).
This study aimed to determine the incidence of PH and identify risk factors associated with pulmonary hemorrhage in newborns admitted at the largest tertiary-level, public hospital in Botswana through a 2-year period.
This cohort study with prospective data collection was conducted in the Princess Marina Hospital (PMH) in Gaborone, Botswana, where predominantly those with low middle income and socioeconomic status are admitted, whereas those with higher socioeconomic status or health insurance, in our experience, deliver in private hospitals. The unit has 38 beds including six neonatal intensive care beds and admits approximately 100 newborns in a month. Most of the admitted newborns are born in the facility, some are referred from local clinics and primary- or secondary-level hospitals around the capital city and southern part of Botswana. The unit provides basic and advanced clinical care for newborns with all types of medical conditions, except for advanced surgery and extracorporeal membrane oxygenation (ECMO). Due to high mortality rates, especially among the smallest, the unit provides intensive care for babies with birth weight >900 g or GA >28 weeks. All newborns admitted to the neonatal unit were enrolled if the mother gave spoken and written consent to allow the collection of clinical data. The recruitment period was from 1 January 2020 to 31 December 2021. Data were collected using a checklist developed on the RedCap database (https//:ehealth.ub.ac.bw/redcap). The data were collected by a pediatrics registrar, consultant pediatrician, and neonatologist on a daily basis for 10 days and then weekly for each admitted newborn until the occurrence of end points: discharge, death, or transfer out from the unit.
The sample size was calculated based on one sample population proportion taking the incidence of pulmonary hemorrhage at 10% from studies done in South Africa (7). Assuming that 10% of the neonates admitted in the unit have pulmonary hemorrhage, and a finite population size of 1,400 neonates admitted in the unit per year, with a 95% level of confidence and with absolute precision of 10%, the study requires a sample size of 997. After looking into the number of newborns admitted to our study, we decided to include all 1,350 study participants in the analysis.
The collected data were exported to SPSS version 27 for Mac (IBM, Chicago, United States) for analysis. Descriptive statistics were presented as frequency and percentage for categorical variables and mean and standard deviation (SD) for continuous variables. The incidence rate was calculated as a proportion with absolute precision 95% confidence interval. Group comparisons were made using X2 and Student’s t-tests for categorical and continuous variables, respectively. A multivariate logistic regression model was used to identify risk factors independently associated with pulmonary hemorrhage. Statistical significance was set at a P-value of <0.05.
The following operational definitions were used.
Pulmonary hemorrhage in the newborn was defined when a discharge of large amounts of bloody fluid from the endotracheal tube or respiratory tract occurred in very sick newborns who showed acute clinical deterioration like blood aspirated from the trachea concurrent with respiratory decompensation that necessitated intubation or escalated support. When traces of blood appeared at suctioning secretions from the respiratory tract, this was not defined as PH.
Anemia was observed prior to PH in this study and according to the definitions in the unit’s protocol. Early (first 1–2 days) anemia was Hb (venous or arterial) < 13.5 g/dl. Later anemia, also before PH happened, depended on the day of life (DOL) and gestational age.
Transfusion of red blood cells was performed before PH occurred when Hb level is <11–12 g/dl and the need for respiratory support, including the need for supplemental oxygen.
Apnea was defined as the absence of breathing/cessation of respiratory airflow in a neonate for a period of >15–20 s often associated with bradycardia and/or desaturation.
Presumed sepsis was defined as the presence of non-specific clinical signs and symptoms consistent with sepsis and therefore starting treatment with antibiotics. Symptoms and signs were any five of the following: difficulty breathing, apnea, increased mean respiratory rate of more than two standard deviations (SDs) above normal for age or the need for mechanical ventilation for an acute process (not related to underlying neuromuscular disease or the need for general anesthesia), temperature lability (core temperature >38.5° or <36°C), low blood pressure and poor perfusion, abdominal distention, hyperglycemia, bloody stools, or convulsions. Abnormal whole blood cell counts and immature neutrophils were part of the evaluation for presumed sepsis.
