- 1Department of Neuroscience and Mental Health, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Medical-Surgical and Transplant Pathophysiology, University of Milan, Milan, Italy
Background: Febrile seizures (FS) and benign convulsions in children with mild gastroenteritis (CwG) are acute symptomatic seizures, transiently occurring in infants and young children, probably related to the immaturity of the brain. Our paper aims to review the literature data on patients with FS and CwG.
Methods: A review of series of patients with FS and CwG was performed by literature search on PubMed January 1960 to October 2022. Several parameters were considered, including epidemiology, pathophysiology, clinical features, electroencephalographic findings and other diagnostic studies, and treatment.
Results: FS and CwG share an age-dependent course, but they show significant differences in the pathophysiology, clinical features, diagnostic studies, and treatment.
Conclusion: Acute symptomatic seizures include seizures that are caused by acute structural brain pathologies, such as stroke, as well as seizures that are provoked by a reversible factor, such as hyponatraemia, although the two groups should be not equated. Furthermore, FS and CwG should be set apart as “age-dependent acute symptomatic seizures”, reinforcing the concept of their self-limited course over a certain period.
1. Introduction
Febrile Seizures (FS) and benign convulsions in children with mild gastroenteritis (CwG) are quite common in infants and young children. The American Academy of Pediatrics (AAP) defines FS as “a seizure accompanied by fever (temperature 100.4°F or 38°C by any method), without central nervous system (CNS) infection, that occurs in infants and children 6 through 60 months of age” (1). Benign convulsions associated with mild gastroenteritis (CwG) have been defined as afebrile seizures occurring in otherwise healthy children with mild acute gastroenteritis, who do not have central nervous system infection, dehydration, or electrolyte imbalances (2). However, in clinical practice, the occurrence of febrile CwG is not uncommon (3). Although the presence of fever seems to influence the clinical characteristics of seizures associated with mild gastroenteritis (4), distinguishing between febrile CwG and FS is not easy for the clinician (5).
FS and CwG are considered situation-related seizures, which are essentially transient and functional disorders of infants and young children, probably related to the immaturity of the brain (6–10). Nevertheless, the two entities show significant differences in clinical features and management (6, 11–15). Our paper aims to review the epidemiology, clinical features, diagnostic investigations and treatment options in patients with FS and CwG.
2. Literature search
A review of a series of patients with FS and CwG was performed by literature search on PubMed from January 1960 to October 2022. This review is not meant to be exhaustive. We have selected some of the more comprehensive articles on FS, febrile CwG and afebrile CwG. We do not pretend to have included all the better contributions, but most of those included have extensive references that should be consulted for more complete coverage. Our search terms included “Febrile seizures,” “Benign convulsions”, “Gastroenteritis”, and “Acute symptomatic seizures”. The main data are summarized in Table 1.
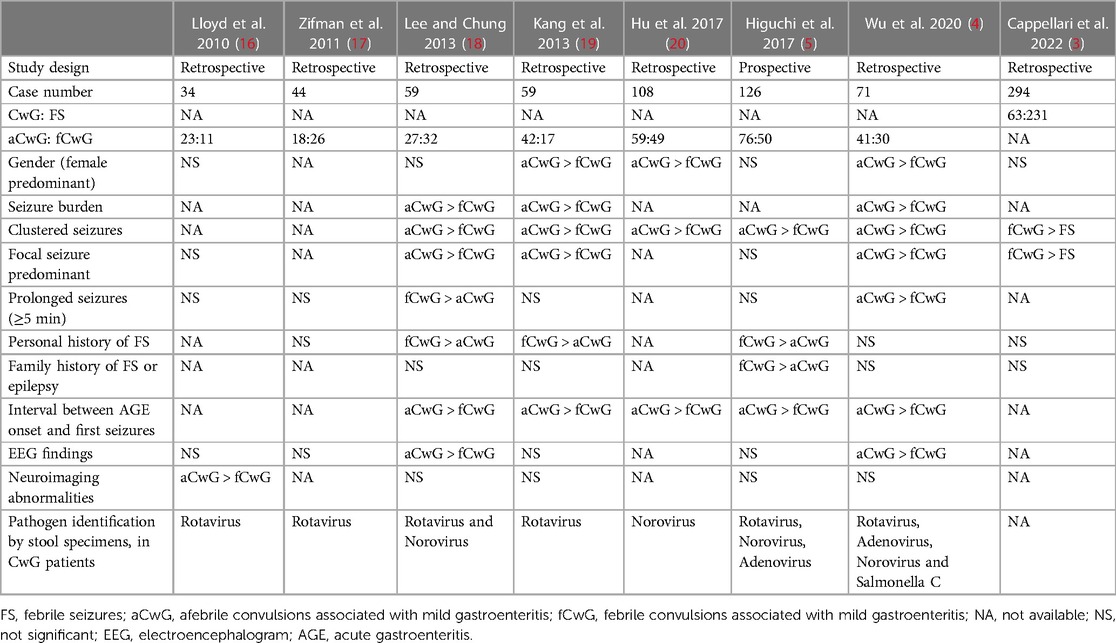
Table 1. Summary of literature data on patients with febrile seizures, afebrile convulsions associated with mild gastroenteritis and febrile convulsions associated with mild gastroenteritis. Only studies including more than 30 patients have been included.
