- Department of Pediatrics, The First Affiliated Hospital of Yangtze University, Jingzhou, China
Background: This study aimed to gather evidence from clinical trials on the efficacy and safety of the available treatments for intravenous immunoglobulin (IVIG)-resistant Kawasaki disease (KD) in children.
Methods: This work adopted the Newcastle–Ottawa scale to analyse the quality of the enrolled articles. A network meta-analysis was performed using clinical trials that compared drugs used to treat IVIG-resistant KD. Aggregate Data Drug Information System software v.1.16.5 was employed to analyse whether infliximab, second IVIG infusions, and intravenous pulse methylprednisolone (IVMP) were safe and effective.
Results: Ten studies, involving 704 patients with IVIG-resistant KD, were identified and analysed. Overall, infliximab exhibited remarkable antipyretic activity compared with the second IVIG infusions (2.46, 1.00–6.94). According to the drug rank, infliximab was the best option against IVIG-resistant KD. Regarding adverse effects, the infliximab group was more prone to hepatomegaly. A second IVIG infusion was more likely to result in haemolytic anaemia. IVMP treatment was more susceptible to bradycardia, hyperglycaemia, hypertension, and hypothermia. In addition, infliximab, IVMP, and the second IVIG infusions showed no significant differences in the risk of developing a coronary artery aneurysm (CAA).
Conclusion: Infliximab was the best option against IVIG-resistant KD, and IVMP, infliximab, and second IVIG infusions have not significant differences of prevent CAA in patients with IVIG-resistant KD.
Systematic Review Registration: Identifier: https://osf.io/3894y.
Introduction
Kawasaki disease (KD) is an acute, self-limiting, systemic vascular inflammation mainly occurring in small arteries, particularly the coronary arteries (1, 2). In the acute stage, immunoglobulins administered at high doses may decrease coronary artery injury, but 15%–20% of such cases will develop intravenous immunoglobulin (IVIG)-resistant KD (3). According to the literature, coronary artery aneurysm (CAA) incidence is 9-fold higher in IVIG-resistant KD cases than that in IVIG-sensitive cases (4). IVIG-resistant KD may have an increased risk of coronary artery injury compared to IVIG-sensitive KD. Therefore, the risk of coronary artery injury and hospitalisation duration and costs are reduced if IVIG-resistant KD cases are detected, and appropriate treatment is administered prior to further IVIG therapy.
For febrile IVIG-resistant cases, no clear guidelines are available for treatment, which presents a typical challenge. Patients with IVIG-resistant KD should be treated with the second IVIG infusions (2 g/kg for 1 day). An alternative approach is either 30 mg/kg intravenous pulse methylprednisolone (IVMP; 30 mg/kg for 2–3 h once daily for 3 days) or infliximab (5 mg/kg for 1 day) (5). Infliximab is the drug of choice for treating IVIG-resistant KD (6). However, no uniform treatment guidelines are available, and many different treatments exist among diverse medical centres (7). In addition, drug-related adverse effects (AEs) remain unclear.
Several studies have investigated different drugs for treating IVIG-resistant KD. Previous meta-analyses showed that IVMP and infliximab exhibited higher efficacy than the second IVIG infusions (8, 9). However, the previous pairwise meta-analysis could only analyse two drugs. Network meta-analysis (NMA) can analyse multiple drugs based on clinical research. It has a high reference value for evaluating the advantages of interventions and can provide the best evidence for clinical decision-making. This study aimed to perform a systematic review and Bayesian NMA on paediatric patients reported in studies published in several databases over the past 15 years to investigate the efficacy and safety of different drug regimens for treating IVIG-resistant KD.
Methods
The present study performed NMA and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines extended to NMA (10). Additionally, this study utilised a population-intervention-comparison-outcome framework to include studies describing the treatment of IVIG-resistant KD. Our study protocols were registered in the OSF Registries (https://osf.io/3894y).
Database search
Relevant databases, such as PubMed, Embase, ScienceDirect, ProQuest, ClinicalTrials.gov, ClinicalKey, Cochrane CENTRAL, and Web of Science, were comprehensively searched until 1 May, 2022 to identify relevant studies. The search strategy was approved by the review teams (LH and QF). Regarding the search strategy, the Medical Subject Headings used were (Mucocutaneous Lymph Node Syndrome OR Kawasaki disease) AND (methylprednisolone OR intravenous immunoglobulin/IVIG OR infliximab OR corticosteroids OR steroids OR glucocorticoids OR TNF blockers). No study design or language restrictions were imposed. Additionally, the reference lists of the enrolled studies were manually searched. Finally, two reviewers (LH and QF) reviewed the studies and extracted relevant data.
