- 1Lifecourse Epidemiology of Adiposity and Diabetes (LEAD) Center, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 2Department of Pediatrics, Department of Environmental Medicine, New York University Grossman School of Medicine, New York, NY, United States
- 3Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 4Department of Medical Social Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
- 5Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
- 6Avera Research Institute, Department of Pediatrics, University of South Dakota School of Medicine, Sioux Falls, SD, United States
- 7Division of Research, Kaiser Permanente Northern California, Oakland, CA, United States
- 8Department of Epidemiology, Geisel School of Medicine at Dartmouth, Hanover, NH, United States
- 9Departments of Pediatrics, Albert Einstein College of Medicine, Bronx, NY, United States
- 10Department of Pediatrics, Hackensack Meridian School of Medicine, Nutley, NJ, United States
- 11Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, United States
- 12Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 13Department of Food Science and Human Nutrition, Michigan State University, East Lansing, MI, United States
- 14Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, GA, United States
- 15Department of Psychological and Behavioral Sciences, The George Washington University, Washington, DC, United States
- 16Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
- 17Departments of Pediatrics & Occupational and Environmental Health Sciences, University of Washington, Seattle, WA, United States
- 18Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
- 19AJ Drexel Autism Institute, Drexel University, Philadelphia, PA, United States
- 20Department of Pediatrics, University of North Carolina School of Medicine, Chapel Hill, NC, United States
- 21Department of Psychiatry and Behavioral Sciences, University of California Davis School of Medicine, Sacramento, CA, United States
- 22Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, San Francisco, CA, United States
Objective: Ongoing pediatric cohort studies offer opportunities to investigate the impact of the COVID-19 pandemic on children's health. With well-characterized data from tens of thousands of US children, the Environmental influences on Child Health Outcomes (ECHO) Program offers such an opportunity.
Methods: ECHO enrolled children and their caregivers from community- and clinic-based pediatric cohort studies. Extant data from each of the cohorts were pooled and harmonized. In 2019, cohorts began collecting data under a common protocol, and data collection is ongoing with a focus on early life environmental exposures and five child health domains: birth outcomes, neurodevelopment, obesity, respiratory, and positive health. In April of 2020, ECHO began collecting a questionnaire designed to assess COVID-19 infection and the pandemic's impact on families. We describe and summarize the characteristics of children who participated in the ECHO Program during the COVID-19 pandemic and novel opportunities for scientific advancement.
Results: This sample (n = 13,725) was diverse by child age (31% early childhood, 41% middle childhood, and 16% adolescence up to age 21), sex (49% female), race (64% White, 15% Black, 3% Asian, 2% American Indian or Alaska Native, <1% Native Hawaiian or Pacific Islander, 10% Multiple race and 2% Other race), Hispanic ethnicity (22% Hispanic), and were similarly distributed across the four United States Census regions and Puerto Rico.
Conclusion: ECHO data collected during the pandemic can be used to conduct solution-oriented research to inform the development of programs and policies to support child health during the pandemic and in the post-pandemic era.
Introduction
Research studies are needed to understand whether and how the social and economic disruption of the COVID-19 pandemic and/or SARS-CoV-2 infection affected the health of children in the United States (US) (1). Environmental exposures, health behaviors, and health status during the pandemic may have implications for children's future health, especially if environmental exposures or health behaviors altered by the pandemic persist in the post-pandemic era (e.g., parent unemployment, shuttered community resources, new modes of physical activity) (2–4). Moreover, pandemic-related exposures (e.g., food insecurity, parent illness) occurring during developmentally sensitive periods (5) may have implications for health across the life course, even if the exposures are time-limited (6).
As of September 2022, there were over 300,000 COVID-19-related publications in PubMed, yet only a small proportion (around 10% or less) are focused on children's health. There is evidence of both adverse and favorable changes in children's environments, behaviors, and health status during the pandemic (7, 8). However, there are concerns about the quality of the evidence and representativeness of study samples. Most studies that included primary data collection in children during the pandemic were conducted outside the US (9–14). Early studies conducted among US children provided some insights into whether the social and economic disruption of the pandemic had implications for children's health status and behaviors, with a focus on the initial lockdown stage (15, 16). However, most studies were conducted among small and homogenous samples, and had methodological limitations, such as the use of unvalidated questionnaires to assess health outcomes or cross-sectional study designs with a risk of recall bias (17, 18). While more recent work has capitalized on national samples in the US, such as the Adolescent Brain and Cognitive Development (ABCD) cohort (19–23), there is still a relative dearth of high-quality studies that include children at all stages of development.
The data sources needed to conduct these types of high-quality analyses on pediatric health and the COVID-19 pandemic are limited because public health precautions, such as physical distancing requirements, or hesitancy to attend in-person research visits among potential study participants, reduced the feasibility of conducting primary data collection during the pandemic. For example, field operations were halted during the pandemic for large national surveys that had planned to assess variables relevant for child health in 2020 and 2021. This included the National Health and Nutrition Examination Survey (NHANES) and the American Community Survey, conducted by the Centers for Disease Control and Prevention and the US Census Bureau, respectively (24, 25). This is concerning because the cessation of national studies that include diverse samples of children in regard to race, ethnicity, and socioeconomic status potentially created a gap in understanding of inequities in the COVID-19 experience (26, 27).
