- 1Department of Dermatology, Affiliated Haikou Hospital Xiangya School Central South University and Haikou Municipal Municipal People’s Hospital, Haikou, Hainan, China
- 2Department of Hematology, Affiliated Haikou Hospital Xiangya School Central South University and Haikou Municipal Municipal People’s Hospital, Haikou, Hainan, China
Background: Although the association between maternal exposure to antibiotics and the risk of atopic dermatitis (AD) in childhood has been studied extensively, there still is a lack of clarity on the topic. The aim of this study was to summarize the published data and to examine if maternal exposure to antibiotics increases the risk of AD in childhood.
Methods: Systematic search was performed in PubMed, Scopus, Web of Science, and Embase for all types of studies on the review subject independent of any language restrictions and published up to 28th December 2022. Data was analyzed using random-effects model and presented as pooled odds ratio (OR) with 95% confidence intervals (CI).
Results: A total of 18 studies (5,354,282 mother-child pairs) were included. Maternal exposure to antibiotics was associated with an increased risk of AD in childhood (OR: 1.14, 95% CI: 1.06, 1.22, I2 = 85%, p = 0.0003). The significance of the results was not affected by the location of the study (Asia or Europe). While subgroup analysis based on exposure assessment or diagnosis of AD demonstrated a tendency of increased risk of AD, the association was not statistically significant in multiple subgroups. Segregating data based on the timing of exposure did not affect the significance of the results for studies on all trimesters. However, there was no association between antibiotic exposure in the third trimester or just before delivery and the risk of childhood AD.
Conclusion: The results of this meta-analysis suggest that maternal exposure to antibiotics may lead to a modestly increased risk of AD in offspring. The evidence is limited by high interstudy heterogeneity and bias in exposure and outcome assessment. Future studies are needed to explore if the timing of exposure, the dose, the number of prescriptions, and the type of antibiotic affect this association.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023387233.
Introduction
Atopic dermatitis (AD) is a common, chronic, and recurring inflammatory skin disease of childhood. It is characterized by intense itching, dryness, and eczematous lesions (1). Studies show that AD affects around 20% of children, 7%–14% of adult population, and the incidence of the disease has increased dramatically in the last decade due to lifestyle changes (2). AD significantly impacts the quality of life of children and their parents (3). The intense pruritis associated with the disease leads to sleep disturbances, adverse psychosocial effects, and increased financial burden on the patients and on the healthcare system (4).
During the last decade, clinicians focused on identifying risk factors for AD which can be modified to reduce disease prevalence. One such factor is maternal exposure to antibiotics which has been linked with several allergic diseases (5–8). Women's' susceptibility to infections increase during pregnancy and over 40% of women receive antibiotics before the delivery. Recent meta-analyses showed that maternal exposure to antibiotics can lead to an increased risk of asthma and food allergies in childhood (6, 8). Numerous studies suggest that exposure to antibiotics alters maternal microbiota which in turn can influence infant immunity and increase the risk of allergies (9–11).
The relationship between maternal exposure to antibiotics and the risk of AD has been a subject of research in multiple studies (6–8) but with conflicting results. While few studies have reported a positive association (12–14), others have not detected the correlation between maternal exposure to antibiotics and AD (15–17). Several meta-analyses (6–8) have attempted to summarize the evidence. However, all of them had a very small number of included studies, ranging from five to seven. Therefore, the current updated review was designed to examine if maternal exposure to antibiotics is associated with the increased risk of AD in childhood.
Material and methods
The PROSPERO registration (No. CRD42023387233) of the review was completed before the beginning of the study. The review conformed to the standard instructions of the PRISMA statement (18).
Literature search
The search strategy was completed independently by two reviewers. Systematic electronic literature search of PubMed, Scopus, Web of Science, and, Embase was done. Since these databases are not comprehensive, a Google Scholar search was also conducted for gray literature. The search was independent of any language restrictions and publication dates, and was completed on 28th December 2022. The terms “eczema”; “atopic dermatitis”; “pregnancy”; “maternal”; “prenatal”; “antimicrobials”; and “antibiotics” formed the various search strings used for the literature search (Supplementary Table S1). The search strings were common to all databases. Two reviewers screened the studies, identified by the systematic search. After removal of duplicates, titles and abstracts of the remaining articles were assessed. Full texts of relevant articles were retrieved and checked for the eligibility by both reviewers. All discrepancies between the reviewers were resolved by discussion.
Inclusion criteria
The standardized PECOS inclusion criteria were used to select eligible studies. The criteria for each domain were as follows:
Population- Mother-child pairs
Exposure- Exposure to antibiotics before birth
Comparison- No exposure to antibiotics
Outcome- AD in childhood (<18 years of age) [Outcome was to be reported as adjusted effect size with 95% confidence intervals (CI)]
Study type- All types
Exclusion criteria were: (1) studies not specifically on AD and reporting data on all allergic diseases combined (2) studies not reporting adjusted outcomes (3) studies not reporting exclusively on AD in childhood (4) studies with overlapping or duplicate data. In such cases, the study with the largest sample or the most comprehensive data was selected.
