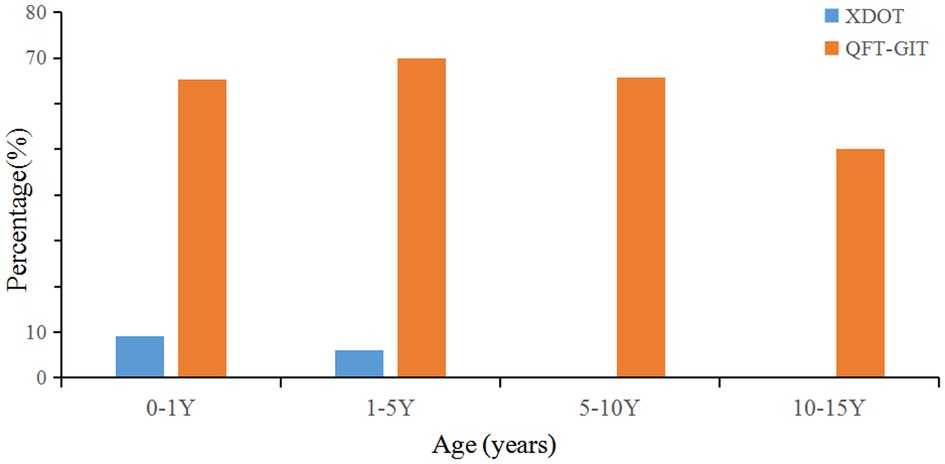- 1Laboratory of Respiratory Diseases, Beijing Key Laboratory of Pediatric Respiratory Infection Diseases, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, Key Laboratory of Major Diseases in Children, Ministry of Education, National Clinical Research Center for Respiratory Diseases, National Center for Children’s Health, Beijing, China
- 2Department of Cardiology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China,
Objective: We aimed to compare QuantiFERON-TB Gold In-Tube (QFT-GIT) and X.DOT-TB for screening latent tuberculosis infection (LTBI) in kawasaki patients, and to identify the risk factors associated with indeterminate IGRA results.
Methods: We conducted a retrospective study on children with KD, who were screened for mycobacterium tuberculosis (Mtb) infection by either ELISA-based QFT-GIT or ELISPOT-based X.DOT-TB tests, admitted in Department of Cardiology, Beijing Children's Hospital from July 2019 to April 2022.
Results: A total of 1327 cases were included. Among them, 932 cases were tested by QFT-GIT and 395 cases by X.DOT-TB. The positive rate of children was 0.1% and 0.2%, and the indeterminate rate was 68.2% and 6.1% for QFT-GIT and X.DOT-TB, respectively. Patients with hypoproteinemia had a higher risk of indeterminate X.DOT-TB result. Female, critical ill, shock or hypoproteinemia presented statistically significant associations with an increased risk of indeterminate QFT-GIT result. High-dose of IVIG inhibited the release of IFN-γ by more than 90%, which might account for the high indeterminate incidence.
Conclusion: It is recommended to perform X.DOT-TB rather than QFT-GIT to screen LTBI in patients with high level of the mitogen that can inhibit IFN-γ release. For KD children with positive IGRA results, it has a higher risk of activation TB infection when treated with immunosuppressive therapy in the future. Children with KD aged <5 years old had higher frequency of indeterminate IGRA results.
Introduction
Kawasaki disease (KD), an acute systemic vasculitis of unknown etiology, mainly involved in the middle and arterioles in children under 5 years old (1, 2). Current standard treatment includes intravenous immunoglobulin (IVIG), alongside aspirin (3, 4). Other medications include glucocorticoids (e.g., high-dose pulsed intravenous methylprednisolone), tumour necrosis factor (TNF)-alpha inhibitors (e.g., infliximab), interleukin (IL)-1 inhibitors, (e.g., anakinra), or ciclosporin, especially for IVIG-resistant cases (5–8).
