- 1Department of Movement, Human and Health Sciences, University of Rome “Foro Italico”, Rome, Italy
- 2Department of Physiology and Pharmacology “Vittorio Erspamer”, Sapienza University of Rome, Rome, Italy
- 3Department of Theoretical and Applied Sciences, eCampus University, Novedrate, Italy
- 4Departement of Unicusano, Università Degli Studi Niccolò Cusano, Rome, Italy
- 5Department of Health and Exercise Science, Appalachian State University, Boone, NC, United States
Introduction: The aim of the study was to investigate the influence of training status on cardiovascular function in young male recreational and competitive rowers.
Methods: Ejection duration in percentage to the heart rate period (ED%), subendocardial viability ratio (SEVR), augmentation index at 75 bpm (AIx75) and carotid to femoral pulse wave velocity (cf-PWV) of competitive rowers (CR) (age 17.6 ± 4.1 years), recreational rowers (RR) (age 16.7 ± 2.70 years) and athletes practicing other recreational sports (ORS) (age 15.3 ± 1.4 years) were assessed.
Results: ED% was lower in CR compared to ORS (31.9 ± 3.9% vs. 38.4 ± 4.8%; p = 0.026) and cf-PWV was higher in CR compared to ORS (5.5 ± 1.0 m/s vs. 4.7 ± 0.5 m/s; p = 0.032). SEVR was higher in CR compared to RR and ORS (165.8 ± 33.7% vs. 127.4 ± 30.4% and 128.3 ± 27.8%; p = 0.022) and AIx75 was lower in CR compared to RR and ORS (−15.7 ± 8.6% vs. 1.2 ± 9.9% and 1.5 ± 9.1; p = 0.001).
Discussion: Healthy, young competitive male rowers reported higher myocardial performance and better cardiovascular health than recreational athletes. Interpretations of cf-PWV in competitive rowers should be performed alongside other cardiovascular indicators.
Introduction
Rowing is considered a unique sport due to the combination of high intensity aerobic and muscular effort that is required during a race. The continuous exertion force using large muscle groups leads rowers to gain both large aerobic power and muscle strength (1, 2). Research has shown that the cardiovascular system of this type of athletes may undergo significant remodelling changing both the structure and function of heart and arteries (3–8).
Previous studies conducted in adults have shown that professional rowing may induce an increase in cardiac volume and mass and that these adaptations differ between male and female athletes (3, 9–11). Although cardiac remodelling occurs in both competitive and recreational rowers, this sport seems to increase specific aspects of cardiac structure and function in elite adult athletes consequently to the higher volume and intensity of training (12). These morphological adaptations can improve the mechanical performance of the heart which consequently increases the systolic flow velocity, the emptying velocity of the left ventricle, and the maximum volume ejected during the left ventricle systole (5, 13). Additionally, recreational and competitive adult rowers reported favourable elastic properties of the arteries and larger vascular diameters (5–8, 14). However, there is also inconclusive evidence on the impact of rowing on cardiovascular functions. Other studies have shown that this discipline may exhibit no-effects or negative effects on vascular characteristics in competitive and recreational adults (8, 15–17). Moreover, the fact that athletes undergo high-intensity training from an early age to compete professionally (18, 19) suggests that more research in adolescent rowers is needed to uncover the cardiovascular adaptations occurring at an early age. Research in adolescents reported that, among different disciplines, competitive male rowers show the highest left ventricular wall thickness and cavity dimension (4). However, little is known about the vascular adaptations in young rowers as the majority of research has been conducted on adults (7, 15). Thus, the aim of this study was to investigate the influence of training status on cardiovascular functioning in young recreational and competitive male rowers.
Materials and methods
Participants
Twenty-eight, young, healthy males (age = 16.3 ± 2.7 years) were recruited for this study. Participants were divided in three groups: competitive rowers (CR, N = 8), recreational rowers (RR, N = 8) and other recreational sports (ORS, N = 12). The CR group was composed of athletes with 6.0 ± 3.9 years of training experience, who trained at least 5 times per week (12.8 ± 2.3 h per week, 6.8 ± 0.5 training sessions per week), and had regularly participated in regional and/or national competitions in the previous year. The RR group included individuals with 3.4 ± 0.9 years of training experience, who attended 2 or 3 rowing training sessions per week (2.7 ± 0.6 h per week, 2.4 ± 0.5 training sessions per week), and reported no history of competitive sports careers. The ORS group was composed of individuals with 4.0 ± 1.7 years of training experience, who practised recreational activities different from rowing 2 or 3 times per week (2.7 ± 0.4 h per week, 2.3 ± 0.5 training sessions per week), and without history of a competitive sports career. All participants underwent clinical examinations to exclude any risk associated with maximal physical exertion during exercise. Inclusion criteria were healthy individuals reporting no disease, infirmity or underlying medical conditions (20). Exclusion criteria included having medical conditions such as diabetes, heart, respiratory or renal disease and not taking any medications at the time of recruitment. All participants and parents/guardians of minors gave written informed consent to enrol in this research. This study was conducted in accordance with the Declaration of Helsinki and approved by the CAR-IRB—University of Rome “Foro Italico” Committee (Approval No. CAR 37/2020).