Study quality assessment
This study used the Newcastle–Ottawa scale (NOS) to assess the quality of the enrolled observational studies. Typically, we judged the NOS statements on three aspects (selection, outcome, and comparability) involving eight items. The Cochrane Collaboration-recommended risk-of-bias approach was used to assess the quality of the randomised clinical trials. A final score of six or more stars was considered high quality.
Selection criteria
Studies conforming to the criteria below were included: (a) patients with the diagnosis of KD in line with the Japanese diagnostic criteria, as well as common standards from the 2017 American Heart Association (i.e., IVIG resistance was defined as persistent or recrudescent fever [T ≥38.0 °C] at least 36 h after completion of the first IVIG infusion), (b) odds ratios (ORs) together with relevant 95% confidence intervals (CIs) regarding categorical variables or numbers and standard deviations could be obtained from the studies, and (c) statistical approaches were clearly described, and statistical analysis was conducted accordingly. The following studies were excluded: (a) studies with defects or low-quality (NOS score<six stars), (b) no ORs or 95% CIs could be obtained for categorical variables, and (c) reviews, duplicates, or unpublished literature.
Statistical analyses
This study utilised NMA to analyse all enrolled articles. Moreover, Aggregate Data Drug Information System software v. 1.16.6 was used to compare the safety and effectiveness of diverse therapeutic agents (11). The Bayesian method was applied in the NMA, which made it possible to compare diverse treatments among different studies (12). We adopted a random-effects model with the Bayesian method through a Markov chain Monte Carlo simulation to obtain the combined effect sizes. We also drew a consistency model to analyse the outcomes assessed and determined the relative effect sizes of treatments based on ORs. Instead of the fixed-effects model, we utilised the random-effects model because it is suitable and conservative for interpreting interstudy variance. Residual deviance was also used to evaluate the goodness of fit of the models. To increase the accuracy of comparison effect sizes and appropriately explain the relationships of multiarm studies, this study constructed rank probabilities that involved every intervention in every outcome to draw conclusions for diverse outcomes of interest (13). Data were expressed with 95% CIs. Subsequently, diverse treatments were ordered based on the highest to the lowest probabilities.