Proven sepsis was defined when presumed sepsis and if the blood culture was positive.
Septic shock was defined if the condition required vasopressor treatment if presumed or proven sepsis.
Disseminated intravascular coagulation (DIC) was suspected and defined if simultaneous bleeding and clotting occurred during blood sampling.
PDA was confirmed by echocardiographic evaluation of each newborn when clinically suspected of having PDA, performed by a pediatric cardiologist.
Persistent pulmonary hypertension of the newborn (PPHN) in our setting is diagnosed by echocardiography. In PPHN, echocardiography demonstrates normal structural cardiac anatomy with evidence of pulmonary hypertension [i.e., elevated right ventricle pressure (RVp)]. RVp can be estimated based on Doppler measurement of the velocity of the tricuspid regurgitation (TR) jet, if present. If there is no TR, RVp can be assessed qualitatively (e.g., flattened or displaced ventricular septum).
Transient tachypnea is a mild, self-limited respiratory problem in neonates that begins after birth and lasts about 3 days, also named “wet lungs” or type II respiratory distress syndrome, often without carbon dioxide retention.
There were 1,350 newborns enrolled during the study period, of which 729 (54%) were male newborns. The mean (SD) birth weight was 2,154 (±997.5) g, and the gestational age was 34.3 (±4.7) weeks. In addition, 80% of the newborns were delivered in PMH. Other deliveries were home deliveries or born at clinics outside PMH (Table 1).
Table 1 also depicts clinically suspected sepsis [886 (65.6%)], RDS [570 (42%)], and neonatal jaundice [469 (34.7%)] that were the most common clinical diagnoses observed in the admitted newborns.