Anthropometric parameters assessment
Weight, height and sitting height were measured using a scale and a stadiometer to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as the ratio between weight in kg and the square of height in metres (kg/m2). Fat mass (FM) and fat free mass (FFM) were assessed through Bioelectrical Impedance Analysis (BIA AKERN 101 Anniversary, Pontassieve, FI, Italy). Participants were measured with minimal, tight-fitting clothes and without shoes, socks or jewellery. Measurements were taken with participants in a supine position with adhesive gel electrodes at defined sites on the dorsal surfaces of the hand, wrist, ankle, and foot. Maturity offset and age at peak height velocity (HPV) was determined for each individual through the Mirwald equation which included three somatic dimensions (height, sitting height, and leg length) and their interactions (21).
Cardiovascular assessment
Cardiovascular health was assessed through pulse wave analysis (PWA) and pulse wave velocity (PWV) using the SphygmoCor XCEL® (SphygmoCor XCEL, AtCor Medical, Naperville, IL, United States). After arriving at the laboratory, participants laid supine for five minutes in a dimly lit room before measurements of brachial systolic (BSBP) and diastolic blood pressure (BDBP) were taken. Brachial pulse pressures (BPP), mean brachial pressure (MBP), aortic pulse pressures (APP), mean aortic pressure (MAP), resting heart rate (RHR), HR period, augmentation index at 75 bpm (AIx75), ejection duration in milliseconds (EDms) and in percentage of the cardiac cycle (ED%), and subendocardial viability ratio (SEVR) were provided by the SphygmoCor XCEL software (22).
Carotid-femoral pulse velocity (cf-PWV) in m/s was assessed through applanation tonometry while the participant was laid in a supine position. The cf-PWV was calculated as the vascular distance divided by the pulse wave transit time (m/s). The vascular distance was determined by the SphygmoCor XCEL software using the distance between the carotid artery to the sternal notch, the sternal notch to the leg cuff, and the femoral artery to the leg cuff. Transit time was measured using volumetric displacement (femoral artery) and applanation tonometry (carotid artery) and distances were measured using a tape measure by a trained technician.
SphygmoCorXCEL showed a high intra-test (intraclass correlation = 0.996 and 0.983, p < 0.0001; cf-PWV and AIx, respectively) and a day to-day reliability (intraclass correlation = 0.979 and 0.939, p < 0.0001; cf-PWV and AIx, respectively). XCEL measures are valid, highly reliable and not affected by body side (23).
Cardiorespiratory fitness assessment
Cardiorespiratory fitness was assessed through a cardiopulmonary exercise test (CPET) on an electronically braked cycle ergometer (Monark 939 E, Monark Sport & Medical, Vansbro, Sweden) and by a calibrated respiratory gas analysis system (Quark CPET Cosmed, Rome, Italy). The exercise protocol included a 1-minute resting period followed by a15 or 20 Watts per minute graded exercise test starting at 0 Watts. The participants were asked to maintain a cadence of 65–70 rpm throughout the entire test. The 15 W/min incremental exercise protocol was used for the participants in the RR and ORS group while the 20 W/min protocol for CR. HR during the exercise test was continuously recorded using GARMIN HR chest belt (GARMIN, United States ) and the rate of perceived exertion was assessed using an OMNI scale 0–10 and recorded 15 s prior to the end of every stage. The test was terminated when one of the following criteria were met: the subject achieved volitional exhaustion or a cadence of 50 rpm, a value of 10 on an OMNI scale 0–10, the 90% of the predicted HRmax (beats/min) or a respiratory exchange ratio (RER) equal to 1.1. Peak Oxygen Uptake (VO2peak) was calculated as the 30-second average of the highest VO2 during the last minute of the exercise test before the test was terminated.