According to world health organization (WHO), there was approximately 1.2 million TB cases in children in 2021 (9). Children and young adolescents (aged below 15 years) represent about 11% of all people with tuberculosis (TB) globally. This means that 1.1 million children become ill with TB every year, almost half of them below five years of age (10). According to a previous report in Beijing, the average annual TB morbidity was 31.67/10,000 (11). Although mycobacterium tuberculosis (Mtb) infected individuals have a possibility of 5%–10% to develop active TB, immunosuppressive therapy by glucocorticoids, TNF-alpha inhibitors, and some others, have been proved to increase the risk of active TB disease in latent tuberculosis infection (LTBI) individuals. Though being preventable and curable, TB remains one serious threat to children's health (12–14). Young children, whose immune system is still at the stage of development, are much more likely to develop severe or disseminated TB following infection, and the situation could be more complicated if it occurs in combination with basic diseases. So it is necessary and highly recommended to screen Mtb infection in KD children before immunosuppressive therapy.
The interferon-γ release assays (IGRAs) detects the production of interferon-gamma (IFN-γ) by effector T cells stimulated by Mtb-specific antigens, and are reliable immune tests for identification of Mtb infection. At present, there are two main kinds of IGRAs with different experimental process and interpretation of the results. One measures the concentration of IFN-γ via an Enzyme-linked Immunosorbent Assay (ELISA) and another measures the frequencies of IFN-γ-secreting cells via Enzyme-linked Immunospot Assay (ELISPOT) (15). In our hospital, two commercial kits of IGRAs were used clinically. Among them, QuantiFERON-TB Gold In-Tube (QFT-GIT) assay (QIAGEN, Australia) is ELISA based, while X.DOT-TB (TB Healthcare, Foshan, China) is ELISPOT based (16). Patients were routinely screened in our hospital by either IGRAs before immunosuppressive therapy.
We aimed to compare QFT-GIT and X.DOT-TB for screening LTBI in kawasaki patients admitted in Department of Cardiology, Beijing Children's Hospital from July 2019 to April 2022, and to identify the risk factors associated with indeterminate IGRA results.
Materials and methods
Ethic statements
This study was approved by the Ethics Committee of Beijing Children's Hospital, Capital Medical University.
Study population
We conducted a retrospective study on children aged ≤18 years with KD, who were screened for Mtb infection admitted and hospitalized in Department of Cardiology, Beijing Children's Hospital affiliated to Capital Medical University from July 2019 to April 2022.
Patient categories
The diagnostic criteria for Kawasaki disease is according to Japanese Guidelines for Kawasaki Disease (Sixth revision) (17). Here are six of its latest key clinical features: (1) Fever; (2) Bilateral bulbar conjunctival injection; (3) Changes of lips and oral cavity: reddening of lips, strawberry tongue, diffuse injection of oral and pharyngeal mucosa; (4) Rash [including redness at the site of Bacille Calmette Guerin (BCG) inoculation]; (5) Changes of peripheral extremities: (Initial stage) reddening of palms and soles, edema. (Convalescent stage) periungual desquamation; (6) Non-supparative cervical lymphadenopathy.
Complete KD (Classic KD) subgroup: (1) Fulfill five or six of the above signs is diagnosed with complete KD; (2) Fulfill four of the above signs and coronary artery abnormality by echocardiography is diagnosed with complete KD.
Incomplete KD (Atypical KD) subgroup: KD is classified as incomplete (atypical) when the symptoms and signs of the child satisfy some but not all diagnostic criteria for complete KD (18). When the patients who fulfill three or four signs in the principle clinical features without coronary artery dilation but with some features from the list of “Other significant clinical features can be diagnosed as incomplete KD, if other diseases are ruled out; 2) Incomplete KD may also be considered in the presence of only one or two principal clinical features after excluding other diagnoses (17).”
Refractory KD (IVIG-resistant) subgroup: Refractory Kawasaki disease diagnosed when fever persists or recurs of any magnitude 24–36 h after completion of IVIG therapy (18, 19).
Age groups: According to the who 2022 Guidelines for the Management of tuberculosis in Children and Adolescents, the children were divided into infants (less than 1 year old), younger children (less than 5 years old), children (less than 10 years old), and younger adolescents (10–15 years old) (10).
