- 1Department of Cardiology, Children's Hospital Capital Institute of Pediatrics, Beijing, China
- 2Department of Cardiology, Capital Institute of Pediatrics-Peking University Teaching Hospital, Beijing, China
- 3Graduate School of Peking Union Medical College, Capital Institute of Pediatrics, Beijing, China
Objective: Vasovagal syncope (VVS) is the commonest form of syncope, and malignant VVS draws substantial attention due to its life-threatening cardiac asystolic risk. This study aimed to explore the predictive role of a wide panel of clinical indicators for malignant VVS in children, and further to develop a nomogram model.
Methods: This is a retrospective case-control study. VVS is diagnosed based on head-up tilt test (HUTT). STATA software (version 14.0) was used for statistical analysis, and effect sizes are expressed as odds ratio (OR) and 95% confidence interval (CI).
Results: Total 370 children with VVS were analyzed, and of them 16 children had malignant VVS. Sixteen malignant VVS and 64 non-malignant VVS were matched on age and sex by a 1:4 propensity scores matching method. Mean corpuscular hemoglobin (MCH) and standard deviation of average RR intervals milliseconds (SDANN) were significantly and independently associated with malignant VVS after adjusting for confounders, with OR reaching 1.437 (95% CI: 1.044 to 1.979; P = 0.026) and 1.035 (95% CI: 1.003 to 1.068; P = 0.029), respectively. Calibration and discrimination analyses revealed that the addition of MCH and SDANN can enhance model performance. Then, a nomogram to predict malignant VVS was developed using general characteristics and two above significant factors, and higher values in medical history, number of syncope, MCH and SDANN were associated with a greater risk of malignant VVS.
Conclusion: MCH and SDANN were two promising factors for the development of malignant VVS, and modeling of significant factors in a nomogram can provide strong reference to aid clinical decision-making.
1. Introduction
Vasovagal syncope (VVS) is the commonest form of syncope (60%–70%), and its onset peaks initially in childhood and adolescence (1). In routine clinical practice, head-up tilt test (HUTT) is employed to diagnose suspected reflex syncope after preliminary evaluation (2). Based on HUTT technique, there are several types of VVS, and thereof malignant VVS, defined as syncope episodes with ≥3s sinus arrest (3), draws substantial attention, as it can carry the risk of life-threatening cardiac asystolic and seriously impair children's physical and mental health (4, 5).
The incidence rate of sinus arrest during HUTT in pediatric patients with VVS is estimated to be 4.5%–6.5% (6, 7), with the variance possibly attributable to differences in age, race, area, eligibility criteria or test methodology (8). There is evidence that sinus arrest during HUTT can predict spontaneous asystolic syncope in adults ≥ 40 years of age (9). From clinical aspects, early detection of the potential for sinus arrest in children would be helpful for diagnosis and treatment, since it allows for prompt implementation of preventative measures, despite the challenges of performing HUTT at primary hospitals.
Thus far, few studies have attempted to unravel the risk profiles of sinus arrest in children with malignant VVS, whereas the results of these studies are not often reproducible. For example, Dhala and coworkers found that syncope episodes were comparable between subjects with and without sinus arrest, and subjects with sinus arrest tended to be younger (10). Lacroix and coworkers reported no difference in heart rate during supine time and HUTT-positive time between patients with and without sinus arrest (11). In the study by Takase and coworkers, total power was significantly higher in patients with sinus arrest than those without during HUTT (12). Another study by Miranda and coworkers supported the utility of heart rate variability (HRV) in identifying cardioinhibitory syncope (13). Above studies indicated no universal consensus on a certain factor being consistently associated with sinus arrest, likely due to insufficient power to detect significance and simple analysis of limited number of factors in patients with VVS.
To derive a reliable estimate and yield more valuable data for future investigations, we in this clinical study decided to explore the predictive role of a wide panel of clinical indicators for malignant VVS in children, and further to develop a nomogram model to facilitate application in routine clinical practice.
2. Methods
2.1. Study design and ethical aspects
This is a retrospective case-control study. Ethical approval was obtained from the ethics committee of the Capital Institute of Pediatrics (Approval No. SHERLLM2021025).
2.2. Study participants
All study participants diagnosed with VVS were retrospectively enrolled from the Department of Cardiology, Children's Hospital Capital Institute of Pediatrics during the period from July 2017 to August 2022.
According to medical records, patients with a clinical diagnosis of VVS were abstracted. In patients with VVS, malignant VVS is defined as syncope episodes with ≥ 3s sinus arrest during HUTT.
2.3. Eligibility criteria
Patients must satisfy the following four criteria: (1) children and adolescents under 18 years of age; (2) with a positive history of syncope; (3) with positive HUTT results.