In September 2016, the National Institutes of Health (NIH) launched a 7-year initiative, the Environmental influences on Child Health Outcomes (ECHO) Program (28). ECHO is a national consortium of 69 new and established pregnancy and pediatric cohort studies. The consortium was designed to be a large, population-based cohort of U.S. children unified under a single research protocol. Primary data collection in the ECHO Program, using this standardized protocol among US children and their caregivers, not only continued during the pandemic in-person, as allowed, but also pivoted to remote data collection methods (eg, remote informed consent procedures and collection of biospecimens such as blood spots, urine, and hair samples; online surveys for self-administration; phone-based questionnaires; and administrator-assisted anthropometric assessments via video conferencing). The ECHO Program also expanded to include time-sensitive assessments of COVID-19 infection and pandemic-related psychosocial impacts, thus providing a valuable data source for understanding whether and how the social and economic disruption of the COVID-19 pandemic and/or SARS-CoV-2 infection affected the health of US children. As part of this effort, the ECHO Program conducted rapid response research via COVID-19 Administrative Supplements funded by the NIH, with a focus on using existing and novel tools. Additionally, the ECHO Program fostered innovation in measurement by developing and administering novel, publicly available COVID-19-specific questionnaires for caregivers and children (29).
The objective of this research brief is to provide an overview of what the ECHO Program is uniquely poised to contribute to our understanding of the COVID-19 pandemic and child health. Specifically, we propose to (1) inform the scientific community about the characteristics of parent-child dyads who participated in data collection as part of the ECHO Program during the COVID-19 pandemic, and (2) describe the types of innovative research questions that can be answered with those data, which will be de-identified and publicly available in 2023.
Methods
The ECHO Program aims to examine the effects of physical, chemical, social, behavioral, biological, natural, and built environmental exposures on five key child health outcomes: pre-, peri-, and postnatal; upper and lower airway; obesity; neurodevelopment; and positive health (http://echochildren.org) (30). The ECHO Cohort is described in detail here (31). Briefly, ECHO enrolls children and their caregivers from community- and clinic-based studies. New data were collected under a standardized data collection protocol, the ECHO-Wide Cohort Protocol (EWCP), beginning in 2019 and continuing through 2023. Extant data from each of the pediatric cohorts collected prior to the inception of the EWCP were pooled and harmonized at the centralized ECHO Data Analysis Center at Johns Hopkins University and RTI International. Institutional review boards monitored human subject activities at each cohort site and the Data Analysis Center. Caregivers provided informed consent, and children provided assent or consent, as appropriate. Many cohorts offered an “e-consent” option during the pandemic, in which the informed consent process was conducted via video conference. Additionally, RedCap is a secure, HIPAA- and FISMA-compliant web platform for building and managing online surveys and databases. The ECHO Program encourages the use of RedCap Central, a centralized version of RedCap. The purpose of RedCap Central is to facilitate immediate and automatic transfer of data from the ECHO Cohorts to the centralized ECHO Data Analysis Center to be used in pooled analyses and ECHO-wide scientific publications. De-identified data collected with the EWCP during the COVID-19 pandemic, along with selected extant data collected pre-pandemic, will be available to the public in 2023 via the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH) (https://dash.nichd.nih.gov/) (32). In addition to the data themselves, the publicly available DASH website includes downloadable versions of the full ECHO data collection protocol, data collection forms, data dictionaries and a detailed description of study methodologies. DASH is designed to enhance the accessibility, and expand the reach, of ECHO data to provide opportunities for researchers worldwide to answer important questions about child health.
In addition to ongoing data collection with the EWCP, a novel ECHO COVID-19 questionnaire was developed in April 2020 in English and Spanish for the ECHO Program (29). The questionnaire included three versions: caregiver self-report, adolescent self-report and caregiver-report on child for children 12 years and younger. The original questionnaire was designed to assess SARS-CoV-2 infection, access to health-related services, impact on employment, changes in health behaviors, and the psychosocial impact of the pandemic on caregivers and children (e.g., pandemic-related parent and child stress, coping mechanisms, social connectedness). The ECHO Program leveraged existing infrastructure to quickly mobilize the collection of this new questionnaire, which was subsequently modified in later phases of the pandemic to include additional factors as the pandemic evolved, such as vaccine administration, vaccine hesitancy, and remote schooling.
To summarize the data available, we report characteristics of children from birth to 21 years who participated in the EWCP during the first 17 months of the pandemic. The number of children who completed the protocol and the ECHO COVID-19 questionnaire is reported for the full 17 months (4/1/2020–8/31/2021) and by time period: Period 1 (4/1/2020–5/31/2020), Period 2 (6/1/2020–8/31/2020), Period 3 (9/1/2020–5/31/2021, “2020/21 academic school year”) and Period 4 (6/1/2021–8/31/2021), by age group, child sex, race, ethnicity, level of maternal education and region of residence. The World Health Organization declared COVID-19 a pandemic on March 11, 2020, and the ECHO Program amended its protocol to incorporate the COVID-19 questionnaires in April 2020. April 1 was used as the start date for data described in this paper because the ECHO questionnaires queried behaviors in the preceding week to month. The time periods were selected to generally align with the school year and summer vacation, although there is some variation in the start and end dates of the academic year by school district.