Quality control
The principal investigators completed a 3-month training period prior to the start of the project to ensure consistency with data collection and data reduction. When recording cardiovascular parameters, only PWA recordings with a quality control index equal or greater than 90% were used for the statistical analysis. Each subject received a minimum of three PWA and PWV measures with 1-minute rest in between. The results of two PWA measures reporting the lowest and closest (within ± 5 mmHg) BSBP and BDBP were averaged and used for the final analysis. The average of the two lowest and closest (within ± 0.3 m/s) cf-PWV measurements were also used (22, 24).
Prior to each CPET, a turbine calibration (using a 3-L syringe), a two-point gas calibration (16.00% and 20.93% O2; 5.0% and 0.04% CO2), a CO2 scrubber calibration (0.00% CO2), and a delay calibration were performed on the metabolic cart according to the manufacturer's recommendation. From the CPET, the raw breath-by-breath data from each test were reduced using a 3-breaths moving average (smoothing) and a 10 s time average. Then, data were imported on a shared separate Excel file for further analysis.
Statistical analysis
All results were expressed as mean ± standard deviation. The differences in anthropometric, cardiovascular and fitness parameters among groups were tested using Univariate Analysis of Variance (ANOVA). Differences in AIx75 among the three groups were assessed by a covariance analysis (ANCOVA) model that included height as covariate. These analyses were followed by post-hoc analysis (Bonferroni adjustment) when significant main effects were observed. Effect size was calculated using Cohen's definition of small, medium, and large effect size (as partial η2 = 0.01, 0.06, 0.14) (25). Statistical significance was set at p ≤ 0.05 and all analyses were performed using IBM SPSS statistics version 25.
Results
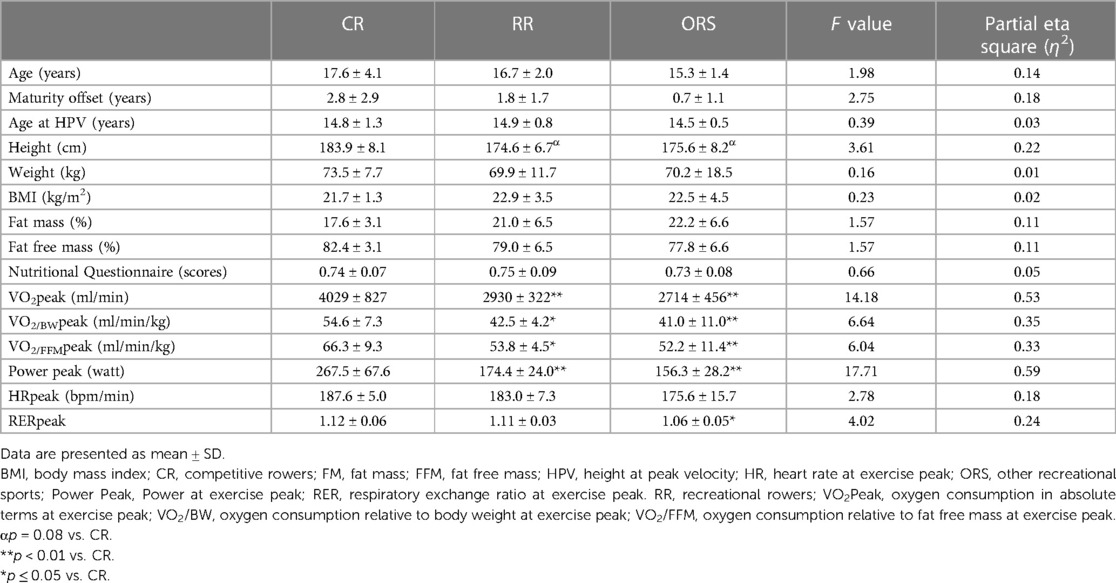
Characteristics of the three groups are shown in Table 1. No significant differences between groups were observed in age, maturity offset, age at HPV, weight, BMI and body composition. However, CR showed a marginal higher height compared to RR and ORS (p = 0.08). CR showed higher absolute and relative VO2peak, and power at peak compared to RR and ORS.