In addition, patients were excluded if they had one or more of the following: (1) cardiopulmonary diseases, such as cardiomyopathy, arrhythmia and congenital heart defects or hypertension; (2) neuropsychological disorder, such as depression and anxiety, epilepsy and so on; (3) genetic metabolic diseases, such as hyperthyroidism, hereditary channelopathies (e.g., brugada syndrome, long QT syndrome, et al.) and so on;(4) any other diagnosis during HUTT, such as orthostatic hypotension, orthostatic hypertension, or the postural orthostatic tachycardia syndrome.
2.4. Head-up tilt test
The HUTT test was performed in a serene setting with low lighting. Cuff blood pressure (BP) and heart rate (HR) was continuously monitored utilizing an electric tilting device (SHUT-100A, STANDARD, Beijing, China). After an initial observation of patient in the supine position for 10 min. The patients were on a 45-min tilt phase without medication at a 60° tilt. If syncope does not occur, administer sublingual nitroglycerin (4–6 μg/kg, maximum ≤ 0.3 mg) for 20 min. If syncope occurs during the test, tilt table is quickly lowered to the prone position. In addition to four key factors, a positive response to HUTT involves syncope or presyncope, including (1) a drop in blood pressure (systolic blood pressure ≤ 80 mmHg, diastolic blood pressure ≤ 50 mmHg, or a mean decrease in blood pressure ≥ 25%); (2) sinus arrest with a junctional escape rhythm; (3) atrioventricular block ≥ II° or cardiac arrest for 3 s; (4) the heart rate of children aged 4–6 is decreased < 75 bpm, and the heart rate of children aged 7–8 is decreased < 65 bpm(14).
2.5. Data collection
Baseline characteristics from assessable children admitted for the first time to our hospital were collected, including age, sex, body mass index (BMI), medical history, family history of syncope, number of syncope, from medical records. Medical history is defined as the first symptoms of syncope or presyncope presented in our hospital.
In addition, blood tests, cardiac enzyme markers, 24-h urine output, 24-h Holter and echocardiography were completed and collected before HUTT. The supine BP/HR was recorded when the subject was in that position, and the tilt BP/HR was obtained during the first minute after tilt, when the subject was stable. All examinations are conducted by the auxiliary departments of our hospital.
2.6. Statistical analysis
Study children were grouped into malignant VVS and non-malignant VVS patients. To balance between-group bias in sample sizes, a 1:4 propensity score matching (PSM) analysis was conducted by equating groups based on age and sex. Continuous data are expressed as median (interquartile range) and compared using the Mann-Whitney test in case of deviation from normal distributions and as mean (standard deviation) and using the t-test otherwise. Number (percent) is used to express categorical data and the χ2 test or Fisher test was used for between-group comparison.
Univariate Logistic regression analyses were used to identify single factors in significant association with malignant VVS, and then multivariable Logistic regression analyses were used to check the independent association of these significant factors after adjusting for age, sex, BMI, number of syncope, family history of syncope and medical history. Effect sizes are expressed as odds ratio (OR) and 95% confidence interval (95% CI).
Predication performance gained by adding significant factors identified aforementioned to the basic model (including age, sex, medical history, number of syncope and family history of syncope) was appraised from both calibration and discrimination aspects. Correlation analysis was done to quantify the magnitude of collinearity across factors under study. Regarding corrections, Akaike Information Criterion (AIC) (15), Bayesian Information Criterion (BIC) (16) and likelihood ratio tests were used to assess the global fit of predicted probability to the reflectance of the actual observed risk and the revised risk model, by adding significant factors identified. The lower values of AIC and BIC indicate a better model. From the discrimination aspects, net reclassification improvement (NRI), integrated discrimination improvement (IDI), change in area under the curve (ΔAUC) and decision curve analysis (DCA) were used to evaluate the clinical benefits and utility of the full model relative to the basic model (17–19). DCA can calculate the net benefit of models across various risk thresholds by taking account of weighted risks and benefits. The net benefit increases with distance from both the horizontal line with no malignant VVS and the solid curve line under all malignant VVS.
Finally, a nomogram model in predicting malignant VVS risk was developed, and the predictive accuracy of this model was quantified by concordance index (C-index), defined as the area under the receiver operating characteristics curve. The nomogram and accuracy assessment were implemented by the regression modeling strategies (RMS) package (available at the website https://cran.r-project.org/web/packages/rms/index.html) in R programming environment (version 4.2.1).
