- 1Health Management and Economics Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Social Determinants of Health Research Center, Health Management and Safety Promotion Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
- 3Preventive Medicine and Public Health Research Center, Psychosocial Health Research Institute, Iran University of Medical Sciences, Tehran, Iran
- 4Social Determinants of Health Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Background: The heart is the first fully developed organ in early pregnancy, especially in the first trimester of pregnancy, so any factor that contributes to heart failure is life-threatening. Thus, it is important to identify the risk and preventive factors related to this disease and to provide a scientific basis for the control, prevention, management and treatment of Child with Congenital Heart Diseases (CHD).
Objectives: As the etiology of CHD is multifactorial, to identify the risk and preventive factors, this study aimed to investigate the factors related to CHD in Tehran, Iran.
Methods: The present case-control study was performed on 600 people including 200 mothers of children with CHD. Simple random sampling was performed in 2020. The control group was matched with the case group, and the data were analyzed by SPSS software at a significance level of 0.5.
Results: The results showed that low socioeconomic status, low education, history of abortion, smoking, alcohol consumption are risk factors, and consumption of folic acid, and prenatal care are the protective factors against CHD.
Conclusion: According to the findings, our emphasis should be on preventive strategies, education of mothers and public health experts on the need for folic acid and pregnancy care, and cessation or reduction of alcohol and tobacco use, especially in families with low socioeconomic status and low level of education.
Background
Congenital heart disease (CHD) is the most common congenital defect in the world, which refers to the abnormal growth of the heart and arteries. Although in the western world, progress, innovation and prenatal diagnosis play a major role and have significantly reduced the mortality figures and in southern Europe the incidence of CHD is lower than in northern Europe and Anglo-Saxon countries (1), but this disease accounts for 1% of all live births and one-third of all congenital abnormalities (2), 94% of which occur in developing countries (3). In some studies in developing countries, the prevalence of CHD has been reported at 4–12 cases per 1,000 live births (4–6). CHD is the cause of 20%–30% of infant mortality and 20% of stillbirths (3). It is also a major global economic health threat (7) so in 2012, hospital costs for children with CHD were estimated to be higher than $ 6 billion (8). In 2021 in China, the cost of pediatric CHD was estimated to be $12.6 billion (5). Despite the stillbirths and infant deaths, even with improved prognosis and quality of life in CHD children using innovative medical techniques, congenital abnormalities are still the main causes of lifelong mental and physical disability in children, which can have a significant impact on their families and societies (3, 9).
The heart is the first fully developed organ in early pregnancy, especially in the first trimester of pregnancy, so any factor that contributes to heart failure is life-threatening. Although the main causes of CHD abnormalities remain unclear, it is obvious that 80% of the CHD cases are due to multiple environmental and genetic factors or the interaction between the two (10). It seems that an embryo that is genetically exposed to environmental stimuli may develop morphogenetic abnormalities of the heart during intrauterine development. In fact, the interaction between genetic predisposition and exposure to environmental stimuli may lead to congenital heart disease (11).
Various studies have linked factors, such as alcohol use, sex hormones, occupational exposure to rubella virus, maternal diabetes and the use of certain drugs, including thalidomide, lithium and folic acid antagonists, to varying degrees of CHD (11–13). Strengthening protective strategies such as avoiding autogenous substances, infection screening and treatment of the mother and her proper diet, including vitamins, and folic acid (14), can control about 70% of cases of this disease (15). Therefore, we can say that the etiology of CHD is multifactorial and in this regard, identifying the risk and preventive factors of this disease is important and necessary in order to provide a scientific basis for control, prevention, management and treatment of CHD. Thus, the aim of this study was to identify the risk and preventive factors related to CHD in Tehran, Iran.
Methods
Study design
This case-control study was carried out in 2020 on the mothers of children with CHD who were admitted to the Shahid Rajaie Cardiovascular Medical and Research Center in Tehran, Iran. This hospital is one of the largest cardiac referral hospitals in Asia and admits patients with cardiovascular diseases from various parts of Iran. Pediatric heart care is one of the most important clinical services offered in this hospital. The pediatric cardiology department, as a well-known and best-equipped clinic, provides educational, treatment, and research services for children and adolescents with heart diseases.