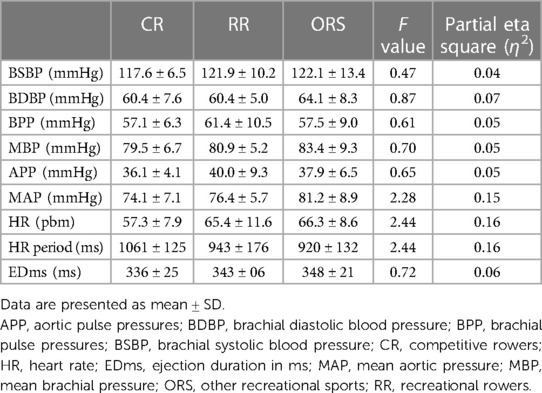
Compared to ORS, CR reported lower ED% (F2,25 = 4.24, p = 0.026, η2 = 0.25; 38.4 ± 4.8% and 31.9 ± 3.9%, respectively) (Figure 1), and higher PWV (F2,25 = 3.96, p = 0.032, η2 = 0.24; 4.7 ± 0.5 m/s and 5.5 ± 1.0 m/s, respectively) (Figure 3). Moreover, CR also reported higher SEVR compared to RR and ORS (F2,25 = 4.47, p = 0.022, η2 = 0.26; 165.8 ± 33.7%, 127.4 ± 30.4% and 128.3 ± 27.8%, respectively) (Figure 2) and lower AIx75 (F2,24 = 9.13, p = 0.001, η2 = 0.43; −15.7 ± 8.6%, 1.2 ± 9.9% and 1.5 ± 9.1, respectively) (Figure 4). No significant differences between groups were observed for brachial and aortic blood pressure, RHR, HR period and EDms (Table 2).
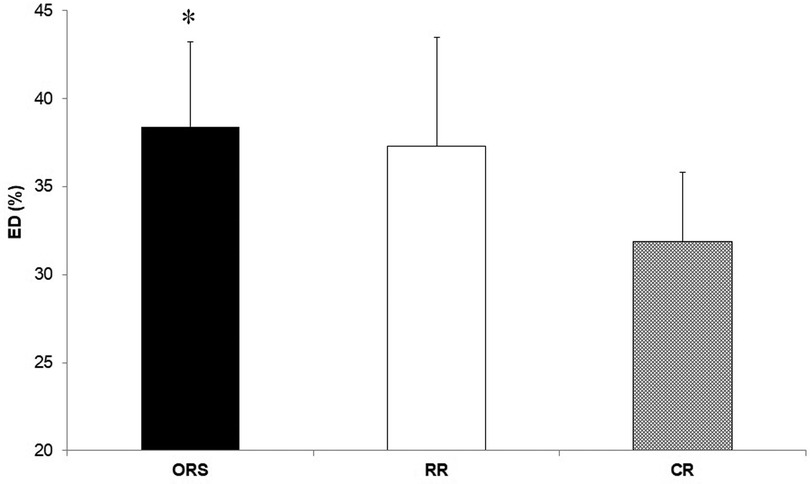
Figure 1. Percentage of ejection duration in ORS, RR and CR groups. *p < 0.05 vs. CR. CR, competitive rowers; ED, ejection duration; ORS, other recreational sports; RR, recreational rowers.
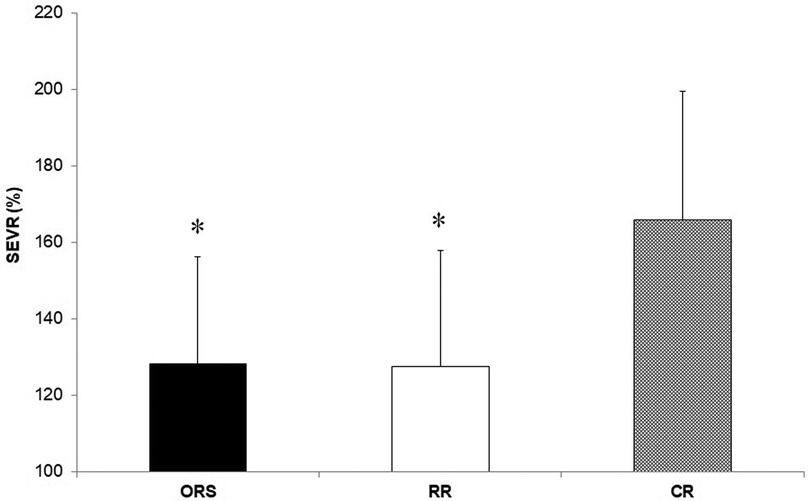
Figure 2. Subendocardial viability ratio values in ORS, RR and CR groups. *p ≤ 0.05 vs. CR. CR, competitive rowers; ORS, other recreational sports; RR, recreational rowers; SEVR, subendocardial viability ratio.