All statistical analyses were conducted using the Stata software (version 14.0, Stata Corp, TX, USA) unless otherwise indicated. A two-sided P value less than 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of study patients
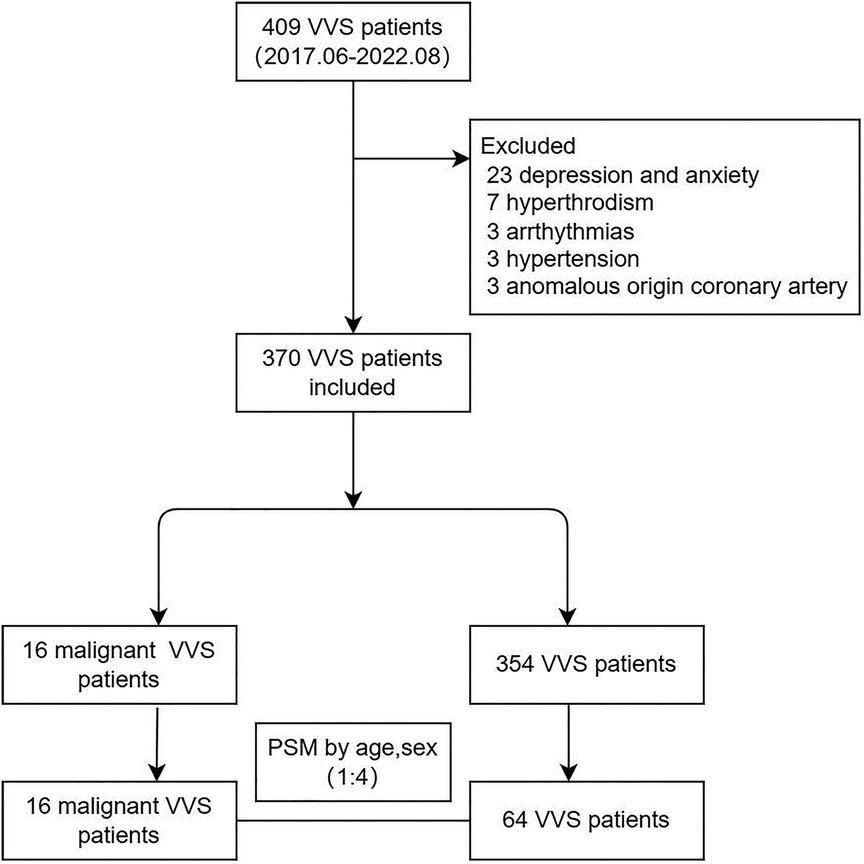
Among 409 children diagnosed with VVS in this study, 37 were eliminated due to depression and anxiety (n = 23), hyperthyroidism (n = 7), arrhythmias (n = 3), hypertension (n = 3) and anomalous origin coronary artery (n = 3). Ultimately, total 370 children with VVS were analyzed, and of them 16 children had malignant VVS. (Figure 1) Children with malignant VVS had a median age of 10 [interquartile range (IQR): 8.98 to 12.34] years, significantly lower than that of 354 children free of malignant VVS (P < 0.05). The baseline characteristics of children with and without malignant VVS are compared in Supplementary Table S1.
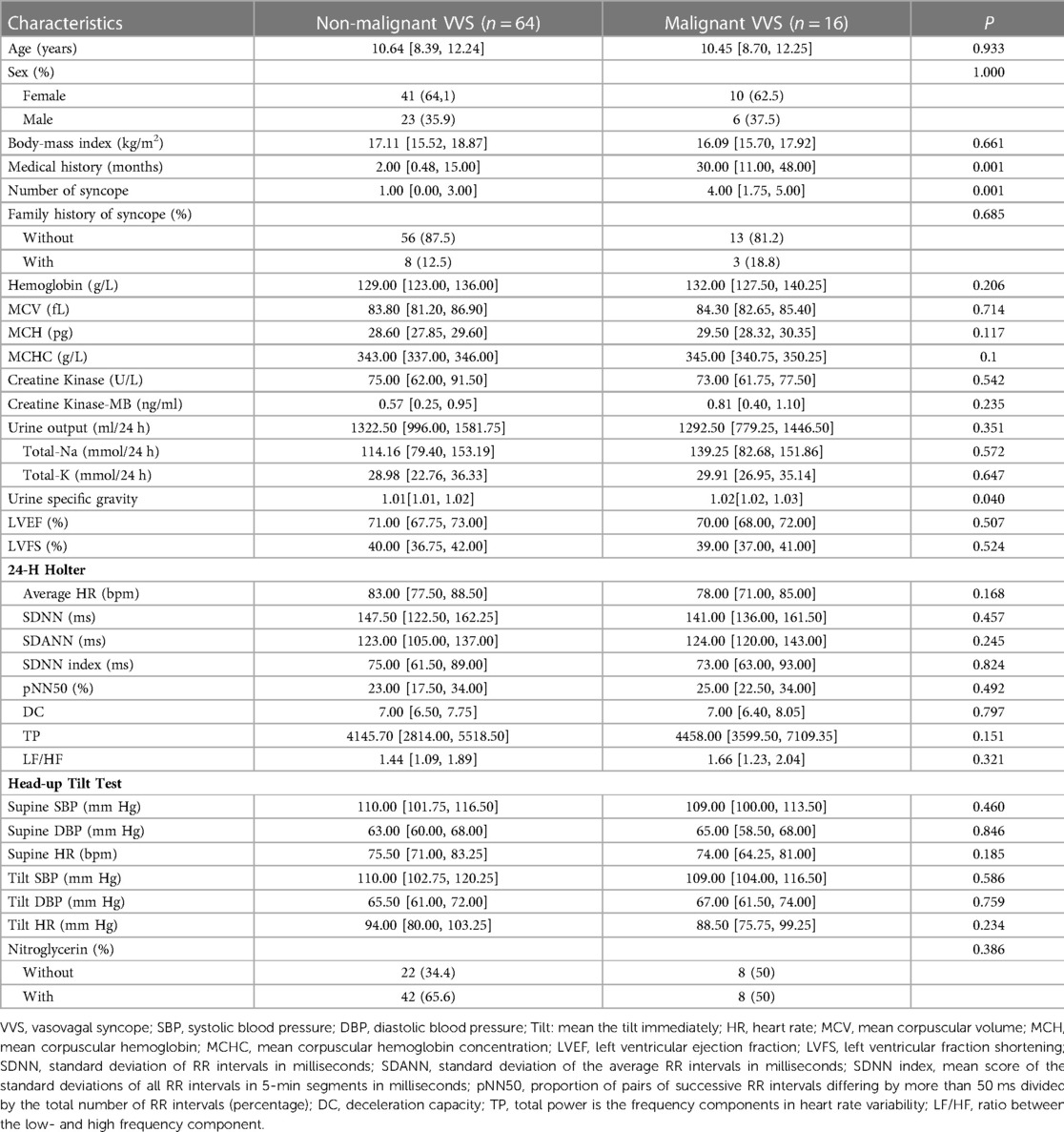