Results
Characteristics of children from birth to age 21 years who participated in the ECHO-Wide Cohort Protocol during the first 17 months of the pandemic and completed a COVID-19 questionnaire are shown in Table 1. Sixty cohorts contributed data from 13,725 children between April 1, 2020 and August 31, 2021. Participants were included from multiple life stages: early childhood (31%), middle childhood (41%), and adolescence up to age 21 (16%). The lower proportion of participants from the adolescent life stage compared to younger life stages is consistent with overall ECHO Cohort, and likely does not represent a selection bias. The pediatric sample was 49% female, 22% Hispanic, and children were similarly distributed across the four United States Census regions and Puerto Rico. Child race was 64% White, 15% Black, 3% Asian, 2% American Indian or Alaska Native, <1% Native Hawaiian or Pacific Islander, 10% Multiple race and 2% Other race. Fifty-seven percent of caregivers had a bachelor's degree or higher. Most data were collected during the 2020–2021 academic school year (n = 10,058), followed by Summer 2020 (n = 3,548), Summer 2021 (n = 2,180), and the first two months of the pandemic (n = 708). The lower number of cohorts administering COVID-19 questionnaires in the first two months of the pandemic (n = 21 cohorts) compared to the other time Periods (n ≥ 50 cohorts) reflects the staggered timing across the US when clinical research units were permitted to resume data collection. Nineteen percent of participants completed more than one COVID-19 questionnaire during the 17-month period. As shown in Figure 1, COVID-19 questionnaires were administered during different phases of the pandemic (e.g., the initial lockdown period, as well as periods of less restrictive public health precautions), all seasons of the year, during both the academic school year and school vacations, and before and after COVID-19 vaccines became available to adults and youth.
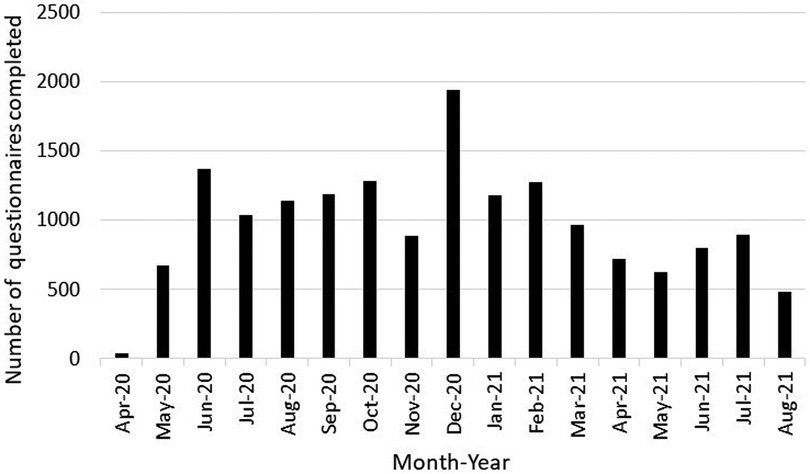
Figure 1. Number of COVID-19 questionnaires administered to children birth to 21 years and their caregivers who participated in the ECHO-wide common protocol during the first 17 months of the COVID-19 pandemic by month of administration.
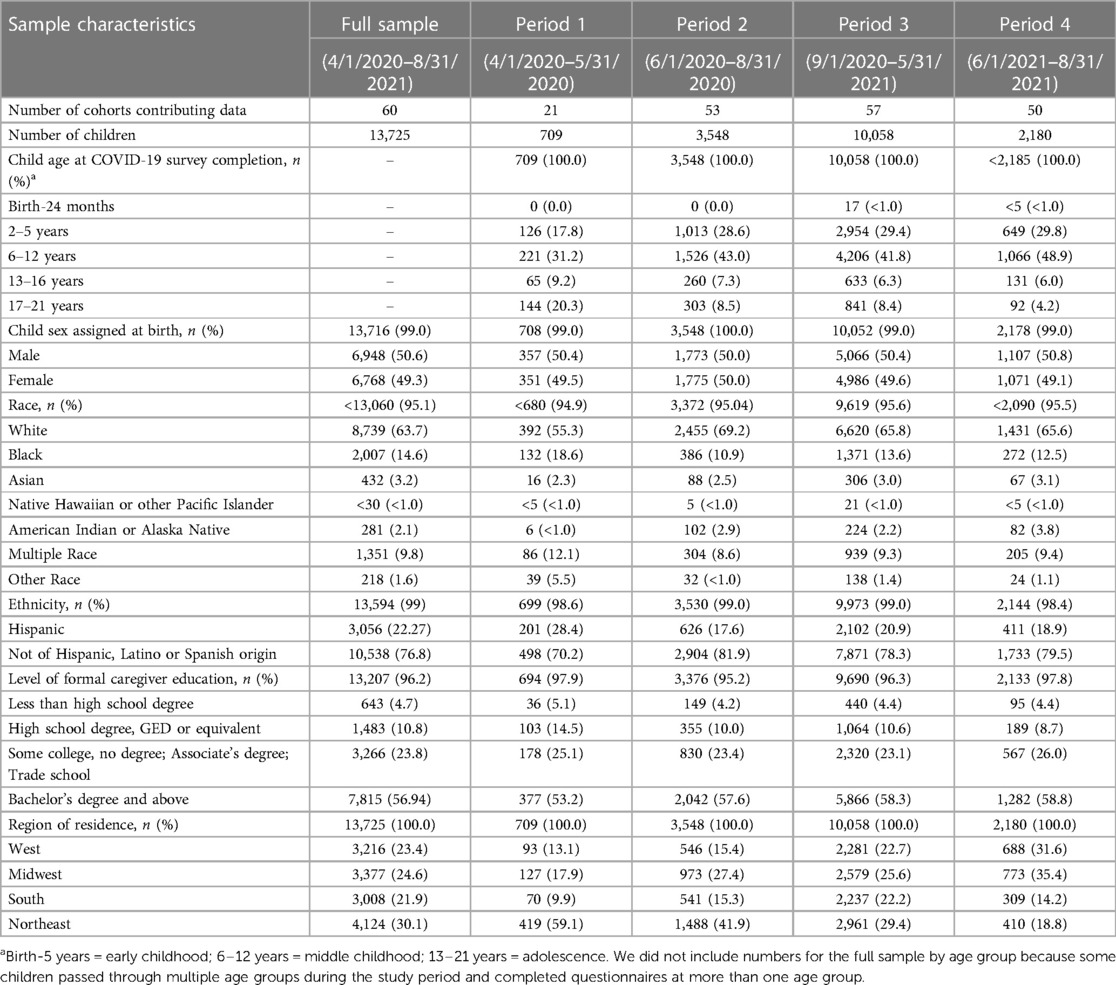
Table 1. Characteristics of children birth to 21 years who participated in the ECHO-wide cohort protocol during the first 17 months of the COVID-19 pandemic and completed an ECHO COVID-19 questionnaire via self-report or caregiver-proxy report.