3. Epidemiology
Both FS and CwG are transient and functional disorders occurring in infants and young children (6).
3.1. FS
FS affects 2%–5% of children under five years of age (21), with a peak incidence around 18 months (22). Viral infections are the most frequent cause of febrile illnesses associated with FS (up to 82% of cases) (23). Some vaccinations have also been linked to FS, with the risk period following vaccination varying between vaccines (24). Other risk factors for FS include fever above 38°C, shorter fever duration and a positive family history of FS or epilepsy (25, 26). Up to 41% of children who have febrile status epilepticus (FSE) go on to experience another FS in the future, increasing their risk of other negative outcomes (27).
3.2. CwG
CwG occur in children aged one month to 6 years, with a peak incidence at 1–2 years of age (28). CwG mostly occur during the winter and early spring months, which is the period of the year associated with the largest circulation of several viruses such as rotavirus, norovirus and adenovirus (28–31). Furthermore, CwG have been more frequently reported in East Asian countries, suggesting that the genetic characteristics of the host may contribute to the occurrence of seizures in children with gastroenteritis (28, 32).
4. Pathophysiology
The rapid development of the immature CNS in childhood may increase the susceptibility to seizures in infants and young children with FS and CwG (6).
4.1. FS
The height of the body temperature is considered to play a major role in the pathogenesis of FS, compared to the rapidity of its rise (33, 34). Increasing the temperature of the brain has been suggested to increase neuronal firing, which in turn increases the likelihood of synchronised neuronal activity that leads to seizure induction (35). The seizure threshold varies between patients. A lower seizure threshold to excitatory input both in vivo (kainic acid) and in vitro (electrical stimulation) had been associated with prolonged FS (36). Children prone to FS produce more pro-inflammatory cytokines in the CNS, such as IL-1Beta, which might induce seizures (37). Also, the type of infection seems to play a role in the pathogenesis of FS, with human herpes virus 6 (HHV6), viral upper respiratory tract infections and gastroenteritis frequently involved.
4.2. CwG
Among the various organisms causing gastroenteritis, rotavirus has been detected in a wide number of stool specimens of CwG patients (28, 38–41). There are different hypotheses on the relationship between rotavirus infection and its effect on the CNS. One hypothesis is that rotavirus may directly invade the CNS via the bloodstream (42, 43). Indeed, rotavirus RNA in the cerebrospinal fluid (CSF) has been reported in patients with gastroenteritis and seizures (43, 44). Another hypothesis is that rotavirus may indirectly provoke seizures by various mediators produced in the gastrointestinal tract and released in the general circulation (45).
5. Clinical features
FS and CwG are benign conditions sharing several clinical and prognostic features (6).
5.1. FS
Children with FS can develop seizures within one hour of the onset of the fever (21% of cases), between 1 and 24 h of the fever (57%) or more than 24 h after the fever (22%) (46). However, the fever can occur at any time, not infrequently after the seizure. Children with FS have higher temperatures with illness compared with febrile controls (47). Seizure types include tonic-clonic seizures, which can be asymmetrical, or focal impaired awareness seizures. FS have been classified as simple FS or complex based on duration, recurrence, and presence of focal features (48). Febrile seizures have an average duration of 4–7 min, with only 10%–15% of them lasting longer than 10 min (49). Simple FS are generalized seizures, with duration <15 min, and not recurrent within 24 h. Complex FS are seizures either focal, with a duration >15 min, or repetitive within 24 h. Most FS are simple FS, while 25%–35% are complex FS (50).