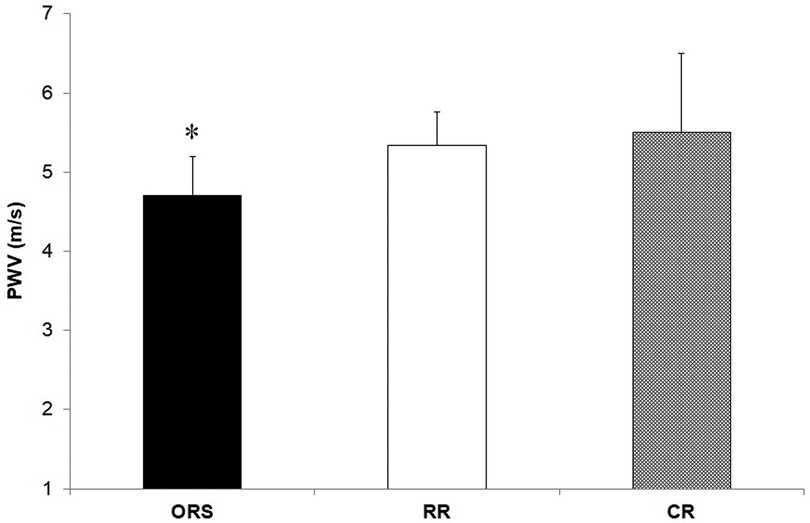
Figure 3. Pulse wave velocity in ORS, RR and CR groups. *p = 0.05 vs. CR. CR, competitive rowers; ORS, other recreational sports; RR, recreational rowers; PWV, pulse wave velocity.
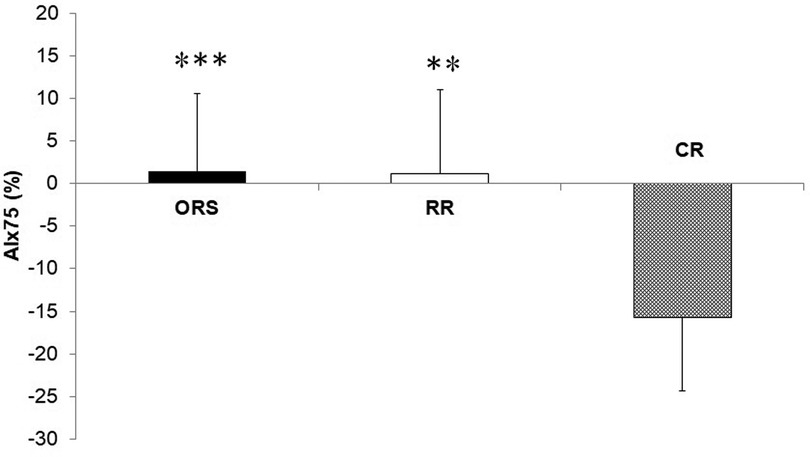
Figure 4. Augmentation index corrected to a heart rate of 75 bpm in ORS, RR and CR groups. ***p < 0.001 vs. CR. **p < 0.01 vs. CR. AIx, Augmentation index; CR, competitive rowers; ORS, other recreational sports; RR, recreational rowers.
Discussion
In this study, young competitive male rowers reported greater myocardial performance (ED%), higher cardiorespiratory fitness (VO2peak), and better indices of cardiovascular health (AIx, cf-PWV, and SEVR) when compared to recreational athletes. To our knowledge, this is the first study that investigated differences in cardiovascular functions between young recreational and competitive male rowers using non-invasive and indirect cardiovascular assessment methods.
The higher cardiorespiratory fitness in CR was expected since physiological attributes of professional rowers are among the highest recorded for any discipline (1, 2, 26). However, CR also showed a lower ED% compared to ORS (Figure 1) suggesting that continuous high-intensity exercise might result in positive myocardial adaptations in young athletes that are observable in resting conditions. Previous research showed that ejection duration is associated with high left ventricular wall thickness and cavity dimensions (13) and that greater left ventricular remodelling can occur in male adolescent rowers compared to controls (4). In addition, we found that competitive rowers showed higher SEVR compared with the two recreational groups (Figure 2) which signifies that rowing may improve the myocardial oxygen supply-demand ratio positively affecting cardiovascular health (27, 28).