Discussion
During a period of unprecedented public health disruptions and precautions in the US, the ECHO Program examined COVID-19-related environmental conditions and health outcomes among 13,725 socioeconomically and racially diverse children and their caregivers. This included a standardized data collection protocol that was initially implemented pre-pandemic, as well as an expanded protocol to include novel approaches to assessing pandemic-related exposures and outcomes. Strengths of these data include the large sample size and diversity by child age, sex, race, Hispanic ethnicity, and US region of residence. The high proportion of caregivers with a Bachelor's degree (57%) suggests that this sample may not be representative of the general population (38% in general US) (33), but this limitation is attenuated by the large number of caregiver-child dyads who participated (n = 13,723) and heterogeneity (e.g., over 5,000 caregivers who participated in ECHO during the pandemic did not have a Bachelor's degree) facilitating robust analyses of socioeconomic issues.
Future publications can leverage these richly characterized data from the ECHO Program to: (1) Examine the short- and long-term impacts of SARS-CoV-2 infection among pregnant mothers and children on children's birth outcomes, airways, obesity, neurodevelopment, and positive health using harmonized data collected before and during the pandemic; (2) Evaluate societal changes during the pandemic and their effects on the five child health outcomes which are a focus of ECHO; (3) Follow COVID-infected subpopulations for other subclinical effects of infection and/or other pandemic-related exposures that may be revealed through biospecimen assays or quantification of other biomarkers; (4) Compare a broad range of physical, chemical, social, behavioral, biological, natural, and built environmental exposures and health outcomes before and during the pandemic; (5) Provide insight into whether and how societal changes associated with the pandemic may differentially impact the health of children from different socioeconomic, racial, and ethnic groups and, in turn, exacerbate existing health inequities; (6) Describe geographic variation in environmental exposures and health outcomes during the pandemic using geocoded data, and describe how geographic location may modify the association between pandemic-related exposures and child health; (7) Identify early life factors associated with health outcomes during the pandemic, and related indicators of resilience and susceptibility, using a life course approach and harmonized extant data; and (8) Characterize the prevalence, disparities, and risk factors for SARS-CoV-2 infection among children. The ECHO Program has already begun leveraging these data to describe families' experiences during the COVID-19 pandemic, and the impact of the pandemic on the health (34–42), but many innovative research questions remain to be answered.
Importantly, ECHO data collected during the pandemic can be used to conduct solution-oriented research to inform the development of programs and policies that are customized to the “new normal” in the post-pandemic era. Societal changes spurred by the pandemic (e.g., caregiver remote working), and some economic impacts of the pandemic (e.g., shuttered community resources) persist, even as the number and proportions of vaccinated persons increases and public health precautions are lifted (43). The ECHO Program offers the unique opportunity to leverage well-characterized data from the largest ongoing pediatric multi-cohort research consortia in the US to understand the impact of the COVID-19 pandemic on child health.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: De-identified data from the ECHO Program are available through NICHD's Data and Specimen Hub (DASH). DASH is a centralized resource that allows researchers to access data from various studies via a controlled-access mechanism. Researchers can now request access to these data by creating a DASH account and submitting a Data Request Form. The NICHD DASH Data Access Committee will review the request and provide a response in approximately two to three weeks. Once granted access, researchers will be able to use the data for three years. See the DASH Tutorial for more detailed information on the process. Requests to access these datasets should be directed to https://dash.nichd.nih.gov/.
Ethics statement
The studies involving human participants were reviewed and approved by Properly constituted Institutional Review Boards—either the ECHO single IRB or the ECHO cohort's local IRB—are accountable for compliance with regulatory requirements for the ECHO-wide Cohort Data Collection Protocol at participating cohort sites. Governing IRBs review ECHO protocols and all informed consent/assent forms, HIPAA authorization forms, recruitment materials, and other relevant information prior to the initiation of any ECHO-wide Cohort Data Collection Protocol-related procedures or activities. ECHO Cohort Investigators (or their designated study personnel) obtain written informed consent or parent's / guardian's permission along with child assent as appropriate, for ECHO-wide Cohort Data Collection Protocol participation and for participation in their specific cohorts. The work of the ECHO Data Analysis Center is approved through the Johns Hopkins Bloomberg School of Public Health Institutional Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All authors have contributed substantially to the conception or design of the work; or the acquisition, analysis, or interpretation of data. The manuscript was drafted by TB. All authors revised and critically reviewed the manuscript, approved the version to be published, and agree to be accountable for the work. No honorarium, grant, or other payment was provided to produce this manuscript. All authors contributed to the article and approved the submitted version.
Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 with co-funding from the Office for Behavioral and Social Sciences Research (PRO Core), UH3OD023251 (Alshawabkeh), UH3OD023320 (Aschner), UH3OD023332 (Trasande), UH3OD023253 (Camargo), UH3OD023248 (Dabelea), UH3OD023313 (Koinis-Mitchell), UH3OD023328 (Duarte), UH3OD023318 (Dunlop), UH3OD023279 (Elliott), UH3OD023289 (Ferrara), UH3OD023282 (Gern), UH3OD023287 (Breton), UH3OD023365 (Hertz-Picciotto), UH3OD023275 (Karagas). UH3OD023271 (Karr), UH3OD023347 (Lester), UH3OD023389 (Leve), UH3OD023268 (Weiss), UH3OD023288 (McEvoy), UH3OD023342 (Lyall), UH3OD023349 (O'Connor), UH3OD023286 (Oken), UH3OD023348 (O'Shea), UH3OD023285 (Kerver), UH3OD023290 (Herbstman), UH3OD023272 (Schantz), UH3OD023249 (Stanford), UH3OD023305 (Trasande), UH3OD023337 (Wright). The funder/sponsor had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the manuscript for publication.
Acknowledgments
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators:
ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Catellier DJ; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D.
ECHO Awardees and Cohorts— Northeastern University, Boston, Massachusetts: Alshawabkeh, AN; Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio: Merhar S; Indiana University, Riley Hospital for Children, Indianapolis, IN: Ren C; University of Buffalo, Jacobson School of Medicine and Biomedical Sciences, Buffalo, NY: Reynolds A; University of California, San Francisco: Keller R; University of Rochester Medical Center, Rochester, NY: Pryhuber G; University of Texas Health Sciences Center, Houston, TX: Duncan A; Vanderbilt Children's Hospital, Nashville, TN: Moore P; Children's Hospital and Clinic Minneapolis, MN: Lampland A; Florida Hospital for Children, Orlando, FL: Wadhawan R; Medical University of South Carolina, Charleston, SC: Wagner C; University of Arkansas for Medical Science: Keller R; University of Florida College of Medicine, Jacksonville, FL: Hudak M; University of Washington, Seattle, WA: Mayock D; Wake Forest University School of Medicine, Winston Salem, NC:: Washburn L; Icahn School of Medicine at Mount Sinai, New York, NY: Teitelbaum SL; Stroustrup A; Cohen Children's Medical Center, Northwell Health: Stroustrup A; Pennsylvania State University, University Park, PA; Gatzke-Kopp; University of North Carolina, Chapel Hill, NC: Swingler M; Boston Children's Hospital, Boston, MA: Mansbach J; Children's Hospital of Philadelphia, Philadelphia, PA: Spergel J; Norton Children's Hospital, Louisville, KY: Stevenson M; Phoenix Children's Hospital, Phoenix AZ: Bauer C; University of Colorado Denver, Denver, CO: Dabelea D; Memorial Hospital of Rhode Island, Providence RI: Koinis-Mitchell; New York State Psychiatric Institute, New York, NY: Duarte C; University of Puerto Rico, San Jaun, PR: Canino G; Kaiser Permanente Northern California Division of Research, Oakland, CA: Ferrara A; Kaiser Permanente Northern California Division of Research, Oakland, CA: Croen L; Henry Ford Health System: Detroit, MI: Zoratti E; University of Wisconsin, Madison WI: Jackson D; Boston Medical Center, Boston MA: Bacharier L, O'Connor G; Children's Hospital of New York: New York, NY: Bacharier L, Kattan M; Johns Hopkins University, School of Medicine, Baltimore, MD: Wood R, Bacharier L; Washington University in St Louis, St Louis, MO: Rivera-Spoljaric K; Cincinnati Children's Hospital Medical Center, Cincinnati, OH: Hershey G; Henry Ford Health System, Detroit, MI: Johnson C; Vanderbilt University, Nashville TN: Hartert T; University of Wisconsin, Madison, WI: Singh A; University of Southern California, Los Angeles, CA: Farzan S; Habre R; University of California Davis Mind Institute, Sacramento, CA: Hertz-Picciotto I; University of Tennessee Health Science Center, Memphis, TN: Mason A; Seattle Children's Research Institute, Seattle, WA: Sathyanarayana S; Women & Infants Hospital of Rhode Island, Providence RI, Lester B; Children's Mercy, Kansas City, MO: Carter B; Emory University, Atlanta, GA: Marsit C; Helen DeVos Children's Hospital, Grand Rapids, MI: Pastyrnak S; Kapiolani Medical Center for Women and Children, Providence, RI: Neal C; Los Angeles Biomedical Research Institute at Harbour-UCLA Medical Center, Los Angeles CA: Smith L; Wake Forest University School of Medicine, Winston Salem, NC: Helderman J; Prevention Science Institute, University of Oregon, Eugene, OR: Leve L; Pennsylvania State University, University Park, PA: Neiderhiser J; Brigham and Women's Hospital, Boston, MA: Weiss S; Boston University Medical Center, Boston, MA: O'Connor G; Kaiser Permanente, Southern California, San Diego, CA: Zeiger R; Washington University of St. Louis, St Louis, MO: Bacharier L; Oregon Health and Science University, Portland, OR: McEvoy C; Indiana University, Riley Hospital for Children: Indianapolis, IN, Tepper R; Johns Hopkins Bloomberg