Up to a third of children with FS have a recurrence, and 75% of these occur within one year (51). Risk factors for recurrent FS include age at onset <18 months, history of FS in a first-degree relative, low grade of fever associated with seizures (<39°C), short duration of fever before a seizure (<1 h) and multiple seizures during the same febrile illness (50). EEG findings have also been reported as an independent risk factor for FS recurrence (50).
5.2. CwG
In many cases of CwG, seizures follow gastrointestinal symptoms, although they can also occur before or simultaneously with the development of diarrhoea (41).
Although the seizures are mostly reported as generalized tonic-clonic, ictal EEG recordings have always demonstrated a focal origin (41, 52). Seizures are mostly brief (<5 min), and often repetitive, usually occurring in clusters ranging from one to 8 seizures within a 24 h period (19). Cluster seizures were observed in 13%–75% of patients with CwG (53).
The overall prognosis of CwG is favourable, and the incidence of seizure recurrence is low (54). The risk of recurrence can be predicted by age at onset <18 months and repeated seizures over 24 h (54).
6. Electroencephalographic findings
EEG could play a role in predicting FS recurrence (50) or epilepsy development (55) in patients with FS, while the role of EEG is of limited value in the evaluation of CwG since most EEG findings return to normal during the follow-up period (41).
6.1. FS
There is a longstanding debate on the usefulness of EEG in children with FS (56). The value of EEG in patients with simple FS has been traditionally denied (1), although some recent papers suggest its usefulness. If an EEG is obtained, it should be taken at least 48 h after the FS to prevent conflating postictal electrical activity with aberrant activity (21). First, pseudo-petit mal discharge (PPMD) pattern and abnormal EEG have been recently reported as independent risk factors for FS recurrence (50). Second, PPMD has been reported as a marker of favourable prognosis in terms of epilepsy development, since epilepsy has been diagnosed in patients without PPMD but not in those with PPMD (55). The value of EEG in patients with complex FS remains controversial (57), and the absence of epileptiform activity does not exclude seizures (58).
6.2. CwG
Interictal EEG findings have been usually reported as normal in CwG, although some patients may initially present slow background activity, focal spikes or epileptiform discharges. Ictal EEG recordings reveal a focal onset, usually with secondary generalization and rarely with persisting focality (9, 41, 59).
7. Other diagnostic studies
The role of diagnostic studies is quite different in FS and CwG.
7.1. FS
Blood tests are not necessary in the presence of typical history and physical examination (60). In patients with simple FS, a lumbar puncture should be performed in children with a history and clinical features of meningitis, while it is an option in infants between 6 and 12 months of age with incomplete or unknown immunization status, as well as in a child pretreated with antibiotics (1). A lumbar puncture should also be considered in children with complex FS (60). Neuroimaging studies, including cranial computed tomography (CT) and magnetic resonance imaging (MRI), are not routinary indicated in children with FS (60–62). Imaging studies should be performed in patients with focal neurological findings, raised intracranial pressure or suspected intracranial structural pathology (60, 63, 64).
7.2. CwG
Diagnosis of CwG is based on clinical findings. Laboratory investigations and neuroimaging are not necessary, except in a few selected cases (65). Reversible abnormalities of high intense signal of the splenium of the corpus callosum on brain MRI have been reported in patients with CwG associated with rotavirus infection (66).
8. Treatment
FS and CwG have significant differences in management (6).
8.1. FS
Most FS are self-limited and stop on their own before patients arrive at the hospital. However, seizures lasting longer than 5 min are unlikely to stop, and a benzodiazepine is required to break the seizure (67). Therefore, intervention to stop the seizure usually is unnecessary in simple FS, since the seizure has typically resolved by the time the child is evaluated by a physician, while the treatment may be required in complex FS if the seizure is still ongoing by the time the child arrives at a medical facility (68). Intravenous lorazepam and diazepam have been reported to have similar rates of seizure resolution and respiratory depression. When intravenous access is unavailable, buccal midazolam or rectal diazepam are good alternatives (69).
Given the usually benign course of FS and the risk of adverse effects with medications, there is currently no role for prophylactic anti-seizure drugs in preventing FS. The use of antipyretics does not decrease the risk of FS (69).