Inclusion criteria were: confirmed diagnosis of the child with CHD, in terms of Diagnosis during pregnancy (fetal echocardiography) and congenital heart disease diagnosed shortly after birth, and children under 5 years of age were included in this study.
Exclusion criteria were: Children with non-congenital heart disease, Children with congenital heart disease whose mothers have died, and Children who had non-Iranian nationality.
Data collection
The data were collected primarily from mothers who attended the pediatric cardiology department between March 2020 and May 2021. Two hundred mothers of children with CHD (Congenital Heart Disease) were selected through simple random sampling. At first, a list of children with congenital heart disease was prepared; a random number was assigned to each child using Excel software. Then, we selected the samples with random numbers from 1 to 200. Finally, the checklist was completed by the mother of the selected child and allocated to the case group. For selecting the controls, a list of children who did not have congenital heart disease was prepared. Then, similar to the case group selection method, random numbers from 1 to 400 were selected using the simple random sampling method by Excel software.
According to the matching criteria, such as mother's age, child's age, child's gender and city of residence, 400 mothers of children without CHD were recruited and allocated to the control group (the ratio of cases to control groups was set to be 1:2).
The CHD diagnosis was made by a cardiologist. Registering the child as a child with congenital heart disease in the medical record. Besides, for more confirmation and certainty, the mother was also asked whether her child had this disease at birth or not. It should be noted that fetal echocardiography records or the child's cardiologist's report are available as documentation of congenital heart disease in the medical report.
Mothers in case and control groups were asked to fill the study checklist. The explanatory variables were selected based on the WHO framework regarding social determinants of health presented by Solar and Irwin (16).
Definition of variables
CHD was chosen as a dichotomous outcome variable (mothers with CHD children or not). The polychoric principal component analysis (PCA) was used to construct the households' socioeconomic status (17). One of the assumptions underlying the classic PCA is that, the input variables are normal. However, as our data regarding the households' socio-economic status were discrete (including binary and ordinal variables), this assumption was clearly violated. As a result, the polychoric PCA was used in this study. The following variables were used in the polychoric PCA model: The mother's education level, father's education level, father's occupation, house ownership, owning a personal computer, and having a kitchen, a bathroom, a vacuum cleaner, a washing machine, and a freezer. Accordingly, five socioeconomic quintiles including the lowest, low, moderate, high, and highest quintiles were identified and used in the subsequent analysis.
The social variables included; the socio-economic status of the household, level of education of mother and father, occupation of mother and father, nationality, place of residence, number of children and family marriage. The medical/biological variables included; the mother's age at delivery, father's age, the number of childbirth deliveries, receiving pregnancy health care, history of abortion, and chronic disease, and the use of folic acid. Also, the lifestyle variables included physical activity (Having or not having physical activity), alcohol consumption, and smoking (whether the mother used alcohol or smoking during pregnancy or not).
Data analysis
Descriptive analysis was conducted in terms of frequencies and percentages. Given that CHD was a binary outcome variable, the Chi-square test and the logistic regression were used to identify the main explanatory variables. To understand the real factors associated with CHD, the significant variables detected through the Chi-square test were first identified and then entered into a multivariate logistic regression model using the forward method. The cutoff point for univariate binary logistic regression analysis was set at ≤0.2. Also, the Hosmer–Lemeshow test was used to examine the goodness of fit for the logistic model against actual outcomes. The results of the Hosmer–Lemeshow test did not show a significant difference/relationship between the variables (p > 0.05), suggesting an adequate overall fit for the model.
Ethical considerations
The current study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.RESEARCH.REC.1399.118). All participants were informed regarding the aim and objectives of the study, and written informed consent was obtained from all of the participants prior to participation.
Results
We analyzed the data of 600 mothers in 2020–21, of whom 33.3% (mothers who had children with CHD) and 66.7% (mothers who had children without CHD) were investigated as the case and control groups, respectively. Most mothers in the control group had a moderate socioeconomic status (SES), whereas most mothers in the case group had the lowest SES. In the case group, most mothers were 18–28-year-old (44.5%), illiterate (32%), housewives (81.5%), and also had two children (43.5%) and had a history of abortion (57%). In the control group, most mothers were 29–39 years old (56.8%), and had a secondary school education (27%) and had no history of abortion (93.5%). Also, having a chronic disease, alcohol use and smoking was more prevalent among mothers with CHD children in comparison with the control group (Table 1).