The incidence of pulmonary hemorrhage among the newborns admitted to our unit was 54/1,350 {4% [95% CI (3–5.2%)]}. The incidence of PH in very low birth weight (VLBW) newborns was 2.96%. The mortality rate among all newborns diagnosed with pulmonary hemorrhage was 29/54 (53.7%). Variables, such as gestational age, birth weight, RDS, bronchopulmonary dysplasia (BPD), sepsis, Apgar score at 5 min <5, and mechanical ventilation, were included in univariate analysis. On univariate analysis, all entered variables were statistically significant at P-value <0.05 (Table 2). Variables with P-value <0.1 were included for multivariate logistic regression analysis. Of these variables, birth weight [aOR (95% CI) 0.85 (0.77–0.94), P = 0.002], anemia [aOR (95% CI) 3.35 (1.4–7.66) P = 0.004], sepsis [aOR (95% CI) 2.62 (1.10–6.24), P = 0.029], and shock [aOR (95% CI) 5.78 (1.82–18.38), P = 0.003] were independently associated with risk of pulmonary hemorrhage (Table 2).
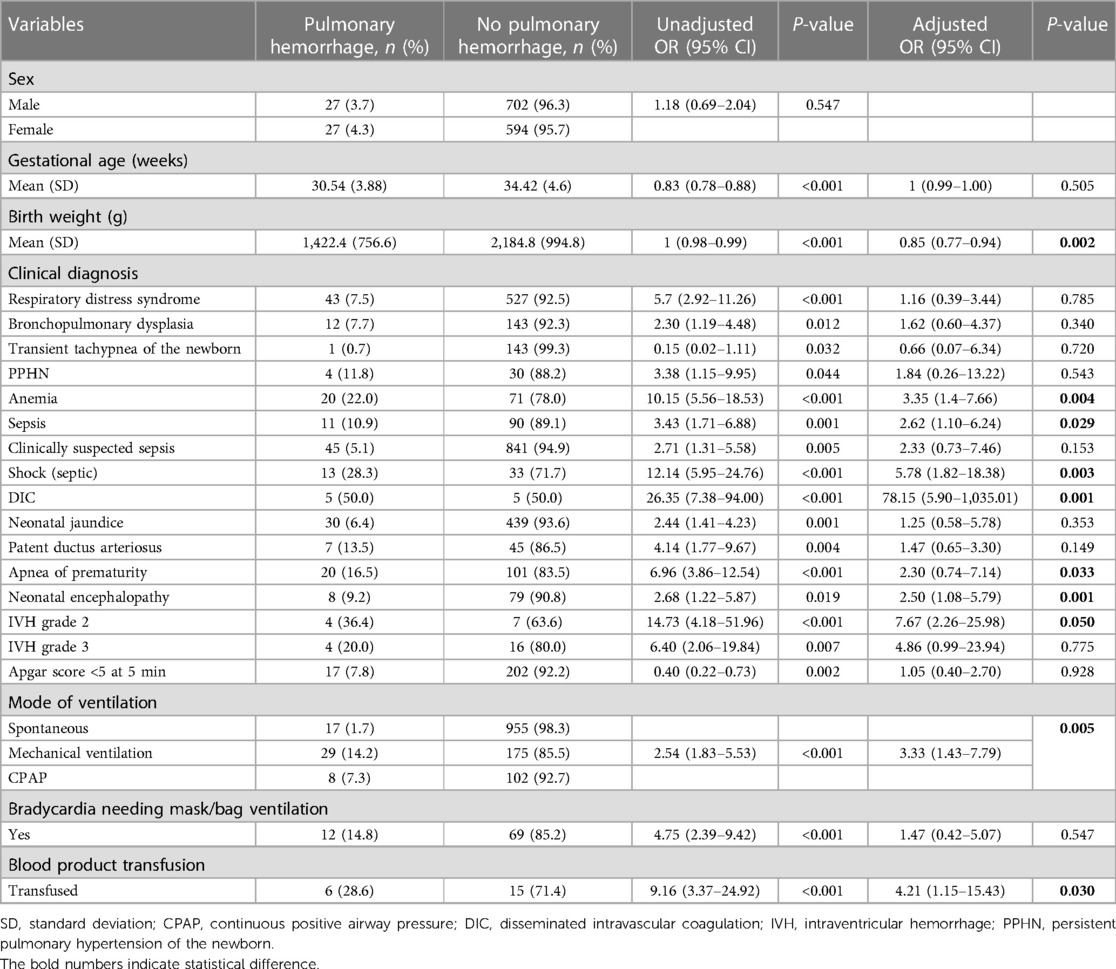
Table 2. Factors associated with pulmonary hemorrhage in newborns admitted to the neonatal care unit.
This study found a 4% high incidence rate of PH in admitted newborns at the largest tertiary hospital in Botswana and about 54% mortality rate in those diagnosed with PH. This incidence and mortality rate was similar to other studies (1, 2, 8). However, there is substantial variation in reported PH incidence and associated mortality rates between high-income countries (HICs) and low middle income countries (LMICs). In a systematic review from HIC in 2012, the incidence of PH was 1–12 per 1,000 live births (9), whereas a study from China reported PH in premature newborns of BW <1,500 g at 6.1% and BW <1,000 g at 22.9%, respectively (1). Also from China, a higher neonatal mortality rate was reported in those with PH and a birth weight less than 1,500 g, 82.1% (96/117), and 67.7% (86/127) in those with a birth weight above 1,500 g (10). In a large retrospective cohort study from multiple neonatal intensive care units in the USA, mortality rates among patients with PH were 40.6% and 54.0% at 7 and 30 days of age, respectively (11).