Considering the wide difference in sample size between children with and without malignant VVS, 64 children with non-malignant VVS were extracted by means of PSM technique to match 16 children with malignant VVS on age and sex. After 1:4 PSM, the comparison of baseline characteristics between 16 children with malignant VVS and 64 children with non-malignant VVS is displayed in Table 1. Medical history and the number of syncope in history differed significantly different between the two groups (both P < 0.05) (Table 1).
3.2. Identification of predictors for malignant VVS
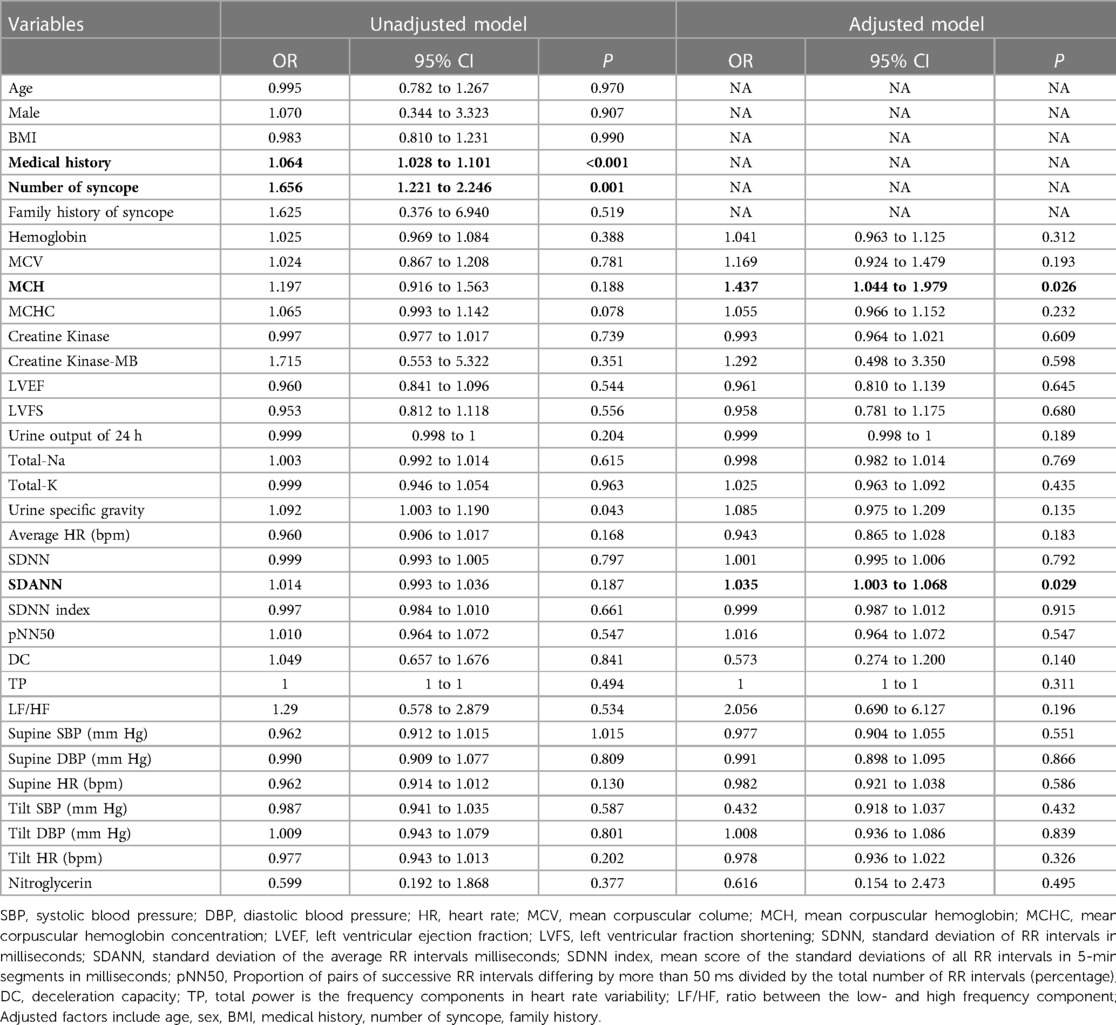
Two factors—mean corpuscular hemoglobin (MCH) and standard deviation of average RR intervals milliseconds (SDANN), were found to be significantly and independently associated with the risk of malignant VVS after adjusting age, sex, BMI, medical history, number of syncope and family history of syncope factors, with OR reaching 1.437 (95% CI: 1.044 to 1.979; P = 0.026) and 1.035 (95% CI: 1.003 to 1.068; P = 0.029), respectively (Table 2).