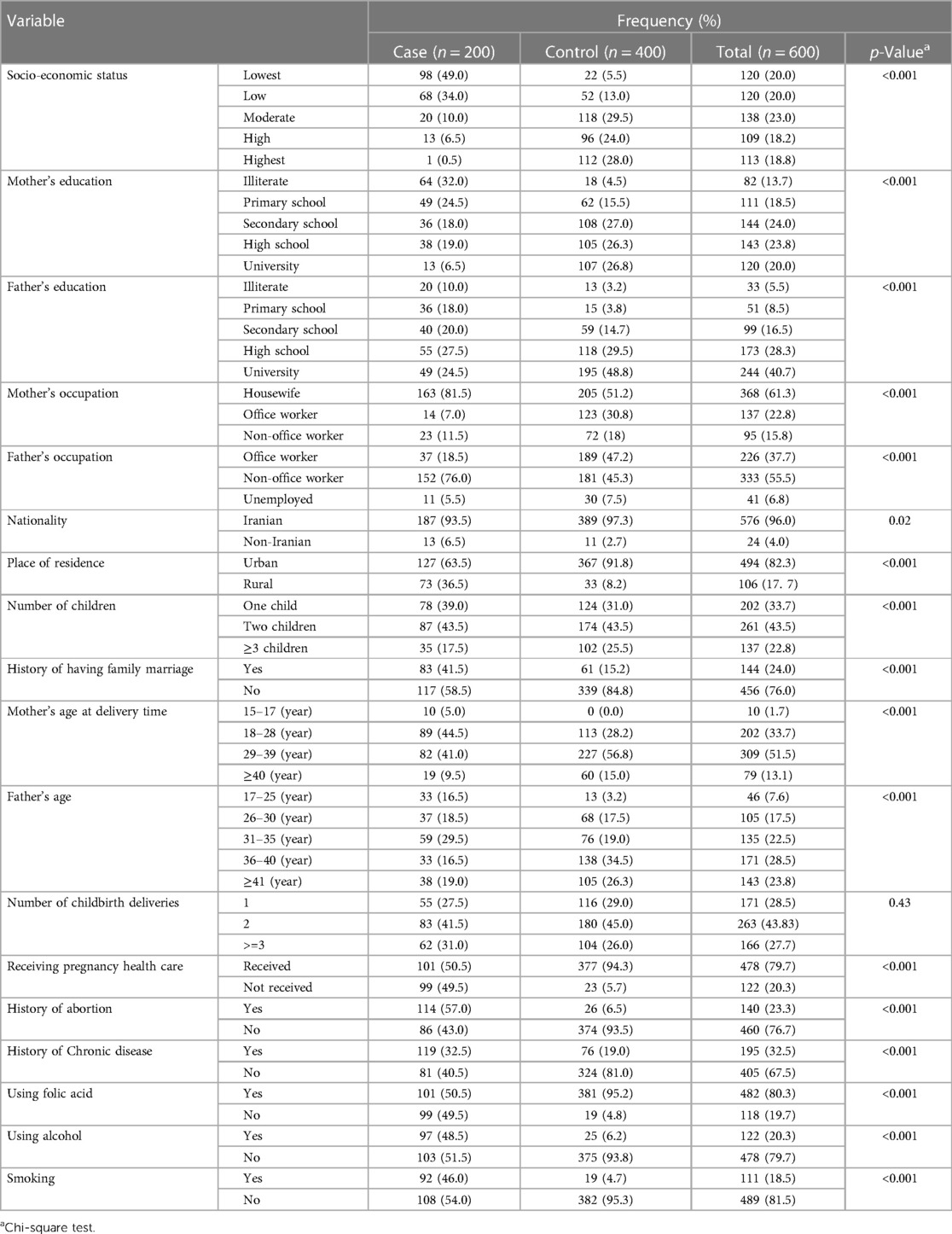
Table 1. Descriptive characteristics of mothers with CHD children in the case and control groups referred to the heart hospital.