PH can start gradually and continue for a long time, or it can be a sudden life-threatening respiratory complication associated with several risk factors (12). We identified multiple risk factors to be independently associated with pulmonary hemorrhage. In our cohort low birth weight, anemia, blood transfusion, apnea of prematurity, neonatal encephalopathy, intraventricular hemorrhage, sepsis, shock, DIC, and mechanical ventilation were all independent risk factors for PH. According to the literature (13), bleeding into the lungs occurs mainly in premature newborns with severe lung disease. Risk factors have been proposed to be respiratory problems (14) and hemodynamically significantly increased blood flow (persistent ductus arteriosus, PDA) in the blood vessels in the lungs (15), like when sepsis impacts on hemodynamics and reopens or enlarges the patent ductus arteriosus. In newborns, infection (8), in accordance with our findings of sepsis and shock being highly associated with PH, as well as the association of PH with apnea of prematurity (16), is in line with the results of our study. Our preterm babies that developed PH were critically ill with clinical conditions like sepsis or neonatal encephalopathy. A retrospective study that reported on perinatal risk factors from China identified a rate of PH in extremely low birth weight infants (ELBW, <1,000 g) to be 18.8%. These preterm babies that experienced PH were critically ill with clinical conditions like sepsis or neonatal encephalopathy. It has been shown that small for gestational age, early onset neonatal sepsis, low birth weight, lower Apgar scores at 1 and 5 min, severe RDS, and surfactant replacement were all risk factors for PH (12). Another study from a neonatal intensive care unit in Brazil reported prior use of blood components to be an independent risk factor for PH (17). Transfusion-related PH and acute illness may be more common than previously thought (18, 19). The evidence from preclinical studies showed that blood products can directly modulate transfusion-related immunomodulation, a mechanism linking transfusion exposure with neonatal morbidities (20). Additionally, the use of blood products prior to the PH episode may cause a sudden increase in blood volume, leading to stress injury of the capillary wall, with the passage of fluid and plasma proteins, which can also lead to left ventricular failure, contributing to an increase in pulmonary capillary blood pressure (17). Preterm newborns represent one of the most frequently transfused patients (21). In accordance with our findings, a large retrospective study in preterm neonates found that those who received any RBC transfusion had a 50% increase in neonatal mortality (20).
In our cohort of 1,350 newborns, we found that recurrent apnea was highly correlated to PH. Apnea is a common condition in premature infants due to the immaturity of respiratory control mechanisms. Recurrent apnea is associated with respiratory failure, pulmonary hemorrhage, abnormal heart and lung function, intracranial hemorrhage, abnormal nervous system development, and even sudden death (22). Studies in infants indicated that negative pressure pulmonary edema because of upper airway obstruction led to pulmonary hemorrhage thought to be a stress failure caused by high negative intrathoracic pressure and mechanical disruption of the alveolar-capillary membrane leading to pulmonary bleeding (23). There could be a similar mechanism to apnea causing PH due to laryngospasm or high negative pleural pressure due to inspiratory effort during upper airway obstruction (24, 25).
In newborns, pulmonary hemorrhage is often a manifestation of pulmonary edema (26), and PDA (5) can range in severity from blood-tinged secretions in the endotracheal tube to life-threatening blood loss with hypovolemic shock. The cause of PH is thought to be due to the rapid lowering of intrapulmonary pressure, which facilitates left-to-right shunting across a PDA and an increase in pulmonary blood flow (27). In our study, we identified PDA as a risk factor for PH with statistical significance on univariate analysis, but on multivariate analysis, we could not show statistical significance.
This study has several limitations. First, this study only included study participants from a single neonatal center. Second, important variables such as the use of surfactant were not captured to give light to the impact of surfactant treatment on the incidence and outcome of PH. Finally, because of the policy of the unit, only ELBW infants of 900–1,000 g were provided intensive care and respiratory support whereas smaller ELBWs (<900 g) were provided nasal oxygen treatment, enteral and parenteral nutrition, and antibiotic treatment. We could not analyze and discuss the outcome of those ELBWs (BW < 900 g) and extremely premature babies based on the treatment provided.