3.3. Calibration and discrimination assessment
Two models were built to assess prediction performance of two significant factors identified: the basic model and the full model. The basic model includes age, sex, medical history, number of syncope and family history of syncope, and the full model additionally includes MCH and SDANN.
As shown in Table 3, according to stepwise regression results (Table 2, Supplementary Table S2), the full model had minimal AIC and BIC. No collinearity was noticed among the variables in full model (Supplementary Figure S1). The predictive performance of the two models was significantly different by the likelihood ratio test (P = 0.013). NRI and IDI statistics showed significant improvement after adding MCH and SDANN to the basic model.
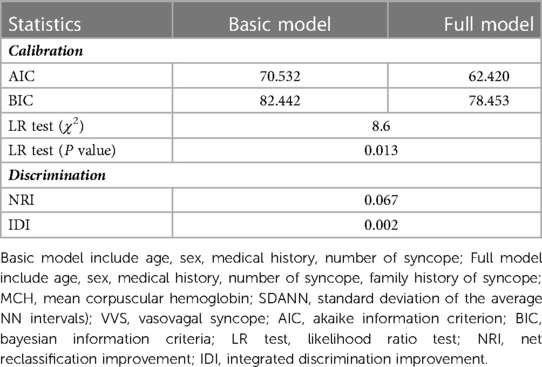
Table 3. Predictive accuracy in models with and without two significant factors when predicting malignant VVS.
Furthermore, DCA indicated the net benefits gained by adding the two significant factors (MCH and SDANN) to the basic model (Figure 2A). Addition of the two significant factors increased the AUC from 0.818 to 0.883, reflecting 6.5 percentage improvement in AUC (Figure 2B).
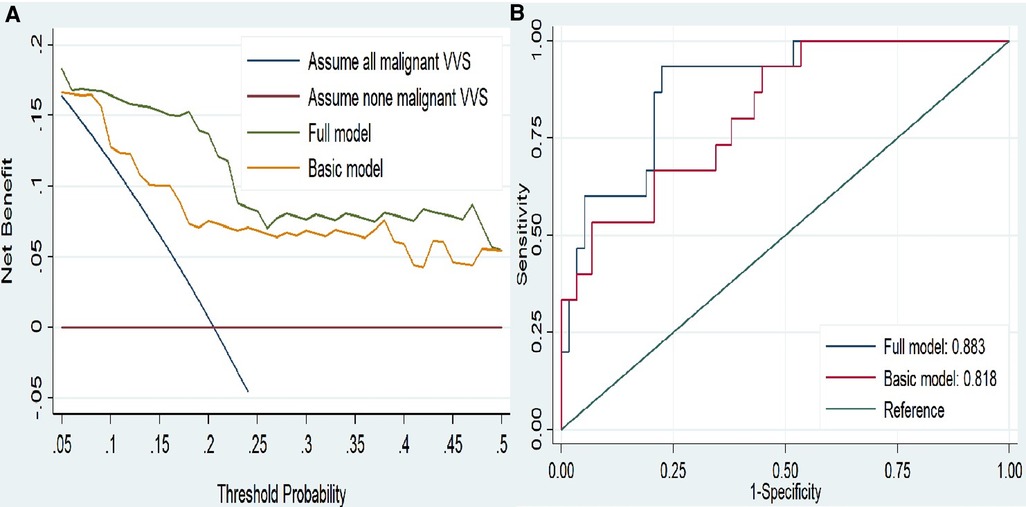
Figure 2. (A) decision curves analysis for malignant vasovagal syncope. (B)ROC curves for the two model. The basic model included age, sex, body mass index, medical history, number of syncope and family history. The full model included indicators in basic model and add MCH and SDANN. Abbreviations: MCH, mean corpuscular hemoglobin; SDANN, standard deviation of the average NN intervals.