Interferon-gamma release assays
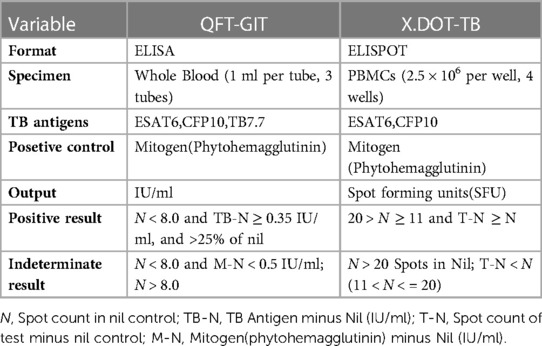
X.DOT-TB: X.DOT-TB is a commercial kit of ELISPOT-based IGRA. Results are reported as the number of IFN-γ-producing T cells (spot-forming cells). Peripheral blood mononuclear cells (PBMCs) obtained from each subject within 4 h of collection were seeded (2.5 × 106 cell/ml) on a plate precoated with the antibody against IFN-γ. A nil control well, a positive control well with mitogen (phytohemagglutinin), and a TB antigen well (containing ESAT-6, CFP-10) were needed for each sample. Plates were incubated for 20–22 h at 37°C in 5% carbon dioxide. After incubation, wells were developed with a conjugate against the antibody used and an enzyme-substrate. Spot-forming cells (SFCs) were counted with an automated ELISpot reader (AID-ispot, Strassberg, Germany). An individual was considered positive for Mtb infection if the spot count in the TB antigen well exceeded a specific threshold relative to the negative control well. The results were interpreted in Table 1.
QFT-GIT: According to the manufacturer's instructions, 1 ml of whole blood was collected into each of the three separate test tubes, including a nil control tube, a positive control tube with phytohemagglutinin, and a TB antigen tube (containing ESAT-6, CFP-10 and TB7.7), followed by incubated for 16–24 h at 37°C. Then the tubes were centrifuged and the supernatant were collected to assess the concentration of IFN-γ (IU/ml) via ELISA. The results were interpreted in Table 1.
Determination of IgG concentrations in plasma
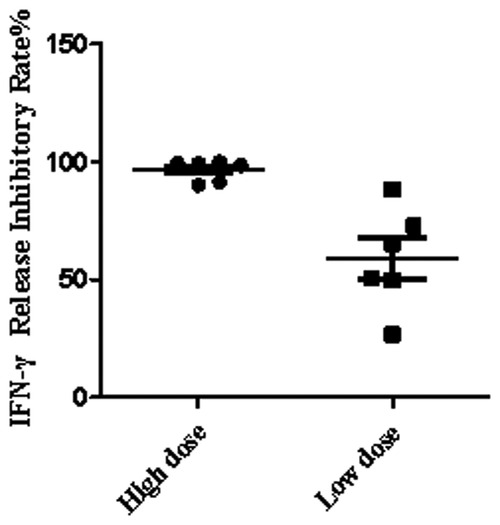
Concentrations of IgG were confirmed using the commercial kit of Tina-quant IgG Gen.2 from Beckman Coulter (USA) Co., Ltd, following the respective manufacturer's instructions. Each blood sample was divided into two parts, one with IVIG and the other with the same volume of saline, and then tested simultaneously. The experiment was divided into high and low dose groups according to the different concentrations of IVIG. The final concentration of IVIG was 15 mg/ml in the high dose group and 5 mg/ml in the low dose group [15.2 ± 1.3 mg/ml (mean ± SD) for high dose; 5.1 ± 1.0 mg/ml for low dose by ELISA determinant].
Statistical analysis
Categorical variables were presented as percentages, while continuous variables were presented as means and standard deviations. P-values <0.05 were considered statistically significant. Multivariable models were built using “Enter” logistic regression procedures. Data analyses were conducted using SPSS version 23.0.
Results
Characteristics of enrolled patients
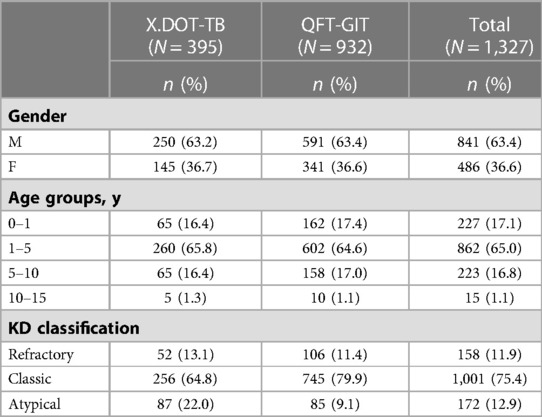
A total of 1,327 hospitalized children with KD, who were screened for Mtb infection by either of IGRAs at admission, were included in this study. Among them, 932 cases were tested by QFT-GIT and 395 cases were tested by X.DOT-TB, respectively. QFT-GIT group includes 591 males and 341 females, while X.DOT-TB group has 250 males and 145 females. Children aged 1 to 5 years (65.0%) and with classic KD (75.4%) were more common in our subjects. Details were presented in Table 2.