8.2. CwG
Antiseizure drugs are not necessary if the patient has seizures of brief duration, even if they occur in clusters. Prolonged seizures may respond to conventional therapy, although some authors report that benzodiazepines were effective in stopping the seizures in only a limited number of cases (38%) (28, 41, 70, 71). Some authors suggest that carbamazepine is the most effective drug at the dose of 5 mg/Kg/die to treat prolonged seizures. Although there is no consensus on the drug of choice for CwG treatment, all authors agree to avoid benzodiazepines in the routinary treatment of patients with CwG (28).
9. Conclusion
FS and CwG are considered situation-related seizures, occurring in infants and young children (6–10). In clinical practice, several terms, such as “situation-related seizure”, “provoked seizure”, and “reactive seizure” are frequently used, but the ILAE has proposed that these terms are synonymous with and should be replaced by the term “acute symptomatic seizure” (72). Acute symptomatic seizures have been defined as seizures that occur within a certain period of an inciting event (73). A major issue in the definition of acute symptomatic seizures is the difficulty in combining, in a single concept, both seizures that are caused by acute structural brain pathologies, such as stroke, and seizures that are provoked by a reversible factor such as hyponatraemia, which should be not equated (74). In our opinion, FS and CwG should not be equalled to other symptomatic seizures too, owing to their propensity to exclusively occur on the immature brain. Indeed, FS and CwG could be set apart as “age-dependent acute symptomatic seizures”, reinforcing the concept of their self-limited course over a certain period. This term is not so far from other terms used to indicate specific age-dependent neurologic disorders, such as “self-limited epilepsies” (75) or transient benign paroxysmal movement disorders in infancy (76). Although the theoretical concept and definition of an acute symptomatic seizure suggests that the seizure recurrence risk should be relatively low, the risk varies according to the causes of acute symptomatic seizures (77). Among the age-dependent acute symptomatic seizures, the prognosis for subsequent epileptic seizures in children with simple FS is similar to that of the general population, while the risk of afebrile seizures is increased to 15%–20% among children with complex FS (78).
10. Open issues
There is controversy regarding whether benign seizures occurring in gastroenteritis with fever should be placed in the category of CwG (6). Seizures occurring in gastroenteritis with fever have been regarded as “FS”, while those occurring during gastroenteritis without fever are classified as “CwG”. However, febrile seizures during viral gastroenteritis may clinically resemble those of CwG rather than those of FS concerning the frequency of clustered seizures and the antiepileptic drug responses, suggesting that they may have a pathogenic mechanism distinct from FS due to other causes (6). Furthermore, we recently reported that children with febrile CwG showed a higher rate of complex seizures as compared with those with FS (3). Overall, these findings suggest that the relationship among FS, febrile CwG and afebrile CwG is quite complex. Although there are some studies investigating the difference between febrile CwG and FS, they are few single-center studies with a small number of patients (5). Future studies and discussions are required to resolve this issue.
Author contributions
AMC and SM: contributed to the conception and writing of the manuscript. GB: contributed to review the literature data. All authors contributed to the article and approved the submitted version.