This cohort study identified a high incidence and mortality rate in neonates with pulmonary hemorrhage. Multiple risk factors, such as low birth weight, anemia, blood transfusion, apnea of prematurity, neonatal encephalopathy, intraventricular hemorrhage, sepsis, shock, DIC, and mechanical ventilation, were identified as independently associated risk factors for PH.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by institutional review boards at the University of Botswana, Botswana Ministry of Health, and Princess Marina Hospital, all addressed in Gaborone, Botswana. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors would like to acknowledge parents and/or caretakers of newborns for their consent to participate in this study. Furthermore, we would like to extend our gratitude to nurses and doctors and administrators in the neonatal unit of the Princess Marina Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li J, Xia H, Ye L, Li X, Zhang Z. Exploring prediction model and survival strategies for pulmonary hemorrhage in premature infants: a single-center, retrospective study. Transl Pediatr. (2021) 10(5):1324. doi: 10.21037/tp-21-64
2. Barnes ME, Feeney E, Duncan A, Jassim S, MacNamara H, O’Hara J, et al. Pulmonary haemorrhage in neonates: systematic review of management. Acta Paediatr. (2022) 111(2):236–44. doi: 10.1111/apa.16127
3. Usemann J, Garten L, Bührer C, Dame C, Cremer M. Fresh frozen plasma transfusion–a risk factor for pulmonary hemorrhage in extremely low birth weight infants? J Perinat Med. (2017) 45(5):627–33. doi: 10.1515/jpm-2016-0309
4. Bozdağ Ş, Dilli D, Gökmen T, Dilmen U. Comparison of two natural surfactants for pulmonary hemorrhage in very low-birth-weight infants: a randomized controlled trial. Am J Perinatol. (2015) 32(03):211–8. doi: 10.1055/s-0034-1389090
5. Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. (2000) 137(1):68–72. doi: 10.1067/mpd.2000.106569
6. Yen T-A, Wang C-C, Hsieh W-S, Chou H-C, Chen C-Y, Tsao P-N. Short-term outcome of pulmonary hemorrhage in very-low-birth-weight preterm infants. Pediatr Neonatol. (2013) 54(5):330–4. doi: 10.1016/j.pedneo.2013.04.005
7. Sankar MJ, Gupta N, Jain K, Agarwal R, Paul VK. Efficacy and safety of surfactant replacement therapy for preterm neonates with respiratory distress syndrome in low- and middle-income countries: a systematic review. J Perinatol. (2016) 36(1):S36–48. doi: 10.1038/jp.2016.31
8. Lin T-W, Su B-H, Lin H-C, Hu P-S, Peng C-T, Tsai C-H, et al. Risk factors of pulmonary hemorrhage in very-low-birth-weight infants: a two-year retrospective study. Acta Paediatr Taiwanica Taiwan er ke yi xue hui za zhi. (2000) 41(5):255–8.
9. Zahr RA, Ashfaq A, Marron-Corwin M. Neonatal pulmonary hemorrhage. Neoreviews. (2012) 13(5):e302–6. doi: 10.1542/neo.13-5-e302
10. Li L, Yu J, Wang J, Zhang X, Shen H, Yuan X, et al. A prediction score model for risk factors of mortality in neonate with pulmonary hemorrhage: the experience of single neonatal intensive care unit in southwest China. Pediatr Pulmonol. (2008) 43(10):997–1003. doi: 10.1002/ppul.20897
11. Ahmad KA, Bennett MM, Ahmad SF, Clark RH, Tolia VN. Morbidity and mortality with early pulmonary haemorrhage in preterm neonates. Arch Dis Childhood Fetal Neonatal Ed. (2019) 104(1):F63–8. doi: 10.1136/archdischild-2017-314172
12. Wang T-T, Zhou M, Hu X-F, Liu J-Q. Perinatal risk factors for pulmonary hemorrhage in extremely low-birth-weight infants. World J Pediatr. (2020) 16(3):299–304. doi: 10.1007/s12519-019-00322-7
13. Tomaszewska M, Stork E, Minich NM, Friedman H, Berlin S, Hack M. Pulmonary hemorrhage: clinical course and outcomes among very low-birth-weight infants. Arch Pediatr Adolesc Med. (1999) 153(7):715–21. doi: 10.1001/archpedi.153.7.715