Despite a large number of studies in adults, the role of training on the arteries of young rowers is still not clear (29, 30). Our results showed that PWV was higher in competitive rowers compared to recreationally trained athletes (Figure 3) and no differences in blood pressure between groups (Table 2). In clinical research, high PWV signifies higher arterial stiffness, and it is commonly associated with elevated blood pressure and RHR (31, 32). In the present study, competitive rowers showed higher PWV but lower blood pressure and HR compared to recreational athletes. This result may be explained by an improved myocardial performance, which results in a more powerful ventricular systole, rather than an increased arterial stiffness as shown by previous research conducted on highly trained individuals with enlarged heart volume and increased myocardial mass (4, 13, 33). Moreover, previous research has shown that the left ventricular ejection time can represent a potential determinant of PWV in young healthy males (34). According to these findings, the interpretation of PWV in healthy and athletic individuals should be done in conjunction with blood pressure, HR, and ED. However, more research on this topic is necessary as professional adults athletes seem not to report differences in PWV when compared to a control group (8, 16).
Another indicator of arterial stiffness that should be studied together with PWV is augmentation index (35, 36). AIx indicates the increase in aortic systolic pressure resulting from the reflected wave generated in the periphery (35, 37). In fact, competitive rowers in our study reported lower AIx75, an consequently lower arterial stiffness, compared to recreational athletes (Figure 4). These findings are in accordance with research conducted in adults which reports a significant increase in peripheral artery diameters and baroreflex sensitivity, and a decrease in vascular peripheral resistance in rowers compared to a non-athletic group (5, 7, 8). However, another study reported higher AIx in adult rowers compared to a control group underlining the need of additional investigations in this specific topic (16).
Our results also showed that the sport of rowing, when performed at the recreational level, may induce cardiovascular adaptations in adolescents that are similar to those caused by other sports. This finding is different from the results of previous research conducted on recreational adult rowers (6, 12, 14). This difference might be explained by the fact that the long-term adaptations occurring in adult rowers after years of training may not manifest in adolescent recreational athletes. However, the exercise intensity and volume used by young competitive rowers may affect cardiac functions as shown by the higher SEVR compared to their recreational counterpart even without changes in ED%. These findings are different from research in adults showing enhanced systolic and diastolic function in competitive rowers compared to their recreational counterparts (12).
In the present study some limitations need to be considered. Firstly, the relatively small sample size and the absence of a group composed by competitive athletes from other sport disciplines and of a control sedentary group. Secondly, it was possible to investigate only cardiac and vascular functioning, therefore next investigations should also assess the morphological structure of the cardiovascular system. Furthermore, future studies should use a longitudinal design to assess the course of rowers' cardiovascular adaptations from childhood to adulthood.
Conclusions
To conclude, young competitive male rowers showed lower arterial stiffness and enhanced left ventricular performance compared to recreationally trained individuals. Moreover, PWV in healthy, competitive athletes should be interpreted differently from the one of general population and together with other indices of cardiovascular health as blood pressure, RHR and ejection duration.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by CAR-IRB—University of Rome “Foro Italico” Committee (Approval No CAR 37/2020). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
FL, GMC and MM conceived the study's design. FL and MM performed the data analysis, interpreted the results, and wrote the manuscript. GMC, GL and BC contributed to the development of the study design, and revised the final version of the manuscript. FL, CL, MC and FD conducted the data collection. LG, approved the statistical analysis, and the interpretation of the results. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank all the coaches and administrators of the sport club “Circolo Canottieri Tirrenia Todaro” because this work could not have been completed without their help. Most of all, we thank the athletes and families for their participation and co-operation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIx, Augmentation index; APP, aortic pulse pressures; BDBP, brachial diastolic blood pressure; BMI, body mass index; BPP, brachial pulse pressures; BSBP, brachial systolic blood pressure; CR, competitive rowers; HPV, height at peak velocity; HR, heart rate; EDms, ejection duration in ms; FM, fat mass; FFM, fat free mass; MAP, mean aortic pressure; MBP, mean brachial pressure; ORS, other recreational sports; RER, respiratory exchange ratio; RHR, resting heart rate; RR, recreational rowers; PWV, pulse wave velocity; SEVR, subendocardial viability ratio; VO2 Peak, oxygen consumption in absolute terms at exercise peak.