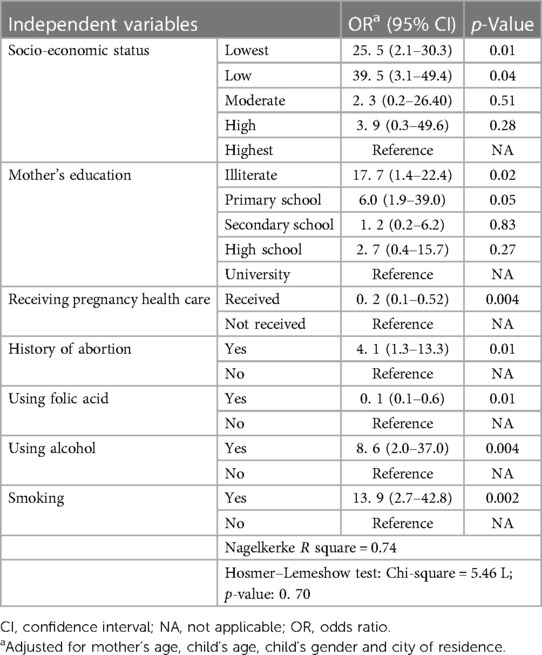
To understand the real factors associated with CHD in children, all the significant variables in the chi-square test (except physical activity) were entered into a multivariate logistic regression model using the forward method. As shown In Table 2, the results of logistic regression revealed that among the variables with a statistically significant OR, the leading cause of CHD in children was related to the lowest SES quintile. The prevalence of CHD in the lowest and low SES group was 39.5 and 25.5 times higher than the highest SES group. Moreover, illiterate mothers and mothers with primary school education had 17.7 and 6.0 times higher odds of having children with CHD than mothers with university degrees, respectively. Having children with CHD was 13.9, 8.6 and 4.1 times higher in mothers with a history of smoking, alcohol use and abortion, respectively. In contrast, using pregnancy health care and folic acid had a protective effect on having children with CHD by up to 0.2 and 0.10 times, respectively.
According to the Nagelkerke R square, the study model explained 74% of the variance of having children with CHD. Moreover, the Hosmer–Lemeshow test was used to examine the goodness of fit for the logistic model against actual outcomes. The Hosmer–Lemeshow test suggested an adequate overall fit for the model (Table 2).
Discussion
The aim of this study was to investigate the factors associated with having a child with CHD. The findings of this study statistically showed that low socioeconomic status, low education, history of abortion, smoking, and alcohol consumption, were the risk factors, and folic acid use and utilizing pregnancy health care were the protective factors against having a child with CHD.
The findings of the present study showed that low socioeconomic status is related to the odds of having a child with CHD. This finding is consistent with the study of Morales-Suárez-Varela et al. and Qu et al. (18, 19). The low socioeconomic status is not the risk factor for having a child with CHD in itself, but the circumstances in which the person lives, including the unfavorable living environment and exposure to various infectious and chemical substances, both at home and at work, increase the chances of having a child with CHD. Although several studies have concluded that having a job is associated with a better pregnancy (20, 21), other studies suggest that low socioeconomic status and work conflicts are associated with a risk of having a child with CHD (18, 22). Contrary to the results of the present study, the findings of a study by Egbe and colleagues showed that the incidence of CHD is lower in the lower socioeconomic classes (23). This discrepancy can be attributed to the unique features of the mentioned study, because they had only entered the diagnoses of CHD made during the hospital stay, into the study. Also, the mean age of patients at the time of diagnosis was 2 days. They had also conducted their study on specific ethnic groups such as Caucasians, and their study was a retrospective study that only had analyzed the available data. As a result, these findings may not have a high sensitivity. Finally, it seems that low socioeconomic status can be associated with fewer visits to health centers and facilities to receive health care services, resulting in less use of supplements and eventually the occurrence of diseases such as CHD (24, 25). It can be said that poor nutrition and low education are the results of living in the poverty among low socio-economic classes.
The present study showed that illiteracy and primary education (low education) increase the chances of having a child with CHD compared to higher education. This finding is consistent with the study of Kafian Atary et al., and Qu et al. (19, 26). Qu and colleagues in a study demonstrated that education for less than 12 years increases the odds of having a child with CHD. It can be said that people with low education are usually from the lower socio-economic classes of society, live in high-risk environments, and have less knowledge and awareness about using folic acid and utilizing prenatal health care, therefore they are more likely to have children with CHD than people with higher education.