Funding
Open Access article charges are covered by “Ricerca Corrente’ (IRCCS RC-2023 Grant no. 01) from the Italian Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Subcommittee on Febrile Seizures; American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. (2011) 127(2):389–94. doi: 10.1542/peds.2010-3318
2. Khosroshahi N, Rahbarimanesh A, Boroujeni FA, Eskandarizadeh Z, Zoham MH. Afebrile benign convulsion associated with mild gastroenteritis: a cohort study in a tertiary children Hospital. Child Neurol Open. (2018) 1(5):2329048X18773498. doi: 10.1177/2329048X18773498
3. Cappellari AM, Cucchetti MF, Alicandro G, Consonni D, Laicini E, Dell’Era L, et al. A comparative study of febrile seizures and febrile convulsions associated with mild gastroenteritis. Pediatr Neurol. (2022) 135:1–3. doi: 10.1016/j.pediatrneurol.2022.06.021
4. Wu YZ, Liu YH, Tseng CM, Tseng YH, Chen TH. Comparison of clinical characteristics between febrile and afebrile seizures associated with acute gastroenteritis in childhood. Front Pediatr. (2020) 16(8):167. doi: 10.3389/fped.2020.00167
5. Higuchi Y, Kubo T, Mitsuhashi T, Nakamura N, Yokota I, Komiyama O, et al. Clinical epidemiology and treatment of febrile and afebrile convulsions with mild gastroenteritis: a multicenter study. Pediatr Neurol. (2017) 67:78–84. doi: 10.1016/j.pediatrneurol.2016.05.011
6. Ueda H, Tajiri H, Kimura S, Etani Y, Hosoi G, Maruyama T, et al. Clinical characteristics of seizures associated with viral gastroenteritis in children. Epilepsy Res. (2015) 109:146–54. doi: 10.1016/j.eplepsyres.2014.10.021
7. Uemura N, Okumura A, Negoro T, Watanabe K. Clinical features of benign convulsions with mild gastroenteritis. Brain Dev. (2002) 24(8):745–9. doi: 10.1016/s0387-7604(02)00097-9
8. Specchio N, Vigevano F. The spectrum of benign infantile seizures. Epilepsy Res. (2006) 70(Suppl 1):S156–67. doi: 10.1016/j.eplepsyres.2006.01.018
9. Komori H, Wada M, Eto M, Oki H, Aida K, Fujimoto T. Benign convulsions with mild gastroenteritis: a report of 10 recent cases detailing clinical varieties. Brain Dev. (1995) 17(5):334–7. doi: 10.1016/0387-7604(95)00074-l
10. Imai K, Otani K, Yanagihara K, Li Z, Futagi Y, Ono J, et al. Ictal video-EEG recording of three partial seizures in a patient with the benign infantile convulsions associated with mild gastroenteritis. Epilepsia. (1999) 40(10):1455–8. doi: 10.1111/j.1528-1157.1999.tb02020.x
12. Fukuyama Y. Borderland of epilepsy with special reference to febrile convulsions and so-called infantile convulsions. Seishin Igaku. (1963) 5:211–23.
13. Sakauchi M. A clinical, genetical and electroencephalo-graphic study of benign infantile convulsions (fukuyama). J Tokyo Women’s Med Univ. (1997) 6:111–28.
14. Okumura A, Katou T, Watanabe K. The clinical features in convulsions with mild gastroenteritis. Syounika Rinsyo (Tokyo). (1999) 52:51–5.
15. Matsufuji H, Ichiyama T, Isumi H, Furukawa S. Low-dose carbamazepine therapy for benign infantile convulsions. Brain Dev. (2005) 27:554–7. doi: 10.1016/j.braindev.2005.01.005
16. Lloyd MB, Lloyd JC, Gesteland PH, Bale JF Jr. Rotavirus gastroenteritis and seizures in young children. Pediatr Neurol. (2010) 42(6):404–8. doi: 10.1016/j.pediatrneurol.2010.03.002
17. Zifman E, Alehan F, Menascu S, Har-Gil M, Miller P, Saygi S, et al. Clinical characterization of gastroenteritis-related seizures in children: impact of fever and serum sodium levels. J Child Neurol. (2011) 26(11):1397–400. doi: 10.1177/0883073811409222
18. Lee EH, Chung S. A comparative study of febrile and afebrile seizures associated with mild gastroenteritis. Brain Dev. (2013) 35(7):636–40. doi: 10.1016/j.braindev.2012.09.014