School of Public Health Kennedy Krieger Institute, Baltimore, MD: Landa R; University of California, UC Davis Medical Center Mind Institute, Sacramento, CA: Ozonoff, S; University of California, UC Davis Medical Center Mind Institute, Davis, CA: Schmidt R; University of Washington: Dager S; Children's Hospital of Philadelphia—Center for Autism Research: Schultz R; University of North Carolina at Chapel Hill: Piven J; Johns Hopkins Bloomberg School of Public Health: Volk H; University of Rochester Medical Center Rochester, NY: O'Connor T; University of Pittsburgh Medical Center, Magee Women's Hospital, Pittsburgh, PA: Simhan H; Harvard Pilgrim Health Care Institute, Boston, MA: Oken E; Baystate Children's Hospital, Springfield, MA: Vaidya R; Beaumont Health Medical Center, Royal Oak, MI: Obeid R; Boston Children's Hospital, Boston, MA: Rollins C; East Carolina University Brody School of Medicine, Greenville, NC: Bear K; Helen DeVos Children's Hospital, Grand Rapids, MI: Pastyrnak S; Michigan State University College of Human Medicine, East Lansing, MI: Lenski, M; University of Chicago, Chicago IL: Msall M; University of Massachusetts Medical School, Worcester, MA: Frazier J; Wake Forest Baptist Health (Atrium Health), Winston Salem, NC: Washburn, L; Yale School of Medicine, New Haven, CT: Montgomery A; Michigan State University, East Lansing, MI: Kerver J; Henry Ford Health System, Detroit, MI: Barone, C; Michigan Department of Health and Human Services, Lansing, MI: McKane, P; Michigan State University, East Lansing, MI: Paneth N; University of Michigan, Ann Arbor, MI: Elliott, M; Columbia University Medical Center, New York, NY: Herbstman J; University of Illinois, Beckman Institute, Urbana, IL: Schantz S; University of California, San Francisco:, San Francisco, CA: Woodruff T; University of Utah, Salt Lake City, UT: Stanford J; University of Utah, Salt Lake City, UT: Porucznik C; University of Utah, Salt Lake City, UT: Silver R; University of Utah, Salt Lake City, UT: Conradt E; Icahn School of Medicine at Mount Sinai, New York, NY: Wright RJ; Boston Children's Hospital, Boston MA: Bosquet-Enlow M; George Mason University, Fairfax, VA: Huddleston K; University of California, San Francisco, San Francisco CA: Bush N; University of Minnesota, Minneapolis, MN: Nguyen R; University of Rochester Medical Center: Rochester, NY: Barrett E; Columbia University Medical Center, New York, NY: Miller R.
Conflict of interest
All authors report funding from the National Institutes of Health.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Larcher V, Brierley J. Children of COVID-19: pawns, pathfinders or partners? J Med Ethics. (2020) 46(8):508–9. doi: 10.1136/medethics-2020-106465
2. Minkus L, Groepler N, Drobnič S. The significance of occupations, family responsibilities, and gender for working from home: lessons from COVID-19. PLoS One. (2022) 17(6):e0266393. doi: 10.1371/journal.pone.0266393
3. Carlson DL, Petts RJ. US Parents’ domestic labor during the first year of the COVID-19 pandemic. Popul Res Policy Rev. (2022) 41(6):2393–418. doi: 10.1007/s11113-022-09735-1
4. Hedderson MM, Bekelman TA, Li M, Knapp EA, Palmore M, Dong Y, et al. Trends in screen time use among children during the COVID-19 pandemic, July 2019 through August 2021. JAMA Netw Open. (2023) 6(2):e2256157. doi: 10.1001/jamanetworkopen.2022.56157
5. Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. (2014) 1308:89–106. doi: 10.1111/nyas.12314
6. Baird J, Jacob C, Barker M, Fall CH, Hanson M, Harvey NC, et al. Developmental origins of health and disease: a lifecourse approach to the prevention of non-communicable diseases. Healthcare (Basel). (2017) 5(1):14. doi: 10.3390/healthcare5010014
7. Bekelman TA, Dong Y, Elliott AJ, Ferrara A, Friesen K, Galarce M, et al. Health behavior changes during the COVID-19 pandemic: a longitudinal analysis among children. Int J Environ Res Public Health. (2022) 19(15). doi: 10.3390/ijerph19159220
8. Knapp EA, Dong Y, Dunlop AL, Aschner JL, Stanford JB, Hartert T, et al. Changes in BMI during the COVID-19 pandemic. Pediatrics. (2022) 150(3). doi: 10.1542/peds.2022-056552
9. Al Hourani H, Alkhatib B, Abdullah M. Impact of COVID-19 lockdown on body weight, eating habits, and physical activity of Jordanian children and adolescents. Disaster Med Public Health Prep. (2021) 16(5):1855–63. doi: 10.1017/dmp.2021.48
10. Androutsos O, Perperidi M, Georgiou C, Chouliaras G. Lifestyle changes and determinants of children’s and adolescents’ body weight increase during the first COVID-19 lockdown in Greece: the COV-EAT study. Nutrients. (2021) 13(3). doi: 10.3390/nu13030930
11. Carroll N, Sadowski A, Laila A, Hruska V, Nixon M, Ma DWL, et al. The impact of COVID-19 on health behavior, stress, financial and food security among middle to high income Canadian families with young children. Nutrients. (2020) 12(8). doi: 10.3390/nu12082352