The present study, in line with other studies, showed a significant relationship between the history of abortion and the increased risk of CHD (11, 27). The previous meta-analysis study showed that a history of abortion increased the risk of congenital miscarriage by 24% (27).
Our study found that mothers who consumed alcohol were 8.6 times more likely to give birth to children with CHD. In 1973, researchers found that alcohol consumption in mothers is a risk factor for heart diseases (28). Every year, more than 10,000 cases of CHD occur in the United States due to alcohol consumption (29). Consistent with the results of the present study, Fung et al. (30), Zhiyan Chen et al. (7), and Sudi Jemal et al. (3) found that alcohol consumption is associated with congenital heart disease so that consuming any amount of alcohol increases the risk of CHD (31). Although a study in Ethiopia (32) has shown that alcohol consumption is rarely associated with congenital anomalies, this finding should be treated with caution, as alcohol is able to be transmitted to the fetus through the placental membrane and therefore can have direct effects on the organogenesis of developing fetus (33, 34).In the present study, we found that smoking increases the chances of having a child with CHD. Studies have shown that smoking or exposure to chemicals substances makes mothers more susceptible to having a child with CHD (22). Other studies have shown that smoking in any way increases the chances of having a child with CHD (3, 26, 31, 32, 35, 36). The results of Nicole's study also indicated that in addition to the mother's smoking during pregnancy, her exposure to secondhand smoke and teratogens such as pesticides, metals and detergents, has a significant relationship with CHD (37). Another study by Deng and colleagues showed that fathers' smoking from the three months before pregnancy to the end of pregnancy increases the risk of having a child with CHD (38). Lijuan Zhao and colleagues also showed that active and passive smoking increases the risk of having a child with CHD (39).
In the present study, folic acid consumption was a protective factor against the development of congenital abnormalities, so it can prevent 90% of CHD cases. This finding is consistent with other studies (19, 37, 40, 41). Other studies have shown that the non-consumption of folic acid increases the chances of having a child with CHD (3). Iran's Ministry of Health has adopted policies through which, pregnant women would have free access to the folic acid supplement, but the lack of folic acid consumption in Iran may be due to low education, awareness, and ignorance of pregnant women and frontline healthcare professionals in regard to the importance of CHD and consumption of folic acid.
The findings of the present study indicate that prenatal health care is a protective factor against having a child with CHD so that it can prevent 80% of CHD cases. To the best of our knowledge, although there was no evidence to compare this finding with previous studies, it can be said that people who receive prenatal care, use folic acid, and are aware of the possibility of their children suffering from various diseases, keep themselves away from the risk factors. It is also possible that during pregnancy examinations, the child is diagnosed with CHD and her mother undergoes abortion, which reduces the chances of having a child with CHD in these women. Ultimately, it is possible that people who do not receive prenatal care live in financial and nutritional poverty, and this increases the risk of having a child with CHD in them.
It seems that the risk factors examined in the present study, such as receiving pregnancy health care, using folic acid, History of abortion, using alcohol, smoking, in interaction with each other and under the influence of important confounding variables of socioeconomic status and education level, this interaction are increase the risk of CHD, and would be need to be more investigated in future cohort and longitudinal studies.
Strengths and limitations
Case-control studies are among the most reliable studies to analyze the relationship between the risk factors of a disease, but they are prone to recall bias, as mothers may be biased or erroneous in answering questions. Also, since in this study only mothers of live births infants in the hospital were included in the study, it is possible that selected bias has occurred in the study. As the evidence shows, these biases are inherent part of case-control studies, although it can be reduced to some extent during the interview by further explaining the variables and helping to remember them, but they cannot be completely eliminated. other possibility and bias is that non-CHD mothers are more prone to recall as well as choose to present a healthier lifestyle. In the present study, we are not investigated the relationship of gene pool in family marriages with CHD, and it is necessary to consider this variable in future studies. There are wide confidence intervals in several findings is another limitation of this study and a risk of low power. Therefore, care should be taken in generalizing the findings of the present study.