Result
Study selection and description
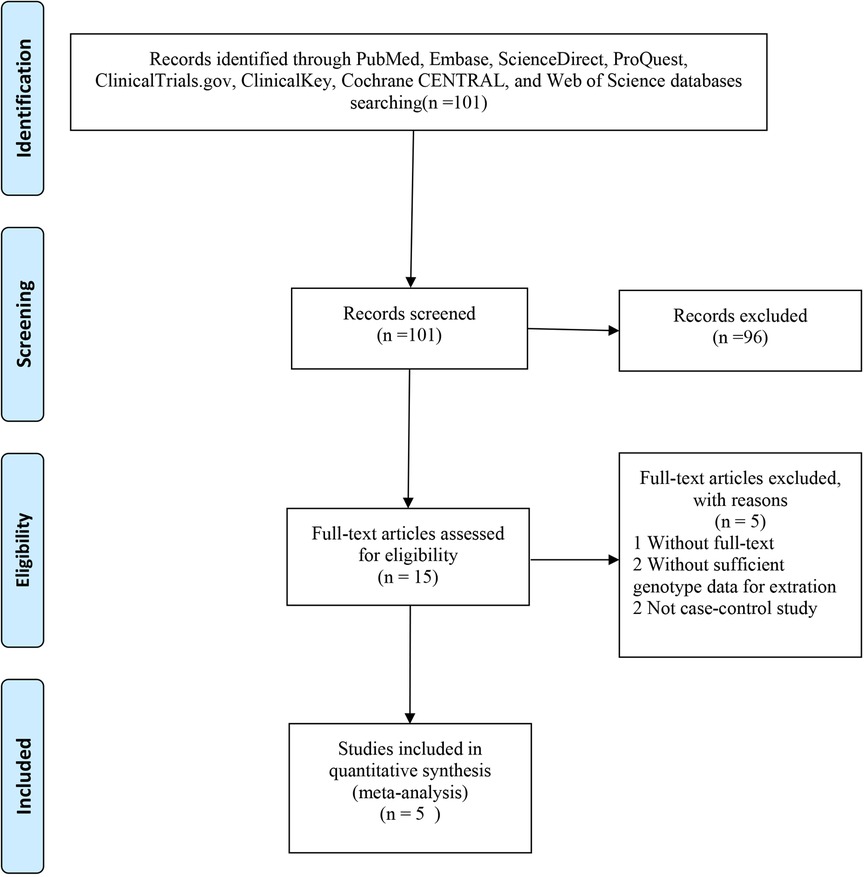
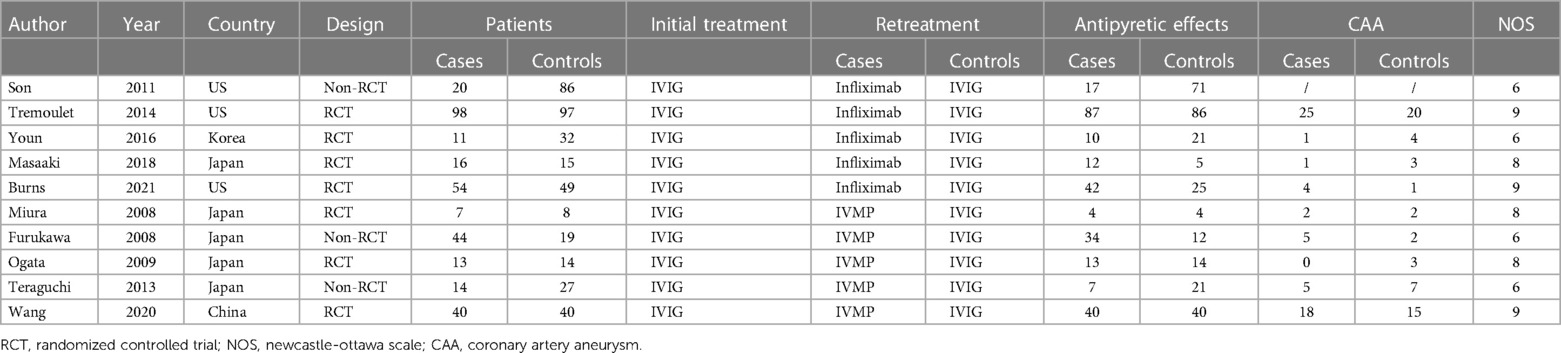
A total of 101 eligible articles were included (Figure 1). Of these, 96 did not meet the inclusion criteria and were not subjected to further examination. We excluded two publications because they did not provide detailed genotypic information. We also excluded two publications because they were not case-control studies. Furthermore, one publication was removed because the full text was not available. In line with our inclusion and exclusion criteria, this work selected 10 articles published between 2003 and 2021 (13–22). Among these, seven were randomised controlled trials (RCTs) (1, 15, 16, 20–22), and three were non-RCTs (14, 18, 19), as determined based on the Cochrane Handbook. The generation of random sequences was not utilised by Furakawa et al. and Teraguchi et al. since some patients were unwilling to receive IVMP and therefore received a second dose of IVIG (14, 19). Data from Son et al. were collected through a retrospective chart review, and all studies were rated as ≥six stars (high quality) (18). Baseline features on admission were comparable among the diverse treatments, such as age at fever onset, sex, race, duration between fever onset and diagnosis, and duration from the first treatment to retreatment. Five studies were conducted in Japan, three in America, and one each in China and Korea (Table 1).
Antipyretic effects
Infliximab was associated with significant antipyretic effects compared with the second IVIG infusion (2.46, 1.00–6.94, Figure 2). No significant differences were recorded between the IVMP and IVIG retreatment groups (0.92, 0.25–3.51). Furthermore, no significant differences were recorded between infliximab and IVMP (2.70, 0.53–14.42). According to the drug rankings (see Figure 3), infliximab had better antipyretic effects than the other drugs. Based on the current research results, infliximab is the best option against IVIG-resistant KD.