12. Horikawa C, Murayama N, Kojima Y, Tanaka H, Morisaki N. Changes in selected food groups consumption and quality of meals in Japanese school children during the COVID-19 pandemic. Nutrients. (2021) 13(8). doi: 10.3390/nu13082743
13. Medrano M, Cadenas-Sanchez C, Oses M, Arenaza L, Amasene M, Labayen I. Changes in lifestyle behaviours during the COVID-19 confinement in spanish children: a longitudinal analysis from the MUGI project. Pediatr Obes. (2020) 16(4):e12731. doi: 10.1111/ijpo.12731
14. Pietrobelli A, Pecoraro L, Ferruzzi A, Heo M, Faith M, Zoller T, et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity (Silver Spring). (2020) 28(8):1382–5. doi: 10.1002/oby.22861
15. Dunton GF, Do B, Wang SD. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health. (2020) 20(1):1351. doi: 10.1186/s12889-020-09429-3
16. Burkart S, Parker H, Weaver RG, Beets MW, Jones A, Adams EL, et al. Impact of the COVID-19 pandemic on elementary schoolers’ physical activity, sleep, screen time and diet: a quasi-experimental interrupted time series study. Pediatr Obes. (2022) 17(1):e12846. doi: 10.1111/ijpo.12846
17. Freiberg A, Schubert M, Romero Starke K, Hegewald J, Seidler A. A rapid review on the influence of COVID-19 lockdown and quarantine measures on modifiable cardiovascular risk factors in the general population. Int J Environ Res Public Health. (2021) 18(16). doi: 10.3390/ijerph18168567
18. Campbell H, Wood AC. Challenges in feeding children posed by the COVID-19 pandemic: a systematic review of changes in dietary intake combined with a dietitian’s perspective. Curr Nutr Rep. (2021) 10(3):155–65. doi: 10.1007/s13668-021-00359-z
19. Nagata JM, Cortez CA, Cattle CJ, Ganson KT, Iyer P, Bibbins-Domingo K, et al. Screen time use among US adolescents during the COVID-19 pandemic: findings from the adolescent brain cognitive development (ABCD) study. JAMA Pediatr. (2022) 176(1):94–6. doi: 10.1001/jamapediatrics.2021.4334
20. Nagata JM, Ganson KT, Liu J, Patel KP, Tai JC, Murray SB, et al. COVID Information and masking behaviors in U.S. adolescents: findings from the adolescent brain cognitive development (ABCD) study. Prev Med Rep. (2022) 28:101900. doi: 10.1016/j.pmedr.2022.101900
21. Stinson EA, Sullivan RM, Peteet BJ, Tapert SF, Baker FC, Breslin FJ, et al. Longitudinal impact of childhood adversity on early adolescent mental health during the COVID-19 pandemic in the ABCD study cohort: does race or ethnicity moderate findings? Biol Psychiatry Glob Open Sci. (2021) 1(4):324–35. doi: 10.1016/j.bpsgos.2021.08.007
22. Pelham WE 3rd, Tapert SF, Gonzalez MR, McCabe CJ, Lisdahl KM, Alzueta E, et al. Early adolescent substance use before and during the COVID-19 pandemic: a longitudinal survey in the ABCD study cohort. J Adolesc Health. (2021) 69(3):390–7. doi: 10.1016/j.jadohealth.2021.06.015
23. Yip SW, Jordan A, Kohler RJ, Holmes A, Multivariate BD. Transgenerational associations of the COVID-19 pandemic across minoritized and marginalized communities. JAMA Psychiatry. (2022) 79(4):350–8. doi: 10.1001/jamapsychiatry.2021.4331
24. Centers for Disease Control and Prevention, National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data Hyattsville, MD (2019).
25. U.S. Census Bureau. American Community Survey (ACS) Available at: www.census.gov/programs-surveys/acs (2022).
26. Webb Hooper M, Napoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. (2020). doi: 10.1001/jama.2020.8598
27. Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet. (2020) 395(10232):1243–4. doi: 10.1016/S0140-6736(20)30893-X
28. Gillman MW, Blaisdell CJ. Environmental influences on child health outcomes, a research program of the national institutes of health. Curr Opin Pediatr. (2018) 30(2):260–2. doi: 10.1097/MOP.0000000000000600
29. Environmental influences on Child Health Outcomes (ECHO). National Institutes of Health (NIH) Environmental influences on Child Health Outcomes (ECHO) COVID-19 Questionnaires: Disaster Research Response (DR2) Resources Portal; Available at: https://tools.niehs.nih.gov/dr2/index.cfm/resource/21805 (2020).
30. Blaisdell CJ, Park C, Hanspal M, Roary M, Arteaga SS, Laessig S, et al. The NIH ECHO program: investigating how early environmental influences affect child health. Pediatr Res. (2021). doi: 10.1038/s41390-021-01574-8
31. Knapp EA, Kress AM, Parker CB, Page GP, McArthur K, Gachigi KK, et al. The environmental influences on child health outcomes (ECHO)-wide cohort. Am J Epidemiol. (2023). doi: 10.1093/aje/kwad071
32. Eunice Kennedy Shriver National Insitute of Child Health and Human Development. Data and Biospecimen Hub (DASH). Available at: https://dash.nichd.nih.gov/ (2022).
33. U.S. Census Bureau. Educational Attainment in the United States: Available at: https://www.census.gov/newsroom/press-releases/2022/educational-attainment.html#:∼:text=The%20high%20school%20completion%20rate,10.5%25%20between%202011%20and%202021 (2021).