Conclusion
Identifying CHD-related risk factors can help policymakers and stakeholders to reduce the prevalence of CHD by taking and strengthening preventive actions. Based on the findings, our emphasis is on preventive strategies, educating mothers and public health professionals about the need for folic acid and pregnancy health care, and stopping or reducing alcohol and tobacco use, especially in families with low socioeconomic status and low education levels.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study protocol and procedures were reviewed and approved by the Research Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.RESEARCH.REC.1399.118). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MA-R and MN conceived the manuscript topic, designed the study, acquired the data, and performed the data analysis. SK and NS provided expert advice regarding the study analysis, drafted the manuscript, and provided critically important intellectual content during manuscript revisions. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the mothers of children with CHD who were admitted to the Shahid Rajaie Cardiovascular Medical and Research Center in Tehran, for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Munoz M-A, Rohlfs I, Masuet S, Rebato C, Cabañero M, Marrugat J. Analysis of inequalities in secondary prevention of coronary heart disease in a universal coverage health care system. Eur J Public Health. (2006) 16(4):361–7. doi: 10.1093/eurpub/cki202
2. Zegkos T, Ntiloudi D, Giannakoulas G. Parental alcohol exposure and congenital heart diseases in offspring: A causal link with controversial evidence. Eur J Prev Cardiol. (2020) 27(4):407–9. 10.1177/2047487319877705
3. Jemal S, Fentahun E, Oumer M, Muche A. Predictors of congenital anomalies among newborns in arsi zone public hospitals, Southeast Ethiopia: a case-control study. Ital J Pediatr. (2021) 47:143. doi: 10.1186/s13052-021-01093-6
4. Hoffman JI. The global burden of congenital heart disease. Cardiovasc J Afr. (2013) 24(4):141–5. doi: 10.5830/CVJA-2013-028
5. Li J, Du Y, Liu Y, Du J, Zhang R, Qu P, et al. Maternal exposure to life events during pregnancy and congenital heart disease in offspring: a case-control study in a Chinese population. BMC Pregnancy Childbirth. (2021) 21(1):1–10. doi: 10.1186/s12884-020-03485-8
6. Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: analysis from FOURIER. Circulation. (2018) 138(8):756–66. doi: 10.1161/CIRCULATIONAHA.118.034309
7. Chen Z, Li S, Guo L, Peng X, Liu Y. Prenatal alcohol exposure induced congenital heart diseases: from bench to bedside. Birth Defects Res. (2021) 113(7):521–34. doi: 10.1002/bdr2.1743
8. Faraoni D, Nasr VG, DiNardo JA. Overall hospital cost estimates in children with congenital heart disease: analysis of the 2012 kid’s inpatient database. Pediatr Cardiol. (2016) 37(1):37–43. doi: 10.1007/s00246-015-1235-0
9. Borjali M, Amini-Rarani M, Nosratabadi M. Nonmedical determinants of congenital heart diseases in children from the perspective of mothers: a qualitative study in Iran. Cardiol Res Pract. (2021) 2021:6647260. doi: 10.1155/2021/6647260
10. Blue GM, Kirk EP, Sholler GF, Harvey RP, Winlaw DS. Congenital heart disease: current knowledge about causes and inheritance. Med J Aust. (2012) 197(3):155–9. doi: 10.5694/mja12.10811
11. Ahmadi A, Gharipour M, Navabi ZS, Heydari H. Risk factors of congenital heart diseases: a hospital-based case-control study in Isfahan, Iran. ARYA Atheroscler. (2020) 16(1):1. doi: 10.22122/arya.v16i1.1941
12. Källén B. Antidepressant drugs during pregnancy and infant congenital heart defect. Reprod Toxicol (Elmsford, NY). (2006) 21(3):221–2. doi: 10.1016/j.reprotox.2005.11.006
13. Liang Q, Gong W, Zheng D, Zhong R, Wen Y, Wang X. The influence of maternal exposure history to virus and medicine during pregnancy on congenital heart defects of fetus. Environ Sci Pollut Res. (2017) 24(6):5628–32. doi: 10.1007/s11356-016-8198-4
14. World Health Organization. World health statistics. Geneva, Switzerland: World Health Organization (2015).
15. Christianson A, Howson CP, Modell B. March of Dimes: global report on birth defects, the hidden toll of dying and disabled children (2005).