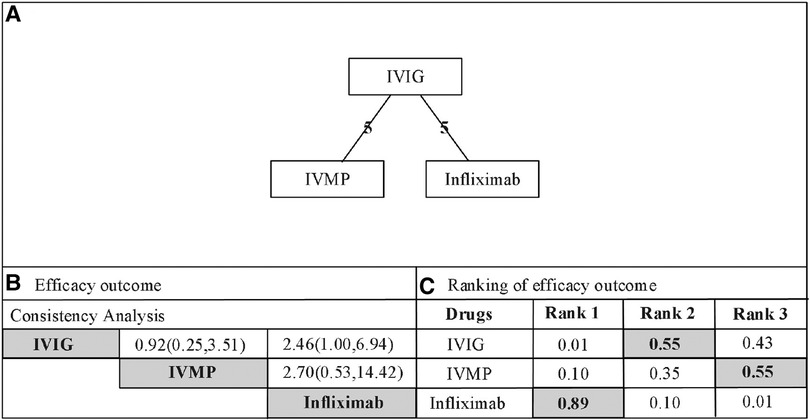
Figure 2. (A) Network of comparisons for efficacy outcome. The nodes (drugs) are represented by circles. The grey circles represent stimulant drug and the white circles represent non-stimulant drugs. The lines connecting each drug represent direct comparisons, while indirect ones were statistically estimated. The thickness of the line represents the amount of existing comparisons and the size of the circles (nodes) indicates the sample-size number. (B) Consistency analysis for the outcome of efcacy. Drugs are reported in alphabetical order. The values presented correspond to the mean diference (MD) associated with its credibility interval (CrI). When the CrI does not cross the 0 null line, there is a statistically signifcant diference between the treatments. Comparisons are made between a first drug (e.g. IVIG) and a second drug (e.g. IVMP) with presentation of the estimated value (0.92 [0.25-3.51]). An MD value of less than 0 demonstrates that the frst drug in the comparison is the more effective. An MD value greater than 0 indicates that the second drug in the comparison is more efective. The highlighted pictures presented statistical diferences. IVIG, intravenous immunoglobulin; IVMP, intravenous pulse methylprednisolone. (C) Rank probabilities of drugs. The values are given as the probability of each treatment occupying a position. Ranking 1 is the best therapy and the last one is the worst treatment for this outcome. IVIG, intravenous immunoglobulin; IVMP, intravenous pulse methylprednisolone.
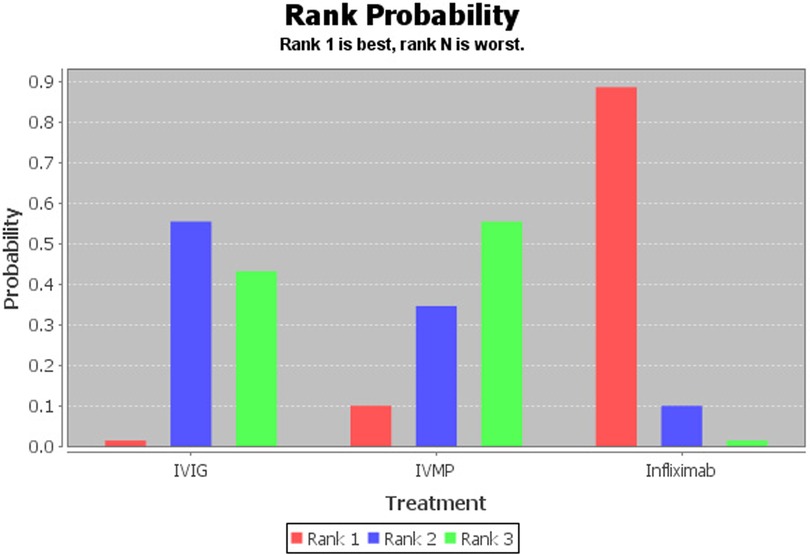
Figure 3. Rank probability graph of drugs. The values are given as the probability of each treatment occupying a position. Ranking 1 is the best therapy (more likely to lead to antipyretic effects) and the last one is the worst treatment for this outcome.