Overall results of IGRA assays
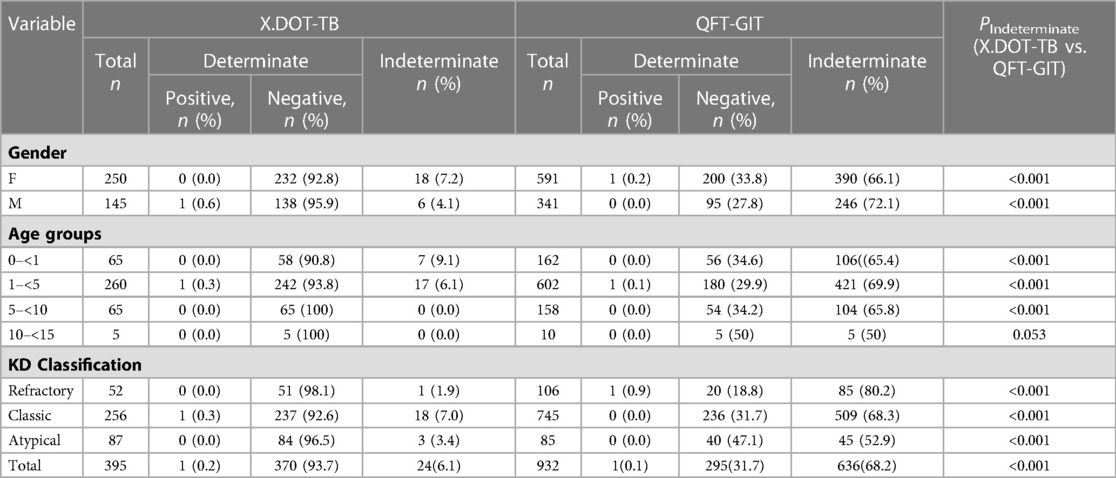
The positive rate of Mtb infection for children with KD was 0.1% detected by QFT-GIT and 0.2% by X.DOT-TB respectively. And the indeterminate rate of QFT-GIT and X.DOT-TB was 68.2% and 6.1% respectively (Table 3). The proportion of QFT-GIT indeterminate results in children aged 1 to 5 years (69.9%) was greater than that in other age groups. And the indeterminate results of X.DOT-TB for children under 1 year (9.1%) was greater than that in other age groups. A comparison of X.DOT-TB vs. QFT-GIT indeterminate results by age groups can be found in Figure 1. The proportion of QFT-GIT indeterminate results was much higher than that of X.DOT-TB results across age groups (P < 0.001).
Risk factors for an indeterminate IGRA result
Univariable logistic regression analysis revealed that children with hypoproteinemia were more likely to have an indeterminate X.DOT-TB result (16. 0%) than that without hypoproteinemia (4. 8%) (P = 0. 02). Multivariate logistic modelling analysis showed that children with hypoproteinemia had a higher risk of indeterminate X.DOT-TB result [adjusted odds ratio (aOR) 8. 90, 95% CI 3. 03–26. 11] (Table 4).