19. Kang B, Kim DH, Hong YJ, Son BK, Kim DW, Kwon YS. Comparison between febrile and afebrile seizures associated with mild rotavirus gastroenteritis. Seizure. (2013) 22(7):560–4. doi: 10.1016/j.seizure.2013.04.007
20. Hu MH, Lin KL, Wu CT, Chen SY, Huang GS. Clinical characteristics and risk factors for seizures associated with norovirus gastroenteritis in childhood. J Child Neurol. (2017) 32:810–4. doi: 10.1177/0883073817707302
21. Tiwari A, Meshram RJ, Kumar Singh R. Febrile seizures in children: a review. Cureus. (2022) 14(11):e31509. doi: 10.7759/cureus.31509
22. Leung AK, Robson WL. Febrile seizures. J Pediatr Health Care. (2007) 21(4):250–5. doi: 10.1016/j.pedhc.2006.10.006
23. Carman KB, Calik M, Karal Y, Isikay S, Kocak O, Ozcelik A, et al. Viral etiological causes of febrile seizures for respiratory pathogens (EFES study). Hum Vaccin Immunother. (2019) 15(2):496–502. doi: 10.1080/21645515.2018.1526588
24. Sun Y, Christensen J, Hviid A, Li J, Vedsted P, Olsen J, Vestergaard M. Risk of febrile seizures and epilepsy after vaccination with diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type B. JAMA. (2012) 307(8):823–31. doi: 10.1001/jama.2012.165
25. Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF, Hauser WA. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. (1995) 36:334–41. doi: 10.1111/j.1528-1157.1995.tb01006.x
26. Rantala H, Uhari M, Hietala J. Factors triggering the first febrile seizure. Acta Paediatr. (1995) 84:407–10. doi: 10.1111/j.1651-2227.1995.tb13660.x
27. Maytal J, Shinnar S. Febrile status epilepticus. Pediatrics. (1990) 86:611–6. doi: 10.1542/peds.86.4.611
28. Castellazzi L, Principi N, Agostoni C, Esposito S. Benign convulsions in children with mild gastroenteritis. Eur J Paediatr Neurol. (2016) 20(5):690–5. doi: 10.1016/j.ejpn.2016.05.014
29. Williams CJ, Lobanov A, Pebody RG. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol Infect. (2009) 137:607e16. doi: 10.1017/S0950268808001714
30. Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. (2013) 368:1121e30. doi: 10.1056/NEJMsa1206589
31. Kotloff KL, Losonsky GA, Morris JG Jr, Wasserman SS, Singh-Naz N, Levine MM. Enteric adenovirus infection and childhood diarrhea: an epidemiologic study in three clinical settings. Pediatrics. (1989) 84:219e25. doi: 10.1542/peds.84.2.219
32. Motoyama M, Ichiyama T, Matsushige T, Kajimoto M, Shiraishi M, Furukawa S. Clinical characteristics of benign convulsions with rotavirus gastroenteritis. J Child Neurol. (2009) 24(5):557–61. doi: 10.1177/0883073808327829
33. van Zeijl JH, Mullaart RA, Galama JM. The pathogenesis of febrile seizures: is there a role for specific infections? Rev Med Virol. (2002) 12(2):93–106. doi: 10.1002/rmv.346
34. Berg AT. Are febrile seizures provoked by a rapid rise in temperature? Am J Dis Child. (1993) 147(10):1101–3. doi: 10.1001/archpedi.1993.02160340087020
35. Dube CM, Brewster AL, Baram TZ. Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev. (2009) 31:366–71. doi: 10.1016/j.braindev.2008.11.010
36. Feng B, Chen Z. Generation of febrile seizures and subsequent epileptogenesis. Neurosci Bull. (2016) 32(5):481–92. doi: 10.1007/s12264-016-0054-5
37. Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1ß (-511) allele 2 in febrile seizures. Pediatr Neurol. (2002) 26(3):192–5. doi: 10.1016/s0887-8994(01)00380-0
38. Kawano G, Oshige K, Syutou S, Koteda Y, Yokoyama T, Kim BG, et al. Benign infantile convulsions associated with mild gastroenteritis: a retrospective study of 39 cases including virological tests and efficacy of anticonvulsants. Brain Dev. (2007) 29:617–22. doi: 10.1016/j.braindev.2007.03.012
39. Narchi H. Benign afebrile cluster convulsions with gastroenteritis: an observational study. BMC Pediatr. (2004) 4:2. doi: 10.1186/1471-2431-4-2