3.4. Nomogram development
A nomogram to predict malignant VVS was developed using the general characteristics and two significant factors (Figure 3). In the nomogram, higher values in medical history, number of syncope, MCH and SDANN were associated with a greater risk of malignant VVS. For example, assuming a child with a medical history at 50 months (50 points), number of syncope at 5 (20 points), family history of syncope (12 points), 10 years old (22 points), female (0 points), MCH at 35.5 pg (50 points) and SDANN at 125 ms (40 points), the probability of malignant VVS was estimated to be 97%.
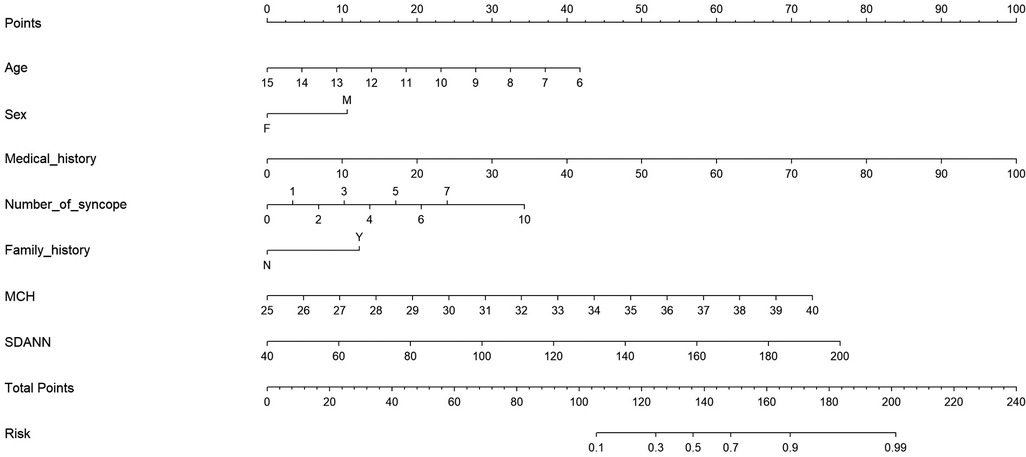
Figure 3. Prediction nomogram of convenient and significant factors for malignant VVS. Abbreviations: Medical history, the medical history of VVS (counts in months); F, female; M, male; MCH, mean corpuscular hemoglobin; SDANN, standard deviation of the average RR intervals in milliseconds.
The nomogram model demonstrated a good accuracy for predicting malignant VVS, with a C-index of 0.883 and calibration curves of good consistency (Supplementary Figure S2).
4. Discussion
The aim of this study was to explore the predictive role of a wide panel of clinical indicators for malignant VVS in children in clinical settings. Our key findings are that two clinical predictors, MCH and SDANN, were significantly and independently associated with the risk of malignant VVS in children. Moreover, integration of convenient characteristics and two significant factors in a nomogram can decently predict malignant VVS. To our knowledge, this study has for the first time developed a prediction nomogram model based on clinical data for malignant VVS in the literature.
Prior studies on malignant VVS suggested some factors can impact its occurrence, such as age and sex (20, 21), which were considered in the present study. In addition, there is evidence that with aging, cardioinhibitory decreased and asystolic pauses occurred less frequently (20), consistent with the results of another study in adult patients (22, 23). By contrast, our findings in children supported these observations, that is, older children were less likely to experience malignant VVS, which could be explained by the hypothesis that autonomic function development is not yet complete in children with malignant VVS at a young age. Furthermore, malignant VVS was found to be associated with a relatively low heart rate at baseline (23), which was not observed in the present study, possibly due to the differences in the characteristics of study participants. As an extension of prior studies, we found that medical history and the number of previous syncope were linked to malignant VVS, which led us to speculate that the likelihood of malignant VVS occurrence increased with the length of disease history.
In this study, it is worth noting that two factors, MCH and SDANN, were in significant and independent association with malignant VVS. On one hand, MCH is a red blood cell index and it is a primary indicator of red blood cell development and hemoglobin content. As indicated by our findings, high levels of MCH were found to be associated with high risk of malignant VVS. From genetic point of view, RBC indices correlated with the expression of many genes such as RPN1, ELL2, MIDN, HBB, HBA1, PIEZO1 and G6PD (24), while these genes are not among the list of candidate genes for VVS (25, 26). We agree that candidate gene approaches focusing on these RBC-correlated genes in susceptibility to VVS or malignant VVS are encouraging. Additionally, increased MCH may be caused by related hypovolemia.