14. Wang L, Zhao L, Xu J, Yu Y, Li Z, Zhang F, et al. Association between pulmonary hemorrhage and CPAP failure in very preterm infants. Front Pediatr. (2022) 10:938431. doi: 10.3389/fped.2022.938431
15. Kappico JM, Cayabyab R, Ebrahimi M, Uzunyan MY, Barton L, Siassi B, et al. Pulmonary hemorrhage in extremely low birth weight infants: significance of the size of left to right shunting through a valve incompetent patent foramen ovale. J Perinatol. (2022) 42(9):1233–7. doi: 10.1038/s41372-022-01464-9
16. Ayebare E, Hanson C, Nankunda J, Hjelmstedt A, Nantanda R, Jonas W, et al. Factors associated with birth asphyxia among term singleton births at two referral hospitals in northern Uganda: a cross sectional study. BMC Pregnancy Childbirth. (2022) 22(1):1–12. doi: 10.1186/s12884-022-05095-y
17. Ferreira CH, Carmona F, Martinez FE. Prevalence, risk factors and outcomes associated with pulmonary hemorrhage in newborns. J Pediatr (Rio J). (2014) 90:316–22. doi: 10.1016/j.jped.2013.12.008
18. Sanchez R, Toy P. Transfusion related acute lung injury: a pediatric perspective. Pediatr Blood Cancer. (2005) 45(3):248–55. doi: 10.1002/pbc.20395
19. Valentine SL, Cholette JM, Goobie SM. Transfusion strategies for hemostatic blood products in critically ill children: a narrative review and update on expert consensus guidelines. Anesth Analg. (2022) 135(3):545–57. doi: 10.1213/ANE.0000000000006149
20. dos Santos AMN, Guinsburg R, de Almeida MFB, Procianoy RS, Leone CR, Marba STM, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J Pediatr. (2011) 159(3):371–6. doi: 10.1016/j.jpeds.2011.02.040
21. Strauss RG. Red blood cell transfusion practices in the neonate. Clin Perinatol. (1995) 22(3):641–55. doi: 10.1016/S0095-5108(18)30273-2
22. Chen J, Jin L, Chen X. Efficacy and safety of different maintenance doses of caffeine citrate for treatment of apnea in premature infants: a systematic review and meta-analysis. Biomed Res Int. (2018) 2018:9061234. doi: 10.1155/2018/9061234
23. Schwartz DR, Maroo A, Malhotra A, Kesselman H. Negative pressure pulmonary hemorrhage. Chest. (1999) 115(4):1194–7. doi: 10.1378/chest.115.4.1194
24. Dolinski SY, MacGregor DA, Scuderi PE. Pulmonary hemorrhage associated with negative-pressure pulmonary edema. J Am Soc Anesthesiol. (2000) 93(3):888–90. doi: 10.1097/00000542-200009000-00042
25. Bersten AD, Patel AR. Pulmonary haemorrhage associated with negative-pressure pulmonary oedema: a case report. Crit Care Resusc. (2006) 8(2):115–6. doi: 10.3316/informit.517611976385693
26. Cole VA, Normand ICS, Reynolds EOR, Rivers RPA. Pathogenesis of hemorrhagic pulmonary edema and massive pulmonary hemorrhage in the newborn. Pediatrics. (1973) 51(2):175–87. doi: 10.1542/peds.51.2.175
Keywords: pulmonary hemorrhage, premature newborns, risk factors, very low birth weight, extreme prematurity
Citation: Gezmu AM, Tefera E, Mochankana K, Imran F, Joel D, Pelaelo I and Nakstad B (2023) Pulmonary hemorrhage and associated risk factors among newborns admitted to a tertiary level neonatal unit in Botswana. Front. Pediatr. 11:1171223. doi: 10.3389/fped.2023.1171223
Received: 21 February 2023; Accepted: 30 May 2023;
Published: 14 June 2023.
Edited by:
Rozeta Sokou, Nikaia General Hospital “Aghios Panteleimon”, Piraeus, GreeceReviewed by:
Aikaterini Konstantinidi, Nikaia General Hospital “Aghios Panteleimon”, Piraeus, Greece© 2023 Gezmu, Tefera, Mochankana, Imran, Joel, Pelaelo and Nakstad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Britt Nakstad YnJpdHQubmFrc3RhZEBtZWRpc2luLnVpby5ubw==; bmFrc3RhZGJAdWIuYWMuYnc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.