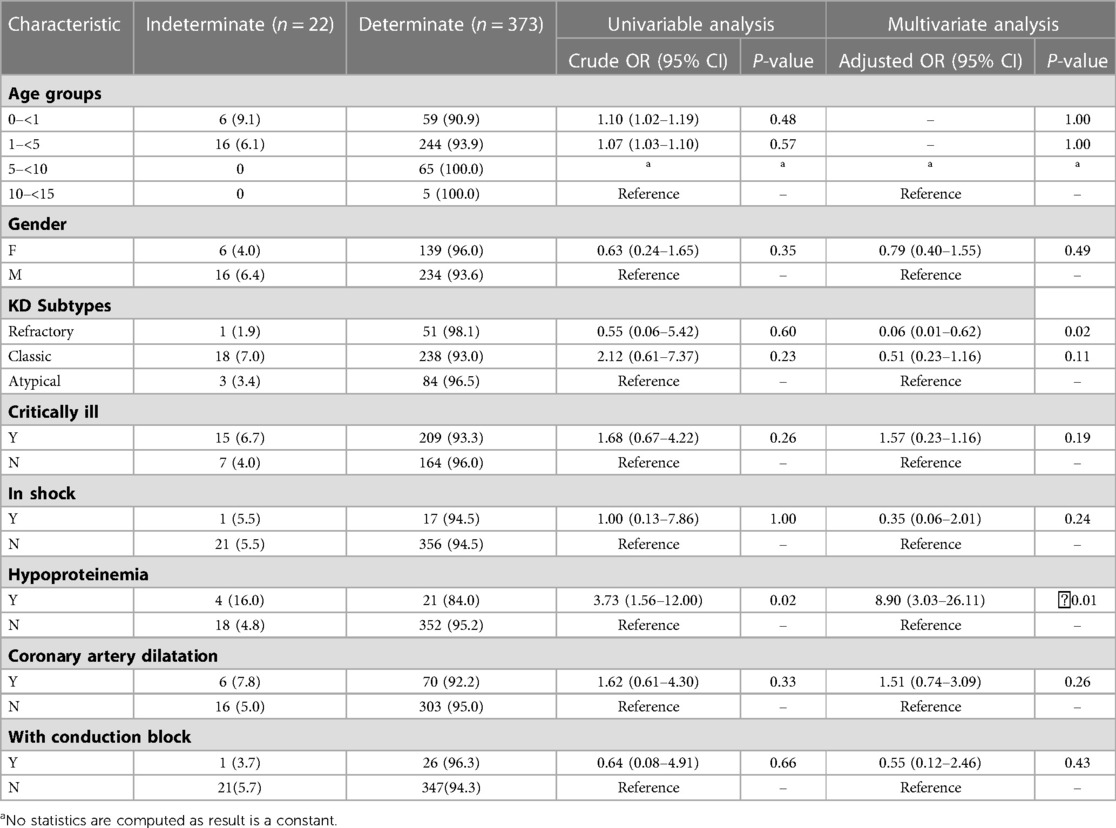
Table 4. Multivariate analysis of risk factors associated with indeterminate X.DOT-TB results in children with KD.
For QFT-GIT, using children with atypical KD as a control group, univariable logistic regression analysis revealed that children with refractory KD and classic KD compared with atypical KD, were more likely to have an indeterminate result (80. 2%, 68. 3%, and 52. 9%, respectively). Besides, children with shock or hypoproteinemia had significantly higher odds of having an indeterminate result than that without shock or hypoproteinemia, respectively. Among the risk factors analyzed through logistic regression, female, children with critical ill, shock or hypoproteinemia presented a statistically significant association with an increased risk of obtaining an indeterminate QFT-GIT result (Table 5).
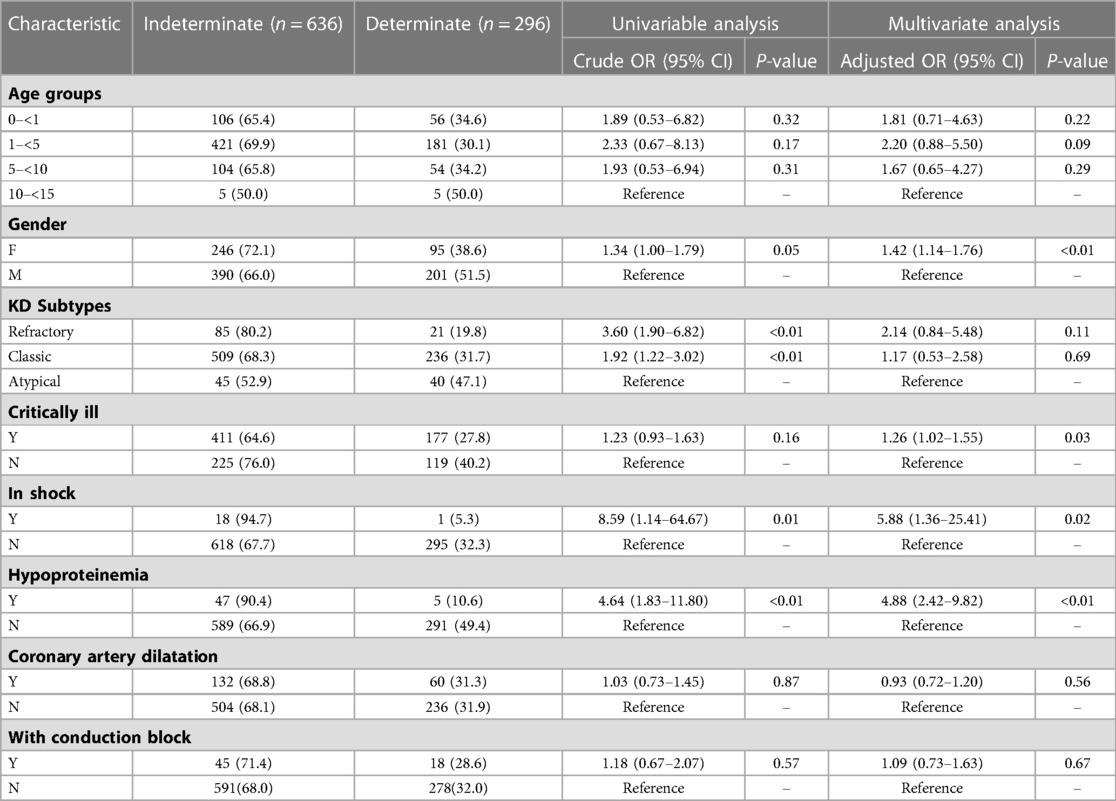
Table 5. Multivariate analysis of risk factors associated with an indeterminate QFT-GIT result in children with KD.
KD classification does not present a statistically significant P-value in multivariate logistic modelling to be associated with the incidence of an indeterminate QFT-GIT result, which may because the disease classification could be somewhat symptoms dependent, but an eighty percent indeterminate rate in refractory KD subgroups is really high.
IVIG concentration in blood and the release of IFN-γ
IVIG is one of the first-line KD therapeutic drugs. It could be given during emergency treatment before hospitalization or in primary hospitals before referral to our hospital. Cases being less response 24–36 h after completion IVIG therapy would be diagnosed as Refractory KD. KD patients, especially those with refractory KD, presented a high indeterminate rate being less responsive to mitogen stimulation in blood by QFT-GIT, but not X.DOT-TB. We then measured the inhibitory effect of IVIG on Mitogen induced IFN-γ release. Compared to group without IVIG, the group added with high-dose IVIG inhibited IFN-γ secretion by more than 90% on average, but only about 50% in the group added with low-dose IVIG (Figure 2).
Discussion