AEs
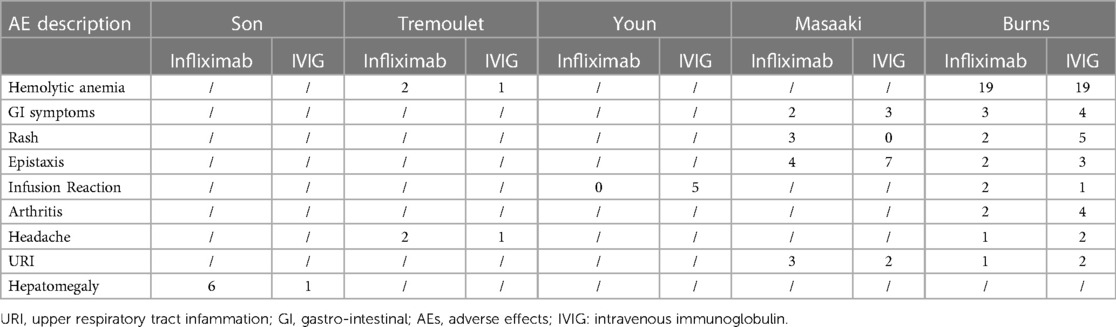
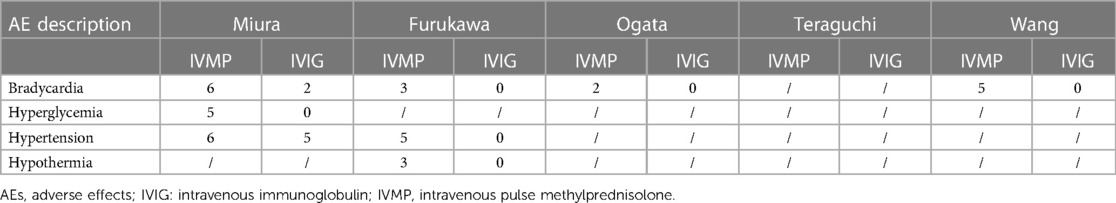
All included studies reported AEs during the disease course, except for the study by Teraguchi et al. (Tables 2, 3). In summary, hepatomegaly was more likely to occur in the infliximab group. Patients undergoing a second IVIG treatment were more likely to develop haemolytic anaemia. Compared with the second IVIG infusion, IVMP treatment was more susceptible to bradycardia, hyperglycaemia, hypertension, and hypothermia.
CAA
All included studies reported CAA, except for the study by Son et al. There were no significant differences in the risk of CAA between infliximab and the second IVIG infusion (1.34, 0.45–4.08). No significant differences were recorded between the IVMP and IVIG-retreatment groups (1.00, 0.25–2.91). Furthermore, no significant differences were observed between infliximab and IVMP (1.37, 0.31–8.29).
Discussion
As reported in a multicentre study, IVIG-resistant cases may have CAA (18.6%), although the first adequate IVIG treatment can reduce IVIG nonresponse (23). Some optimal clinical treatments have been proposed to manage IVIG-resistant cases, among which a second IVIG infusion alone or in combination with corticosteroids, long-course corticosteroids alone, or infliximab plus pulsed therapy has been frequently selected. Continuous or relapsed fever following the initial IVIG dose, but not laboratory measurements of inflammation, has been recognised as an indicator of continuous inflammation. A consensus has been reached that additional treatments should be administered to patients with such symptoms. According to the KD guidelines of the American Heart Association (AHA), a second IVIG dose or steroid therapy is assigned a B evidence level (non-RCTs), whereas infliximab is assigned a C evidence level (expert consensus) (24). The International Society for Pharmacoeconomics and Outcome Research recommends using NMA to compare outcomes among diverse treatment modalities. Thus, a robust NMA is required to guide treatment.
Previous meta-analyses have shown that IVMP and infliximab treatments are more effective than the second IVIG dose in terms of fever resistance (8, 9). Our NMA comparing the three drugs, showed that infliximab was the best option for treating IVIG-resistant KD. The expression of tumour necrosis factor (TNF)-α increases among patients with acute KD, with the greatest expression being observed in patients developing CAA (24). TNF inhibitors can mitigate endarteritis and inflammation by inhibiting the adhesion of neutrophils onto endothelial cells (ECs). Infliximab, the anti-TNF-α chimeric monoclonal antibody, has been used to treat IVIG-resistant KD over the last decade. In retrospective studies from two institutions, IVIG-resistant KD cases receiving infliximab as initial retreatment showed markedly rapid fever resolution and shortened length of stay (LOS) relative to those receiving IVIG (17). Another study in 2021 compared the cost-effectiveness between infliximab and a second IVIG infusion in IVIG-resistant cases; according to the results, for 100 IVIG-resistant cases receiving 10 mg/kg infliximab treatment, US$ 824,759 was saved (25). Such decreased costs were related to a reduction in cost/dose and infusion duration, and 24-h monitoring prior to discharge, which shortened the LOS (14). Therefore, our study further confirmed the potential value of infliximab treatment in patients with IVIG-resistant KD. These results could be conducive to recommending an objective order of these treatment options in future studies and guidelines.