34. Blackwell CK, Mansolf M, Sherlock P, Ganiban J, Hofheimer JA, Barone CJ, et al. Youth well-being during the COVID-19 pandemic. Pediatrics. (2022) 149(4). doi: 10.1542/peds.2021-054754
35. Nozadi SS, Li X, Kong X, Rennie B, Kanda D, MacKenzie D, et al. Effects of COVID-19 financial and social hardships on Infants’ and Toddlers’ development in the ECHO program. Int J Environ Res Public Health. (2023) 20(2). doi: 10.3390/ijerph20021013
36. Bastain TM, Knapp EA, Law A, Algermissen M, Avalos LA, Birnhak Z, et al. COVID-19 Pandemic experiences and symptoms of pandemic-associated traumatic stress among mothers in the US. JAMA Netw Open. (2022) 5(12):e2247330. doi: 10.1001/jamanetworkopen.2022.47330
37. Bekelman TA, Knapp EA, Dong Y, Dabelea D, Bastain TM, Breton CV. Sociodemographic variation in children’s health behaviors during the COVID-19 pandemic. Childhood Obesity. (2022). doi: 10.1089/chi.2022.0085
38. Lucchini M, Bekelman TA, Li M, Knapp EA, Dong Y, Ballard S, et al. Impact of the COVID-19 pandemic on children’s sleep habits: an ECHO study. Pediatr Res. (2022). doi: 10.1038/s41390-022-02309-z
39. McKee KS, Tang X, Tung I, Wu G, Alshawabkeh AN, Arizaga JA, et al. Perinatal outcomes during versus prior to the COVID-19 pandemic and the role of maternal depression and perceived stress: a report from the ECHO program. Am J Perinatol. (2023). doi: 10.1055/a-2033-5610
40. McGowan EC, McGrath M, Law A, O’Shea TM, Aschner JL, Blackwell CK, et al. Health care utilization during the COVID-19 pandemic among individuals born preterm. JAMA Netw Open. (2023) 6(4):e2310696. doi: 10.1001/jamanetworkopen.2023.10696
41. Herbstman JB, Romano ME, Li X, Jacobson LP, Margolis AE, Hamra GB, et al. Characterizing changes in behaviors associated with chemical exposures during the COVID-19 pandemic. PLoS One. (2023) 18(1):e0277679. doi: 10.1371/journal.pone.0277679
42. Hipwell AE, Tung I, Sherlock P, Tang X, McKee K, McGrath M, et al. Impact of sedentary behavior and emotional support on prenatal psychological distress and birth outcomes during the COVID-19 pandemic. Psychol Med. (2023):1–14. doi: 10.1017/S0033291723000314
Keywords: life course approach, environmental exposures, health disparities, parent-child dyads, pediatric health, health behaviors
Citation: Bekelman TA, Trasande L, Law A, Blackwell CK, Jacobson LP, Bastain TM, Breton CV, Elliott AJ, Ferrara A, Karagas MR, Aschner JL, Bornkamp N, Camargo CA, Comstock SS, Dunlop AL, Ganiban JM, Gern JE, Karr CJ, Kelly RS, Lyall K, O’Shea TM, Schweitzer JB and LeWinn KZ (2023) Opportunities for understanding the COVID-19 pandemic and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) program. Front. Pediatr. 11:1171214. doi: 10.3389/fped.2023.1171214
Received: 21 February 2023; Accepted: 30 May 2023;
Published: 15 June 2023.
Edited by:
Eva Moehler, Saarland University Hospital, GermanyReviewed by:
Doina Anca Plesca, Carol Davila University of Medicine and Pharmacy, RomaniaBirgitta Dresp-Langley, Centre National de la Recherche Scientifique (CNRS), France
© 2023 Bekelman, Trasande, Law, Blackwell, Jacobson, Bastain, Breton, Elliott, Ferrara, Karagas, Aschner, Bornkamp, Camargo, Comstock, Dunlop, Ganiban, Gern, Karr, Kelly, Lyall, O'Shea, Schweitzer and LeWinn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Traci A. Bekelman dHJhY2kuYmVrZWxtYW5AY3VhbnNjaHV0ei5lZHU=
†See Acknowledgments for full listing of collaborators
Abbreviations ABCD, Adolescent Brain and Cognitive Development; ECHO, Environmental influences on Child Health Outcomes; EWCP, ECHO-Wide Cohort Protocol; NIH, National Institutes of Health; NHANES, National Health and Nutrition Examination Survey; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; US, United States.
 Traci A. Bekelman
Traci A. Bekelman Leonardo Trasande
Leonardo Trasande Andrew Law3
Andrew Law3 Courtney K. Blackwell
Courtney K. Blackwell Theresa M. Bastain
Theresa M. Bastain Amy J. Elliott
Amy J. Elliott Assiamira Ferrara
Assiamira Ferrara Carlos A. Camargo Jr
Carlos A. Camargo Jr Sarah S. Comstock
Sarah S. Comstock Anne L. Dunlop
Anne L. Dunlop Jody M. Ganiban
Jody M. Ganiban James E. Gern
James E. Gern Rachel S. Kelly
Rachel S. Kelly Kristen Lyall
Kristen Lyall T. Michael O’Shea
T. Michael O’Shea Julie B. Schweitzer
Julie B. Schweitzer Kaja Z. LeWinn
Kaja Z. LeWinn