16. World Health Organization. A Conceptual Framework for Action on the Social Determinants of Health. Discussion Paper. Geneva, Switzerland: WHO Document Production Services. A conceptual framework for action on the social determinants of health. Geneva, Switzerland: WHO Document Production Services (2010). doi: 10.13016/17cr-aqb9
17. Kolenikov S, Angeles G. Socioeconomic status measurement with discrete proxy variables: is principal component analysis a reliable answer? Rev Income Wealth. (2009) 55(1):128–65. doi: 10.1111/j.1475-4991.2008.00309.x
18. Morales-Suárez-Varela M, Kaerlev L, Zhu JL, Llopis-González A, Gimeno-Clemente N, Nohr EA, et al. Risk of infection and adverse outcomes among pregnant working women in selected occupational groups: a study in the danish national birth cohort. Environ Health. (2010) 9(1):1–11. doi: 10.1186/1476-069X-9-70
19. Qu Y, Lin S, Bloom MS, Wang X, Ye B, Nie Z, et al. Maternal folic acid supplementation mediates the associations between maternal socioeconomic status and congenital heart diseases in offspring. Prev Med. (2021) 143:106319. doi: 10.1016/j.ypmed.2020.106319
20. Burdorf A, Brand T, Jaddoe V, Hofman A, Mackenbach J, Steegers E. The effects of work-related maternal risk factors on time to pregnancy, preterm birth and birth weight: the generation R study. Occup Environ Med. (2011) 68(3):197–204. doi: 10.1136/oem.2009.046516
21. Jansen PW, Tiemeier H, Verhulst FC, Burdorf A, Jaddoe VW, Hofman A, et al. Employment status and the risk of pregnancy complications: the generation R study. Occup Environ Med. (2010) 67(6):387–94. doi: 10.1136/oem.2009.046300
22. Spinder N, Prins JR, Bergman JE, Smidt N, Kromhout H, Boezen HM, et al. Congenital anomalies in the offspring of occupationally exposed mothers: a systematic review and meta-analysis of studies using expert assessment for occupational exposures. Hum Reprod. (2019) 34(5):903–19. doi: 10.1093/humrep/dez033
23. Egbe A, Uppu S, Stroustrup A, Lee S, Ho D, Srivastava S. Incidences and sociodemographics of specific congenital heart diseases in the United States of America: an evaluation of hospital discharge diagnoses. Pediatr Cardiol. (2014) 35(6):975–82. doi: 10.1007/s00246-014-0884-8
24. SoleimanvandiAzar N, Kamal SHM, Sajjadi H, Harouni GG, Karimi SE, Djalalinia S, et al. Determinants of outpatient health service utilization according to andersen’s behavioral model: a systematic scoping review. Iran J Med Sci. (2020) 45(6):405. doi: 10.30476/ijms.2020.85028.1481
25. SoleimanvandiAzar N, Kamal SHM, Sajjadi H, Ardakani HM, Forouzan AS, Karimi SE, et al. Outpatient health service utilization and associated factors: a cross-sectional population-based study in Tehran in 2019. Med J Islam Repub Iran. (2021) 35:71. doi: 10.47176/mjiri.35.71
26. Kafian Atary S, Mirshahi A, Amouzeshi A, Ramazani AA, Soleimani Khomartash Z, Bahman B, et al. Epidemiologic study of congenital heart diseases and its related factors in children referred to the pediatric cardiac clinic of Birjand University of Medical Sciences, Iran. Int J Pediatr. (2019) 7(12):10455–63. doi: 10.22038/ijp.2019.41467.3497
27. Feng Y, Wang S, Zhao L, Yu D, Hu L, Mo X. Maternal reproductive history and the risk of congenital heart defects in offspring: a systematic review and meta-analysis. Pediatr Cardiol. (2015) 36(2):253–63. doi: 10.1007/s00246-014-1079-z
28. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. (1973) 301(7815):1267–71. doi: 10.1016/S0140-6736(73)91291-9
29. Burd L, Deal E, Rios R, Adickes E, Wynne J, Klug MG. Congenital heart defects and fetal alcohol spectrum disorders. Congenit Heart Dis. (2007) 2(4):250–5. doi: 10.1111/j.1747-0803.2007.00105.x