In severe KD cases, cardiovascular complications or manifestations have been strongly associated with the incidence and mortality in the acute phase or during long-term follow-up. As revealed by Millar et al., corticosteroid application in acute KD patients who developed CAA possibly induced aggravation of aneurysms, as well as impairment of vascular remodelling (15). As reported by the AHA, steroids only apply to paediatric patients who do not respond to ≥two IVIG infusions for treating continuous fever (26). However, according to previous meta-analyses, infliximab, second IVIG, and IVMP were not significantly different in CAA prevention (8, 9). Similar results were obtained in this study. These drugs may suppress cytokine generation, which is important for reconstructing the affected coronary artery wall (17). It is necessary to further investigate the long-term coronary artery outcomes among treated KD cases and to estimate coronary artery endothelium function in KD cases.
Certain limitations should be noted in this work. First, many articles included in this study were observational RCTs, which may have led to an increased risk of heterogeneity. Second, only infliximab (a TNF inhibitor) was used in every enrolled study, making it impossible to assess the efficacy of additional TNF inhibitors in IVIG-resistant KD. Third, our enrolled articles were collected from published literature, and some unpublished articles might have been missed. Finally, although no significant statistical or clinical heterogeneity was observed across the included studies, potential bias existed because the literature is limited. Most included studies did not completely evaluate the postretreatment incidence of CAAs in patients with IVIG-resistant KD after short-term follow-up. Therefore, large, homogeneous, randomised clinical trials with long follow-up periods are required.
Infliximab was the best options against IVIG-resistant KD, respectively. In addition, IVMP, infliximab and second IVIG infusion have not significant differences of prevent CAA in IVIG-resistant KD patients. More studies will need to be conducted to evaluate the different drug regimens of IVIG-resistant KD.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
YP contributed to the design of the study and reviewed and revised the manuscript. YP followed up with the patient, collected information from the literature, and wrote sections of the manuscript. YP provided assistance for treatment. YP collected the data and followed up with the patient. YP approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Hubei Pediatric Alliance Medical Research Project (HPAMRP202117).
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Son MB, Newburger JW. Kawasaki disease. Pediatr Rev. (2013) 34(4):151–62. doi: 10.1542/pir.34.4.151
2. Pilania RK, Jindal AK, Bhattarai D, Naganur SH, Singh S. Cardiovascular involvement in kawasaki disease is much more than mere coronary arteritis. Front Pediatr. (2020) 8:526969. doi: 10.3389/fped.2020.526969
3. Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, et al. Epidemiologic features of kawasaki disease in Japan: results of the 2009–2010 nationwide survey. J Epidemiol. (2012) 22(3):216–21. doi: 10.2188/jea.JE20110126
4. Campbell AJ, Burns JC. Adjunctive therapies for kawasaki disease. J Inf Secur. (2016) 72(Suppl):S1–5. doi: 10.1016/j.jinf.2016.04.015
5. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–929. doi: 10.1161/CIR.0000000000000484
6. Rife E, Gedalia A. Kawasaki disease: an update. Curr Rheumatol Rep. (2020) 22:75. doi: 10.1007/s11926-020-00941-4
7. Xue LJ, Wu R, Du GL, Xu Y, Yuan KY, Feng ZC, et al. Effect and safety of TNF inhibitors in immunoglobulin-resistant kawasaki disease: a meta-analysis. Clin Rev Allergy Immunol. (2016) 52:389–400. doi: 10.1007/s12016-016-8581-4
8. Chan H, Chi H, You H, Wang M, Zhang G, Yang H, et al. Indirect-comparison meta-analysis of treatment options for patients with refractory kawasaki disease. BMC Pediatr. (2019) 19(1):158. doi: 10.1186/s12887-019-1504-9