Screening for Mtb infection before immune-modulatory treatment is required for many diseases in hospital. A latent Mtb infection without symptoms could progress into active TB disease when the immune system being suppressed. ELISA-based QFT-GIT and ELISPOT-based X.DOT-TB are two types of IGRAs used in our hospital for distinguishing Mtb infected children. QFT-GIT seems to be more convenient and easier to perform than X.DOT-TB, as it uses whole blood rather than mononuclear cells for T cell stimulating (20). The positive rate of children detected by QFT-GIT and X.DOT-TB was not high according to our study, However, in this study, we found that the proportion of indeterminate IGRA results in KD by QFT-GIT was more than ten times higher than that by X.DOT-TB. We noticed all the indeterminate cases by QFT-GIT in this study were because of their response-less to mitogen treatment. QFT-GIT stimulates T cells in the whole blood, while X.DOT-TB stimulates T lymphocytes in extracted PBMCs by maintaining them in culture medium. Thus, increased indeterminate IGRA results by QFT-GIT means there could be certain components in the blood of KD patients that inhibit the stimulated release of IFN-γ. As IVIG is one of standard therapeutic drugs and most of the children especially refractory KD patients received IVIG therapy, we hypothesized that IVIG might influence IFN-γ release directly.
By adding IVIG to whole blood in vitro, we found that the presence of IVIG could directly inhibit the release of IFN-γ by mitogen stimulated T cells in the whole blood, and the effect is somewhat dose dependent. IFN-γ secretion were half reduced with low concentrations of IVIG, and sharply reduced with high-IVIG concentrations. The main principle of QFT-GIT assay is to detect the release level of IFN-γ in blood, whereas X.DOT-TB detects the mononuclear cells without plasma, which is not affected by increased IVIG content in whole blood. Thus, X.DOT-TB could be more suitable for children under IVIG treatment. As IVIG has been widely used in perinatal and neonatal periods in recent years (21, 22), attention should also be paid to children with similar situations. We suggest that a replacement of cell culture median for plasma might be helpful to improve the performance of ELISA-based IGRA method in children under IVIG treatment.