30. Fung A, Manlhiot C, Naik S, Rosenberg H, Smythe J, Lougheed J, et al. Impact of prenatal risk factors on congenital heart disease in the current era. J Am Heart Assoc. (2013) 2(3):e000064. doi: 10.1161/JAHA.113.000064
31. Taylor K, Elhakeem A, Thorbjørnsrud Nader JL, Yang TC, Isaevska E, Richiardi L, et al. Effect of maternal prepregnancy/early-pregnancy body mass index and pregnancy smoking and alcohol on congenital heart diseases: a parental negative control study. J Am Heart Assoc. (2021) 10:e020051. doi: 10.1161/JAHA.120.020051
32. Mekonnen AG, Hordofa AG, Kitila TT, Sav A. Modifiable risk factors of congenital malformations in Bale zone hospitals, Southeast Ethiopia: an unmatched case-control study. BMC Pregnancy Childbirth. (2020) 20(1):1–9. doi: 10.1186/s12884-020-2827-0
33. Skosyreva A. Effect of ethyl alcohol on embyonic development at the stage of organogenesis. Akush Ginekol (Mosk). (1973) 49(4):15–8. PMID: 4805257.4805257
34. Skosyreva A. Comparative study of the direct embryotoxic effect of alcohol and its metabolite acetaldehyde during organogenesis. Akush Ginekol (Mosk). (1982) (1):49–50.7199831
35. Lee LJ, Lupo PJ. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: a systematic review and metaanalysis. Pediatr Cardiol. (2013) 34(2):398–407. doi: 10.1007/s00246-012-0470-x
36. Taye M, Afework M, Fantaye W, Diro E, Worku A. Factors associated with congenital anomalies in Addis Ababa and the Amhara region, Ethiopia: a case-control study. BMC Pediatr. (2018) 18(1):1–11. doi: 10.1186/s12887-018-1096-9
37. Nicoll R. Environmental contaminants and congenital heart defects: a re-evaluation of the evidence. Int J Environ Res Public Health. (2018) 15(10):2096. doi: 10.3390/ijerph15102096
38. Deng K, Liu Z, Lin Y, Mu D, Chen X, Li J, et al. Periconceptional paternal smoking and the risk of congenital heart defects: a case-control study. Birth Defects Res A Clin Mol Teratol. (2013) 97(4):210–6. doi: 10.1002/bdra.23128
39. Zhao L, Chen L, Yang T, Wang L, Wang T, Zhang S, et al. Parental smoking and the risk of congenital heart defects in offspring: an updated meta-analysis of observational studies. Eur J Prev Cardiol. (2020) 27(12):1284–93. doi: 10.1177/2047487319831367
40. Patel SS, Burns TL. Nongenetic risk factors and congenital heart defects. Pediatr Cardiol. (2013) 34(7):1535–55. doi: 10.1007/s00246-013-0775-4
Keywords: congenital heart diseases (CHD), risk factors, protective factors, folic acid, tobacco, alcohol, Iran
Citation: Amini-Rarani M, Karimi SE, SoleimanvandiAzar N and Nosratabadi M (2023) Risk and protective factors related to having a child with congenital heart diseases (CHD): a case-control study in Iran. Front. Pediatr. 11:1170743. doi: 10.3389/fped.2023.1170743
Received: 21 February 2023; Accepted: 19 June 2023;
Published: 3 July 2023.
Edited by:
Umberto Morbiducci, Polytechnic University of Turin, ItalyReviewed by:
Estelle Naumburg, Umeå University, SwedenKatharina Schmitt, Charité University Medicine Berlin, Germany
© 2023 Amini-Rarani, Karimi, SoleimanvandiAzar and Nosratabadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Nosratabadi bm9zcmF0LndlbGZhcmVAZ21haWwuY29t
Abbreviations CHD, congenital heart disease; WHO, World Health Organization; PCA, the polychoric principal component analysis; SES, socio-economic status; CI, confidence intervals; OR, odds ratios; NA, not applicable.
†ORCID Mostafa Amini-Rarani orcid.org/0000-0002-4809-2237 Salah Eddin Karimi orcid.org/0000-0002-1542-0214 Neda SoleimanvandiAzar orcid.org/0000-0002-9840-5588 Mehdi Nosratabadi orcid.org/0000-0002-2977-0426
 Mostafa Amini-Rarani1,†
Mostafa Amini-Rarani1,† Salah Eddin Karimi
Salah Eddin Karimi Mehdi Nosratabadi
Mehdi Nosratabadi