9. Xue LJ, Wu R, Du GL, Xu Y, Yuan KY, Feng ZC, et al. Effect and safety of TNF inhibitors in immunoglobulin-resistant kawasaki disease: a meta-analysis. Meta-analysis. Clin Rev Allergy Immunol. (2017) 52(3):389–400. doi: 10.1007/s12016-016-8581-4
10. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
11. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. (2011) 14(4):417–28. doi: 10.1016/j.jval.2011.04.002
12. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Mak. (2013) 33(5):641–56. doi: 10.1177/0272989X12455847
13. Burns JC, Roberts SC, Tremoulet AH, He F, Printz BF, Ashouri N, et al. Infliximab versus second intravenous immunoglobulin for treatment of resistant kawasaki disease in the USA (KIDCARE): a randomised, multicentre comparative effectiveness trial. Lancet Child Adolesc Health. (2021) 5(12):852–61. doi: 10.1016/S2352-4642(21)00270-4
14. Furukawa T, Kishiro M, Akimoto K, Nagata S, Shimizu T, Yamashiro Y. Effects of steroid pulse therapy on immunoglobulin-resistant kawasaki disease. Arch Dis Child. (2008) 93(2):142–6. doi: 10.1136/adc.2007.126144
15. Mori M, Hara T, Kikuchi M, Shimizu H, Miyamoto T, Iwashima S, et al. Infiximab versus intravenous immunoglobulin for refractory kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. (2018) 8(1):1994. doi: 10.1038/s41598-017-18387-7
16. Miura M, Kohno K, Ohki H, Yoshiba S, Sugaya A, Satoh M. Effects of methylprednisolone pulse on cytokine levels in kawasaki disease patients unresponsive to intravenous immunoglobulin. Eur J Pediatr. (2008) 167(10):1119–23. doi: 10.1007/s00431-007-0642-5
17. Ogata S, Bando Y, Kimura S, Ando H, Nakahata Y, Ogihara Y, et al. The strategy of immune globulin resistant kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. J Cardiol. (2009) 53(1):15–9. doi: 10.1016/j.jjcc.2008.08.002
18. Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, et al. Infliximab for intravenous immunoglobulin resistance in kawasaki disease: a retrospective study. J Pediatr. (2011) 158(4):644–649.e1. doi: 10.1016/j.jpeds.2010.10.012
19. Teraguchi M, Ogino H, Yoshimura K, Taniuchi S, Kino M, Okazaki H, et al. Steroid pulse therapy for children with intravenous immunoglobulin therapy-resistant kawasaki disease: a prospective study. Pediatr Cardiol. (2013) 34(4):959–63. doi: 10.1007/s00246-012-0589-9
20. Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, et al. Infliximab for intensification of primary therapy for kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. (2014) 383(9930):1731–8. doi: 10.1016/S0140-6736(13)62298-9
21. Wang Z, Chen F, Wang Y, Li W, Huang P. Methylprednisolone pulse therapy or additional IVIG for patients with IVIG-resistant kawasaki disease. J Immunol Res. (2020) 2020:4175821. doi: 10.1155/2020/4175821. eCollection 2020 33299898
22. Youn Y, Kim J, Hong YM, Sohn S. Infliximab as the first retreatment in patients with kawasaki disease resistant to initial intravenous immunoglobulin. Pediatr Infect Dis J. (2016) 35(4):457–9. doi: 10.1097/INF.0000000000001039
23. Shulman ST, Rowley AH. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat Rev Rheumatol. (2015) 11:475–82. doi: 10.1038/nrrheum.2015.54
24. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. (2016) 67:1738–4179. doi: 10.1016/j.jacc.2015.12.073
25. Scarlett CJ, Daniel CW, Daniel B, Marshall C, Annie S, Annie LA. A cost comparison of infliximab versus intravenous immunoglobulin for refractory kawasaki disease treatment. Hosp Pediatr. (2021) 11:88–93. doi: 10.1542/hpeds.2020-0188
Keywords: immunoglobulin, resistant kawasaki disease, infliximab, IVIG, methylprednisolone
Citation: Pan Y, Fan Q and Hu L (2023) Treatment of immunoglobulin-resistant kawasaki disease: a Bayesian network meta-analysis of different regimens. Front. Pediatr. 11:1149519. doi: 10.3389/fped.2023.1149519
Received: 22 January 2023; Accepted: 30 June 2023;
Published: 13 July 2023.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Rakesh Kumar Pilania, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaXiaohui Li, Children's Hospital of Capital Institute of Pediatrics, China
© 2023 Pan, Fan and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Pan d29zaGlwYW55YW5AMTI2LmNvbQ==
 Yan Pan
Yan Pan Qihong Fan
Qihong Fan