On the other hand, SDANN, one time-domain indicator of HRV, reflects sympathetic nerve activity (27). Previous studies reported the diagnostic value of HRV for VVS (28). In fact, high SDANN in 24-h Holter before sinus arrest occurred can indicate that children with malignant VVS have higher activity of sympathetic nerve, which may involve the pathogenesis of malignant VVS. Our study also found that a decrease in 24 h urine output and an increase in urine specific gravity were risk factors for malignant VVS (OR: 0.993 and 1.085, respectively), albeit no hints of statistical significance. Nonetheless, this finding suggested that hypovolemia was a risk factor for malignant VVS in the case of normal renal function in children. Altogether, these findings indicate that blood volume, HRV and genetic factors are related to malignant VVS. Considering the fact that the etiology of malignant VVS is complex and the relative risk attributable to a single factor is small.
Besides the obvious strengths of this study, including the successful internal validation and good calibration/discrimination scores, some potential limitations should be recognized. First, this study was conducted at a single center involving a small sample size, and further larger multicenter studies will be required to validate our findings. Second, the presyncope symptoms and accompanying symptoms during syncope were unavailable for us, and so we cannot distinguish malignant VVS symptomatically. However, considering the subjective nature of symptom presentation, we chose a more objective laboratory basis. Third, this study is retrospective in design, and a recall bias cannot be fully excluded. Last but not the least, all clinical indicators were only measured once at baseline, and may not reflect long-term circulating concentrations.
5. Conclusions
To sum up, our findings indicated that MCH and SDANN were two promising factors for the development of malignant VVS, and modeling of significant factors in a nomogram can provide strong reference to aid clinical decision-making. In the long run, past and current findings, annexed with advanced analytical strategies such as artificial intelligence techniques, will provide novel insights about the risk profiles of malignant VVS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical approval was obtained from the ethics committee of the Capital Institute of Pediatrics. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RS, YK and HW. The first draft of the manuscript was written by RS and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the “Peak Climbing” Talents Development Program of Beijing Hospital Authority (DFL20181301).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1158537/full#supplementary-material.
References
1. Tao C, Tang C, Chen S, Jin H, Du J. Autonomic nervous function in vasovagal syncope of children and adolescents. Neurosci Bull. (2019) 35(5):937–40. doi: 10.1007/s12264-019-00383-8
2. Sutton R, Fedorowski A, Olshansky B, Gert van Dijk J, Abe H, Brignole M, et al. Tilt testing remains a valuable asset. Eur Heart J. (2021) 42(17):1654–60. doi: 10.1093/eurheartj/ehab084
3. Xu WR, Jin HF, Du JB. Diagnosis and treatment of malignant vasovagal syncope in children. Chin J Pediatr. (2022) 60(1):64–6. doi: 10.3760/cma.j.cn112140-20211018-00883
4. Engel GL. Psychologic stress, vasodepressor (vasovagal) syncope, and sudden death. Ann Intern Med. (1978) 89(3):403–12. doi: 10.7326/0003-4819-89-3-403
5. Pentousis D, Cooper J, Cobbe S. Prolonged asystole induced by head up tilt test. Report of four cases and brief review of the prognostic significance and medical management. Heart. (1997) 77(3):273–5. doi: 10.1136/hrt.77.3.273
6. Numan M, Alnajjar R, Lankford J, Gourishankar A, Butler I. Cardiac asystole during head up tilt (HUTT) in children and adolescents: is this benign physiology? Pediatr Cardiol. (2015) 36(1):140–5. doi: 10.1007/s00246-014-0977-4
7. Menozzi C, Brignole M, Lolli G, Bottoni N, Oddone D, Gianfranchi L, et al. Follow-up of asystolic episodes in patients with cardioinhibitory, neurally mediated syncope and VVI pacemaker. Am J Cardiol. (1993) 72(15):1152–5. doi: 10.1016/0002-9149(93)90985-L
8. Russo V, Parente E, Groppelli A, Rivasi G, Tomaino M, Gargaro A, et al. Prevalence of asystole during tilt test-induced vasovagal syncope may depend on test methodology. Europace. (2023) 25(2):263–9. doi: 10.1093/europace/euac154
9. Ungar A, Sgobino P, Russo V, Vitale E, Sutton R, Melissano D, et al. Diagnosis of neurally mediated syncope at initial evaluation and with tilt table testing compared with that revealed by prolonged ECG monitoring. An analysis from the third international study on syncope of uncertain etiology (ISSUE-3). Heart. (2013) 99(24):1825–31. doi: 10.1136/heartjnl-2013-304399