40. Caraballo RH, Ganez L, Santos Cde L, Espeche A, Cersosimo R, Fejerman N. Benign infantile seizures with mild gastroenteritis: study of 22 patients. Seizure. (2009) 18:686–9. doi: 10.1016/j.seizure.2009.09.006
41. Verrotti A, Nanni G, Agostinelli S, Parisi P, Capovilla G, Beccaria F, et al. Benign convulsions associated with mild gastroenteritis: a multicenter clinical study. Epilepsy Res. (2011) 93:107–14. doi: 10.1016/j.eplepsyres.2010.11.004
42. Goldwater PN, Rowland K, Thesinger M, Abbott K, Grieve A, Palombo EA, et al. Rotavirus encephalopathy: pathogenesis reviewed. J Paediatr Child Health. (2001) 37:206–9. doi: 10.1046/j.1440-1754.2001.00596.x
43. Keidan I, Shif I, Keren G, Passwell JH. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr Infect Dis J. (1992) 11:773–5. doi: 10.1097/00006454-199209000-00022
44. Nishimura S, Ushijima H, Nishimura S, Shiraishi H, Kanazawa C, Abe T, et al. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. (1993) 15:457–9. doi: 10.1016/0387-7604(93)90088-p
45. Lee YS, Lee GH, Kwon YS. Update on benign convulsions with mild gastroenteritis. Clin Exp Pediatr. (2022) 65(10):469–475. doi: 10.3345/cep.2021.00997
46. Chung S. Febrile seizures. Korean J Pediatr. (2014) 57(9):384–95. doi: 10.3345/kjp.2014.57.9.384
47. Gordon KE, Dooley JM, Wood EP, Bethune P. Is temperature regulation different in children susceptible to febrile seizures? Can J Neurol Sci. (2009) 36(2):192–5.19378713
48. Patel N, Ram D, Swiderska N, Mewasingh LD, Newton RW, Offringa M. Febrile seizures. Br Med J. (2015) 351:h4240. doi: 10.1136/bmj.h4240
49. Eilbert W, Chan C. Febrile seizures: a review. JACEP Open. (2022) 3:e12769. doi: 10.1002/emp2.12769
50. Cappellari AM, Brizio C, Mazzoni MB, Bertolozzi G, Vianello F, Rocchi A, et al. Predictive value of EEG for febrile seizure recurrence. Brain Dev. (2018) 40(4):311–5. doi: 10.1016/j.braindev.2017.12.004
51. Berg AT, Shinnar S, Darefsky AS, Holford TR, Shapiro ED, Salomon ME, Crain EF, Hauser AW. Predictors of recurrent febrile seizures. A prospective cohort study. Arch Pediatr Adolesc Med. (1997) 151(4):371–8. doi: 10.1001/archpedi.1997.02170410045006
52. Capovilla G, Vigevano F. Benign idiopathic partial epilepsies in infancy. J Child Neurol. (2001) 16:874e81. doi: 10.1177/088307380101601202
53. Fan W, Fang C, Yang Y, Zhang C. Comparison of clinical characteristics between cluster and isolated seizures associated with benign convulsions with mild gastroenteritis. Eur J Paediatr Neurol. (2022) 36:26–9. doi: 10.1016/j.ejpn.2021.11.008
54. Wang D, Jiang Y, Hong S, Ma J, Liao S, Cheng M, et al. Prognostic factors for the recurrence of afebrile seizures after benign convulsions associated with mild gastroenteritis. Epilepsia. (2021) 62(12):3068–75. doi: 10.1111/epi.17102
55. Dilber B, Kamaşak T, Arslan EA. Pseudo-petit mal discharge: a marker of favorable prognosis in febrile seizure. Arch Epilepsy. (2022) 28(1):18–22. doi: 10.54614/ArchEpilepsy.2022.35119
56. Natsume J, Hamano SI, Iyoda K, Kanemura H, Kubota M, Mimaki M, et al. New guidelines for management of febrile seizures in Japan. Brain Dev. (2017) 39(1):2–9. doi: 10.1016/j.braindev.2016.06.003
57. Kim H, Byun SH, Kim JS, Lim BC, Chae JH, Choi J, et al. Clinical and EEG risk factors for subsequent epilepsy in patients with complex febrile seizures. Epilepsy Res. (2013) 105:158–63. doi: 10.1016/j.eplepsyres.2013.02.006
58. Sawires R, Buttery J, Fahey M. A review of febrile seizures: recent advances in understanding of febrile seizure pathophysiology and commonly implicated viral triggers. Front Pediatr. (2022) 9:801321. doi: 10.3389/fped.2021.801321
59. Maruyama K, Okumura A, Sofue A, Ishihara N, Watanabe K. Ictal EEG in patients with convulsions with mild gastroenteritis. Brain Dev. (2007) 29(1):43–6. doi: 10.1016/j.braindev.2006.06.002