References
1. Secher NH. Physiological and biomechanical aspects of rowing: implications for training. Sports Med. (1993) 15(1):24–42. doi: 10.2165/00007256-199315010-00004
2. Hagerman FC, Hagerman GR, Mickelson TC. Physiological profiles of elite rowers. Phys Sportsmed. (1979) 7(7):74–83. doi: 10.1080/00913847.1979.11948457
3. Spirito P, Pelliccia A, Proschan MA, Granata M, Spataro A, Bellone P, et al. Morphology of the “athlete's Heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol. (1994) 74(8):802–6. doi: 10.1016/0002-9149(94)90439-1
4. Sharma S, Maron BJ, Whyte G, Firoozi S, Elliott PM, McKenna WJ. Physiologic limits of left ventricular hypertrophy in elite junior athletes. J Am Coll Cardiol. (2002) 40(8):1431–6. doi: 10.1016/S0735-1097(02)02270-2
5. Kowalik T, Klawe JJ, Tafil-Klawe M, Słomko W, Słomko J, Srokowska A, et al. Multiannual, intensive strength-endurance training modulates the activity of the cardiovascular and autonomic nervous system among rowers of the international level. BioMed Res Int. (2019) 2019:1–6. doi: 10.1155/2019/3989304
6. Cook JN, DeVan AE, Schleifer JL, Anton MM, Cortez-Cooper MY, Tanaka H. Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol. (2006) 290:5. doi: 10.1152/ajpheart.01054.2005
7. Naylor LH, O’Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exerc. (2006) 38(1):86–92. doi: 10.1249/01.mss.0000181220.03855.1c
8. Petersen SE, Wiesmann F, Hudsmith LE, Robson MD, Francis JM, Selvanayagam JB, et al. Functional and structural vascular remodeling in elite rowers assessed by cardiovascular magnetic resonance. J Am Coll Cardiol. (2006) 48(4):790–7. doi: 10.1016/j.jacc.2006.04.078
9. Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. (1999) 130(1):23–31. doi: 10.7326/0003-4819-130-1-199901050-00005
10. Finocchiaro G, Dhutia H, D’Silva A, Malhotra A, Steriotis A, Millar L, et al. Effect of sex and sporting discipline on LV adaptation to exercise. JACC Cardiovasc Imaging. (2017) 10(9):965–72. doi: 10.1016/j.jcmg.2016.08.011
11. Wasfy MM, Weiner RB. Differentiating the athlete’s heart from hypertrophic cardiomyopathy. Curr Opin Cardiol. (2015) 30(5):500–5. doi: 10.1097/HCO.0000000000000203
12. Baggish AL, Yared K, Weiner RB, Wang F, Demes R, Picard MH, et al. Differences in cardiac parameters among elite rowers and subelite rowers. Med Sci Sports Exerc. (2010) 42(6):1215–20. doi: 10.1249/MSS.0b013e3181c81604
13. Caselli S, Di Pietro R, Di Paolo FM, Pisicchio C, di Giacinto B, Guerra E, et al. Left ventricular systolic performance is improved in elite athletes. Eur J Echocardiogr. (2011) 12(7):514–9. doi: 10.1093/ejechocard/jer071
14. Sanada K, Miyachi M, Tabata I, Suzuki K, Yamamoto K, Kawano H, et al. Differences in body composition and risk of lifestyle-related diseases between young and older male rowers and sedentary controls. J Sports Sci. (2009) 27(10):1027–34. doi: 10.1080/02640410903081852
15. Kawano H, Iemitsu M, Gando Y, Ishijima T, Asaka M, Aoyama T, et al. Habitual rowing exercise is associated with high physical fitness without affecting arterial stiffness in older men. J Sports Sci. (2012) 30(3):241–6. doi: 10.1080/02640414.2011.635311
16. Franzen K, Reppel M, Köster J, Mortensen K. Acute and chronic effects on central hemodynamics and arterial stiffness in professional rowers. Physiol Meas. (2016) 37(4):544–53. doi: 10.1088/0967-3334/37/4/544
17. Churchill TW. The impact of exercise and athletic training on vascular structure and function. Curr Treat Options Cardiovasc Med. (2020) 22(12):59. doi: 10.1007/s11936-020-00861-7
18. Mikulić P, Ružić L. Predicting the 1000 m rowing ergometer performance in 12–13-year-old rowers: the basis for selection process? J Sci Med Sport. (2008) 11(2):218–26. doi: 10.1016/j.jsams.2007.01.008
19. Arne G, Stephen S, Eike E. Training methods and intensity distribution of young world-class rowers. Int J Sports Physiol Perform. (2009) 4(4):448–60. doi: 10.1123/ijspp.4.4.448
20. Grad FP. The preamble of the constitution of the world health organization. Bull World Health Organ. (2002) 80:981–4. PMID:12571728
21. Mirwald RL, Baxter-Jones ADG, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. (2002) 34(4):689–94. doi: 10.1097/00005768-200204000-00020
22. Butlin M, Qasem A. Large artery stiffness assessment using SphygmoCor technology. Pulse. (2016) 4(4):180–92. doi: 10.1159/000452448
23. Hwang MH, Yoo JK, Kim HK, Hwang CL, Mackay K, Hemstreet O, et al. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens. (2014) 28(8):475–81. doi: 10.1038/jhh.2013.144
24. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals. Hypertension. (2005) 45(1):142–61. doi: 10.1161/01.HYP.0000150859.47929.8e
25. Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Lawrence Erlbaum Associates (1988).