10. Dhala A, Natale A, Sra J, Deshpande S, Blanck Z, Jazayeri MR, et al. Relevance of asystole during head-up tilt testing. Am J Cardiol. (1995) 75(4):251–4. doi: 10.1016/0002-9149(95)80030-V
11. Lacroix D, Kouakam C, Klug D, Guédon-Moreau L, Vaksmann G, Kacet S, et al. Asystolic cardiac arrest during head-up Tilt test: incidence and therapeutic implications. Pacing Clin Electrophysiol. (1997) 20(11):2746–54. doi: 10.1111/j.1540-8159.1997.tb05432.x
12. Takase B, Nagai T, Uehata A, Katushika S, Isojima K, Hakamata N, et al. Autonomic responses to orthostatic stress in head-up Tilt testing: relationship to test-induced Prolonged asystole. Clin Cardiol. (1997) 20(3):233–8. doi: 10.1002/clc.4960200309
13. Miranda CM, Silva R. Analysis of heart rate variability before and during tilt test in patients with cardioinhibitory vasovagal syncope. Arq Bras Cardiol. (2016) 107:568–75. doi: 10.5935/abc.20160177
14. Bartoletti A, Alboni P, Ammirati F, Brignole M, Del Rosso A, Foglia Manzillo G, et al. ‘The Italian protocol’: a simplified head-up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace. (2000) 2(4):339–42. doi: 10.1053/eupc.2000.0125
15. Akaike H. Information theory and an extension of the maximum likelihood principle. Selected Papers of Hirotugu Akaike: Springer. (1998) 2(1):199–213. doi: 10.1007/978-1-4612-1694-0_15
16. Hook EB, Regal RR. Validity of methods for model selection, weighting for model uncertainty, and small sample adjustment in capture-recapture estimation. Am J Epidemiol. (1997) 145(12):1138–44. doi: 10.1093/oxfordjournals.aje.a009077
17. Pencina MJ, D'Agostino SR, D'Agostino JR, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27(2):157–72. doi: 10.1002/sim.2929
18. Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. (2010) 48(12):1703–11. doi: 10.1515/CCLM.2010.340
19. Van Calster B, Wynants L, Verbeek JF, Verbakel JY, Christodoulou E, Vickers AJ, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. (2018) 74(6):796–804. doi: 10.1016/j.eururo.2018.08.038
20. van Dijk JG, van Rossum IA, van Houwelingen M, Ghariq M, Saal DP, de Lange FJ, et al. Influence of age on magnitude and timing of vasodepression and cardioinhibition in tilt-induced vasovagal syncope. Clin Electrophysiol. (2022) 8(8):997–1009. doi: 10.1016/j.jacep.2022.05.009
21. Russo V, Parente E, Rago A, Comune A, Laezza N, Papa AA, et al. Cardioinhibitory syncope with asystole during nitroglycerin potentiated head up tilt test: prevalence and clinical predictors. Clin Auton Res. (2022) 32(3):167–73. doi: 10.1007/s10286-022-00864-3
22. Rivasi G, Torabi P, Secco G, Ungar A, Sutton R, Brignole M, et al. Age-related tilt test responses in patients with suspected reflex syncope. Europace. (2021) 23(7):1100–5. doi: 10.1093/europace/euab024
23. Guaraldi P, Calandra-Buonaura G, Terlizzi R, Cecere A, Solieri L, Barletta G, et al. Tilt-induced cardioinhibitory syncope: a follow-up study in 16 patients. Clin Auton Res. (2012) 22(3):155–60. doi: 10.1007/s10286-011-0153-3
24. Hu Y, Stilp AM, McHugh CP, Rao S, Jain D, Zheng X, et al. Whole-genome sequencing association analysis of quantitative red blood cell phenotypes: the NHLBI TOPMed program. Am J Hum Genet. (2021) 108(5):874–93. doi: 10.1016/j.ajhg.2021.04.003
25. Sheldon RS, Sandhu RK. The search for the genes of vasovagal syncope. Front Cardiovasc Med. (2019) 6:175. doi: 10.3389/fcvm.2019.00175
26. Matveeva N, Titov B, Bazyleva E, Pevzner A, Favorova O. Towards understanding the genetic nature of vasovagal syncope. Int J Mol Sci. (2021) 22(19):10316. doi: 10.3390/ijms221910316
27. Zhao T, Wang S, Wang M, Cai H, Wang Y, Xu Y, et al. Research progress on the predictive value of electrocardiographic indicators in the diagnosis and prognosis of children with vasovagal syncope. Front Cardiovasc Med. (2022) 9:916770. doi: 10.3389/fcvm.2022.916770
Keywords: malignant vasovagal syncope, head up tilt test, children, risk, nomogram
Citation: Sun R, Kang Y, Zhang M, Wang H, Shi L and Li X (2023) Development of a nomogram model to predict malignant vasovagal syncope in Chinese children. Front. Pediatr. 11:1158537. doi: 10.3389/fped.2023.1158537
Received: 4 February 2023; Accepted: 17 March 2023;
Published: 3 April 2023.
Edited by:
Wenquan Niu, China-Japan Friendship Hospital, ChinaReviewed by:
Yue Qi, Capital Medical University, ChinaZhixin Zhang, China-Japan Friendship Hospital, China
© 2023 Sun, Kang, Zhang, Wang, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Li bHhobWFnZ2llQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
 Rui Sun
Rui Sun Yingying Kang1,3,†
Yingying Kang1,3,† Lin Shi
Lin Shi Xiaohui Li
Xiaohui Li