60. Leung AK, Robson WL. Febrile convulsions. How dangerous are they? Postgrad Med. (1991) 89(5):217–8. 221-2, 224. doi: 10.1080/00325481.1991.11700905
61. Di Mario FJ. Children presenting with complex febrile seizures do not routinely need computed tomography scanning in the emergency department. Pediatrics. (2006) 117:528–30. doi: 10.1542/peds.2005-2012
62. Teng D, Dayan P, Tyler S, Hauser WA, Chan S, Leary L, et al. Risk of intracranial pathologic conditions requiring emergency intervention after a first complex febrile seizure episode among children. Pediatrics. (2006) 117:304–8. doi: 10.1542/peds.2005-0759
63. Depiero AD, Teach SJ. Febrile seizures. Pediatr Emerg Care. (2001) 17:384–7. doi: 10.1097/00006565-200110000-00016
64. Millar JS. Evaluation and treatment of the child with febrile seizure. Am Fam Physician. (2006) 73:1761–4.16734052
65. Verrotti A, Tocco AM, Coppola GG, Altobelli E, Chiarelli F. Afebrile benign convulsions with mild gastroenteritis: a new entity? Acta Neurol Scand. (2009) 120(2):73–9. doi: 10.1111/j.1600-0404.2008.01154.x
66. Ogawa C, Kidokoro H, Ishihara N, Tsuji T, Kurahashi H, Hattori A, et al. Splenial lesions in benign convulsions with gastroenteritis associated with rotavirus infection. Pediatr Neurol. (2020) 109:79–84. doi: 10.1016/j.pediatrneurol.2019.05.002
67. Agarwal M, Fox SM. Pediatric seizures. Emerg Med Clin North Am. (2013) 31(3):733–54. doi: 10.1016/j.emc.2013.04.001
68. Leung AK. Febrile seizures: an overview. Drugs Context. (2018) 7(212536). doi: 10.7573/dic.212536
69. Smith DK, Sadler KP, Benedum M. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. (2019) 99(7):445–50.30932454
70. Verrotti A, Moavero R, Vigevano F, Cantonetti L, Guerra A, Spezia E, et al. Long-term follow-up in children with benign convulsions associated with gastroenteritis. Eur J Paediatr Neurol. (2014) 18:572e7. doi: 10.1016/j.ejpn.2014.04.006
71. Okumura A, Uemura N, Negoro T, Watanabe K. Efficacy of antiepileptic drugs in patients with benign convulsions with mild gastroenteritis. Brain Dev. (2004) 26:164e7. doi: 10.1016/S0387-7604(03)00121-9
72. Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. (2010) 51(4):671–5. doi: 10.1111/j.1528-1167.2009.02285.x
73. Romantseva L, Lin N. Acute seizures-work-up and management in children. Semin Neurol. (2020) 40(6):606–16. doi: 10.1055/s-0040-1718718
74. Shorvon S. The concept of symptomatic epilepsy and the complexities of assigning cause in epilepsy. Epilepsy Behav. (2014) 32:1–8. doi: 10.1016/j.yebeh.2013.12.025
75. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58(4):512–21. doi: 10.1111/epi.13709
76. Fernández-Alvarez E. Transient benign paroxysmal movement disorders in infancy. Eur J Paediatr Neurol. (2018) 22(2):230–7. doi: 10.1016/j.ejpn.2018.01.003
77. Mauritz M, Hirsch LJ, Camfield P, Chin R, Nardone R, Lattanzi S, et al. Acute symptomatic seizures: an educational, evidence-based review. Epileptic Disord. (2022) 24(1):26–49. doi: 10.1684/epd.2021.1376
Keywords: febrile seizures, benign convulsions in children with mild gastroenteritis, epilepsy, electroencephalography, PPMD
Citation: Cappellari AM, Mariani S and Bruschi G (2023) Febrile seizures and convulsions with mild gastroenteritis: age-dependent acute symptomatic seizures. Front. Pediatr. 11:1151770. doi: 10.3389/fped.2023.1151770
Received: 26 January 2023; Accepted: 26 June 2023;
Published: 18 July 2023.
Edited by:
Piero Pavone, University of Catania, ItalyReviewed by:
Lorenzo Pavone, University of Catania, ItalyOrkun Tolunay, University of Health Sciences, Türkiye
© 2023 Cappellari, Mariani and Bruschi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto M. Cappellari YWxiZXJ0by5jYXBwZWxsYXJpQHBvbGljbGluaWNvLm1pLml0
 Alberto M. Cappellari
Alberto M. Cappellari Stefano Mariani
Stefano Mariani Gaia Bruschi2
Gaia Bruschi2