26. Cosgrove MJ, Wilson J, Watt D, Grant SF. The relationship between selected physiological variables of rowers and rowing performance as determined by a 2000 m ergometer test. J Sports Sci. (1999) 17(11):845–52. doi: 10.1080/026404199365407
27. Buckberg GD, Fixler DE, Archie JP, Hoffman JIE. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res. (1972) 30(1):67–81. doi: 10.1161/01.RES.30.1.67
28. Tocci ND, Collier SR, Meucci M. Measures of ejection duration and subendocardial viability ratio in normal weight and overweight adolescent children. Physiol Rep. (2021) 9(9) (Cited November 5, 2021). doi: 10.14814/phy2.14852
29. Baumgartner L, Weberruß H, Oberhoffer-Fritz R, Schulz T. Vascular structure and function in children and adolescents: what impact do physical activity, health-related physical fitness, and exercise have? Front Pediatr. (2020) 8:103. doi: 10.3389/fped.2020.00103
30. Ketelhut S, Kirchenberger T, Ketelhut RG. Hemodynamics in young athletes following high-intensity interval or moderate-intensity continuous training. J Sports Med Phys Fitness. (2020) 60(9):1202–8. doi: 10.23736/S0022-4707.20.10814-4
31. Kim EJ, Park CG, Park JS, Suh SY, Choi CU, Kim JW, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens. (2007) 21(2):141–8. doi: 10.1038/sj.jhh.1002120
32. Albaladejo P, Asmar R, Safar M, Benetos A. Association between 24-hour ambulatory heart rate and arterial stiffness. J Hum Hypertens. (2000) 14(2):137–41. doi: 10.1038/sj.jhh.1000961
33. Salvi P, Palombo C, Salvi GM, Labat C, Parati G, Benetos A. Left ventricular ejection time, not heart rate, is an independent correlate of aortic pulse wave velocity. J Appl Physiol. (2013) 115(11):1610–7. doi: 10.1152/japplphysiol.00475.2013
34. Nurnberger J, Saez AO, Dammer S, Mitchell A, Wenzel RR, Philipp T, et al. Left ventricular ejection time: a potential determinant of pulse wave velocity in young, healthy males. J Hypertens. (2003) 21(11):2125–32. doi: 10.1097/00004872-200311000-00022
35. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. (2006) 27(21):2588–605. doi: 10.1093/eurheartj/ehl254
36. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension [published correction appears in Eur Heart J. 2019 Feb 1;40(5):475]. Eur Heart J. (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
Keywords: cardiovascular health, non-invasive measurements, rowing training, young athletes, rower athletes
Citation: Falcioni L, Gallotta MC, Baldari C, Cardinali L, Campanella M, Ferrari D, Guidetti L and Meucci M (2023) Influence of training status on cardiac and vascular functioning in young recreational and competitive male rowers. Front. Pediatr. 11:1162594. doi: 10.3389/fped.2023.1162594
Received: 9 February 2023; Accepted: 10 March 2023;
Published: 6 April 2023.
Edited by:
Stefanos Volianitis, Qatar University, QatarReviewed by:
Marios Hadjicharalambous, University of Nicosia, CyprusFrancesco Bianco, Azienda Ospedaliero Universitaria Ospedali Riuniti, Italy
© 2023 Falcioni, Gallotta, Baldari, Cardinali, Campanella, Ferrari, Guidetti and Meucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Meucci bWV1Y2NpbUBhcHBzdGF0ZS5lZHU=
†These authors have contributed equally to this work and share last authorship
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Lavinia Falcioni
Lavinia Falcioni Maria Chiara Gallotta
Maria Chiara Gallotta Carlo Baldari
Carlo Baldari Ludovica Cardinali1
Ludovica Cardinali1 Matteo Campanella
Matteo Campanella Dafne Ferrari
Dafne Ferrari Laura Guidetti
Laura Guidetti Marco Meucci
Marco Meucci