Due to immature immune function and limited compensatory ability, most children become seriously ill, develop rapidly and are prone to complications. In children with shock or hypoproteinemia, the indeterminate rate of QFT-GIT is more than 90%, so QFT-GIT is not recommended for Mtb infection screening when these conditions occur. In the case of hypoproteinemia, the indeterminate rate of X.DOT-TB was also elevated, reaching 16%. In this case, symptoms should be prioritized, and TB screening should be performed again when the signs are stable. The test results will be more accurate. Hypoproteinemia is associated with a higher risk for an indeterminate IGRA result by X.DOT-TB, while children with critical ill, shock or hypoproteinemia presented a statistically significant association with an increased risk of obtaining an indeterminate IGRA result by QFT-GIT. Albumin is a well-known negative acute phase reactant because its level decreases with inflammation, in which various relevant cytokines, such as IL-1, IL-6, and TNF-α, suppress the synthesis of albumin (23). Since our study did not test children with hypoalbuminemia, it only extrapolated from case statistics. Therefore, further research is needed to elucidate the mechanisms underlying the effects of low albumin levels on IGRA testing (24).
Conclusions
It is recommended to perform X.DOT-TB rather than QFT-GIT to screen LTBI in patients with high level of the mitogen that can inhibit IFN-γ release. For KD children with positive IGRA results, it has a higher risk of activation TB infection when treated with immunosuppressive therapy in the future. Children with KD aged <5 years old had higher frequency of indeterminate IGRA results.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of the Beijing Children's Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC, FL, JX, YY and CS contributed in study design, data collection, and analysis. HC and HZ conducted in manuscript writing. YG, YW, and LC conducted laboratory testing; SC revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Beijing Natural Science Foundation (7224328, 702043), high-level public health technical personnel construction project training plan (subject backbone-02-33).
Acknowledgments
The authors acknowledgement the staff at Laboratory of Respiratory Diseases, Beijing Key Laboratory of Pediatric Respiratory Infection Diseases, Beijing Pediatric Research Institute, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Allergy. (1967) 16(3):178–222. doi: 10.1126/science.113.2937.418-b
2. Marchesi A, Rigante D, Cimaz R, Ravelli A, Jacobis ITD, Rimini A, et al. Revised recommendations of the Italian Society of Pediatrics about the general management of Kawasaki disease. Ital J Pediatr. (2021) 47(1):16. doi: 10.1186/s13052-021-00962-4
3. Gorelik M, Chung SA, Ardalan K, Binstadt BA, Friedman K, Hayward K, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. (1984) 2(8411):1055–8. doi: 10.1016/s0140-6736(84)91504-6
4. Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. (1984) 2(8411):1055–8. doi: 10.1016/S0140-6736(84)91504-6
5. Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. (2006) 55(1):19–26. doi: 10.1002/art.21705
6. Shanxi Provincial Diagnosis and Treatment Center of Kawasaki Disease/Children’s Hospital of Shaanxi Provincial People’s Hospital, Beijing Children’s Hospital, Capital Medical University, Shanghai Children's Medical Center, Children's Hospital of Shanghai Jiao Tong University, Xianyang Children's Hospital of Shaanxi Province, et al. Pediatric expert consensus on the application of glucocorticoids in Kawasaki disease. Chinese J Contemp Pediatr. (2022) 24(3):225–31. doi: 10.7499/j.issn.1008-8830.2112033
7. Burns JC, Roberts SC, Tremoulet AH, He F, Printz BF, Ashouri N, et al. Infliximab versus second intravenous immunoglobulin for treatment of resistant Kawasaki disease in the USA (KIDCARE): a randomised, multicentre comparative effectiveness trial. Lancet Child Adolesc Health. (2021) 5(12):852–61. doi: 10.1016/S2352-4642(21)00270-4
8. Renton WD, Ramanan AV. Strengthening the case for primary adjunctive corticosteroids for Kawasaki disease. Arch Dis Child. (2021) 106(3):209–10. doi: 10.1136/archdischild-2020-320642
9. Sanjeet B. WHO’s global Tuberculosis report 2022. Lancet Microbe. (2023) 4(1):e20. doi: 10.1016/S2666-5247(22)00359-7
10. WHO consolidated guidelines on tuberculosis: Module 5: Management of tuberculosis in children and adolescents. Geneva: World Health Organization; (2022).
11. Wu F, Lai CY, Wang Y, Zhang GQ, Li YQ, Yu SS, et al. Tuberculosis infection and epidemiological characteristics in Haidian District, Beijing, 2005–2018. BMC Public Health. (2020) 20(1):823. doi: 10.1186/s12889-020-08773-8
12. Marais BJ, Obihara CC, Warren RM, Schaaf HS, Gie RP, Donald PR. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis. (2005) 9(12):1305–13. PMID: 16466051
13. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. (2014) 2(8):e453–9. doi: 10.1016/S2214-109X(14)70245-1
14. Mandal N, Anand PK, Gautam S, Das S, Hussain T. Diagnosis and treatment of paediatric tuberculosis: an insight review. Crit Rev Microbiol. (2017) 43(4):466–80. doi: 10.1080/1040841X.2016.1262813
15. Feng Y, Diao N, Shao LY, Wu J, Zhang S, Jin JL, et al. Interferon-gamma release assay performance in pulmonary and extrapulmonary tuberculosis. PLoS ONE. (2012) 7(3):e32652. doi: 10.1371/journal.pone.0032652
16. Pai M, Behr M. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr. (2016) 4(5):TBTB2-0023-2016. doi: 10.1128/microbiolspec.TBTB2-0023-2016
17. Kobayashi T, Ayusawa M, Suzuki H, Abe J, Ito S, Kato T, et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr Int. (2020) 62(10):1135–8. doi: 10.1111/ped.14326
18. Abraham D, Kalyanasundaram S, Krishnamurthy K. Refractory Kawasaki disease a challenge for the pediatrician. SN Compr Clin Med. (2021) 3(3):855–60. doi: 10.1007/s42399-021-00775-w
19. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135(17):e927–99. doi: 10.1161/CIR.0000000000000484
20. Wang L, Tian X, Yu Y, Chen W. Evaluation of the performance of two tuberculosis interferon gamma release assays (IGRA-ELISA and T-SPOT. TB) for diagnosing Mycobacterium tuberculosis infection. Data Brief. (2018) 21:2492–5. doi: 10.1016/j.dib.2018.08.112
21. Qian JH, Zhu JX, Wang MH, Wu SM, Chen TX. Suppressive effects of intravenous immunoglobulin (IVIG) on human umbilical cord blood immune cells. Pediat Allerg Imm. (2011) 22(2):211–20. doi: 10.1111/j.1399-3038.2010.01049.x
22. Sewell WAC, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology. (2002) 107(4):387–93. doi: 10.1046/j.1365-2567.2002.01545.x
23. Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. (1987) 79(6):1635–41. doi: 10.1172/JCI113000
Keywords: interferon-gamma release assays, children, Kawasaki disease, tuberculosis, immunosuppressive therapy
Citation: Chen H, Zheng H, Cui L, Xiao J, Li F, Wang Y, Guo Y, Chen Y, Yuan Y and Shen C (2023) Performance of two interferon-gamma release assays for tuberculosis infection screening in Kawasaki children before immunosuppressive therapy. Front. Pediatr. 11:1162547. doi: 10.3389/fped.2023.1162547
Received: 9 February 2023; Accepted: 13 April 2023;
Published: 18 May 2023.
Edited by:
Xupei Huang, Florida Atlantic University, United StatesReviewed by:
Flavio Sztajnbok, Federal University of Rio de Janeiro, BrazilAnna Starshinova, Saint Petersburg State University, Russia
© 2023 Chen, Zheng, Cui, Xiao, Li, Wang, Guo, Chen, Yuan, Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Yuan eXVhbnl1ZWJqMjJAc2luYS4gQ29t Chen Shen c2hlbmNoZW4xMTEwQDEyNi4gY29t
†These authors have contributed equally to this work
 Hao Chen1,†
Hao Chen1,† Huiwen Zheng
Huiwen Zheng Jing Xiao
Jing Xiao Feina Li
Feina Li Chen Shen
Chen Shen