Data extraction
Two authors extracted the following data in separate forms: author's last name, database of the study, country of origin, study type, sample size, children's age range, method of antibiotic exposure assessment, the timing of exposure, diagnosis of AD, factors adjusted in the data analysis, and effect size. If studies reported multiple ratios of the association for different subgroups, these were combined using the meta-analysis software to generate a single combined ratio. No restrictions were placed on the method of exposure assessment, the timing of exposure, or the diagnosis of AD. All methods reported by the studies were acceptable.
Quality assessment
Quality assessment was conducted using the Newcastle-Ottawa scale (NOS) (19). Every study was examined for the following: the selection of study population, comparability, and outcomes. These parameters were given a maximum of four, two, and three points respectively. Quality assessment was also carried out separately by the two authors of this review. Any discordant scoring was resolved by consensus.
Statistical analysis
The meta-analysis was performed using “Review Manager” [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014]. Effect ratios from the studies were combined in a random-effects model to calculate pooled odds ratio (OR) with 95% confidence intervals (CI). The I2 statistic tool was used for inter-study heterogeneity. Values of >50% indicated high heterogeneity. Funnel plots were used for publication bias. The robustness of the results was checked by excluding one study at a time in a sensitivity analysis. P-values <0.05 were considered statistically significant. Subgroup analyses were conducted based on study location, study type, sample size, exposure assessment, diagnosis of AD, and timing of exposure.
Results
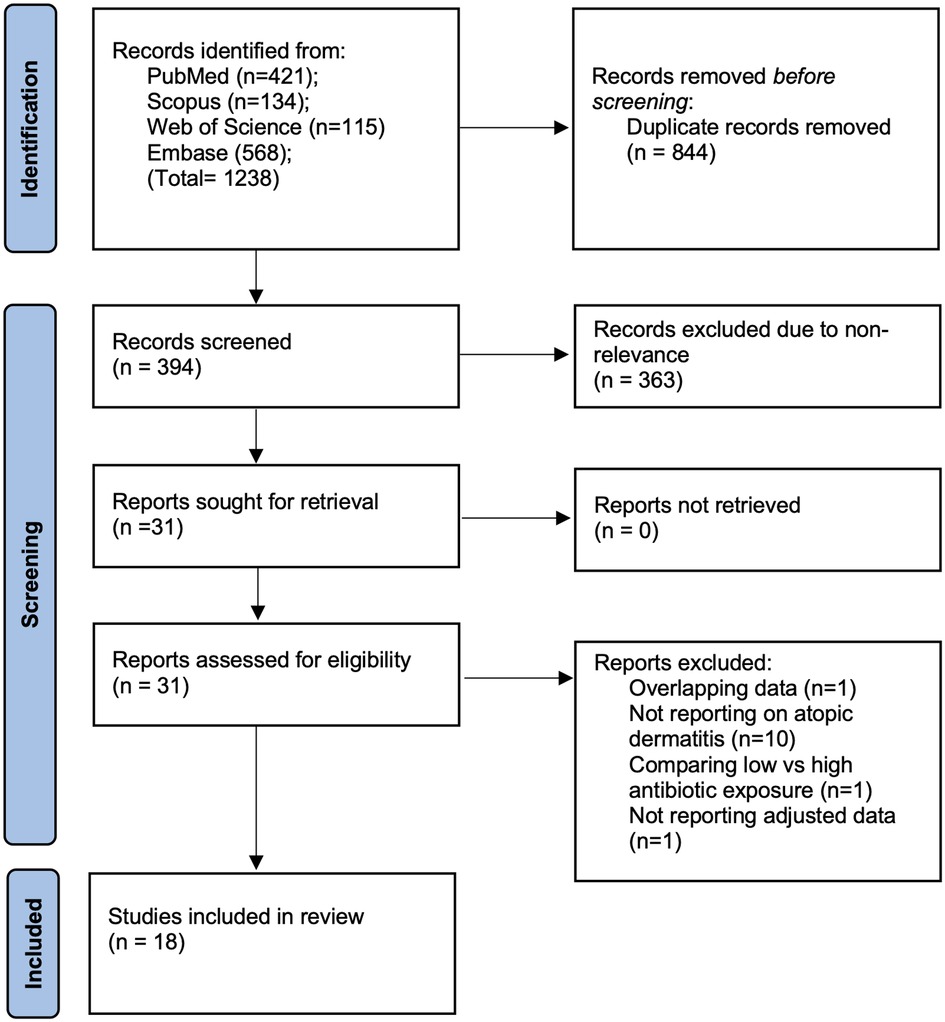
Systematic literature search across all databases resulted in 1,238 studies. Of them, 844 were removed as duplicates. Of 394 articles, 31 studies were selected for complete text review. Thirteen studies failed to meet the eligibility criteria. Finally, 18 studies were included in the review (12–17, 20–31) (Figure 1). The inter-reviewer reliability for the selection of studies was high (κ = 0.9).
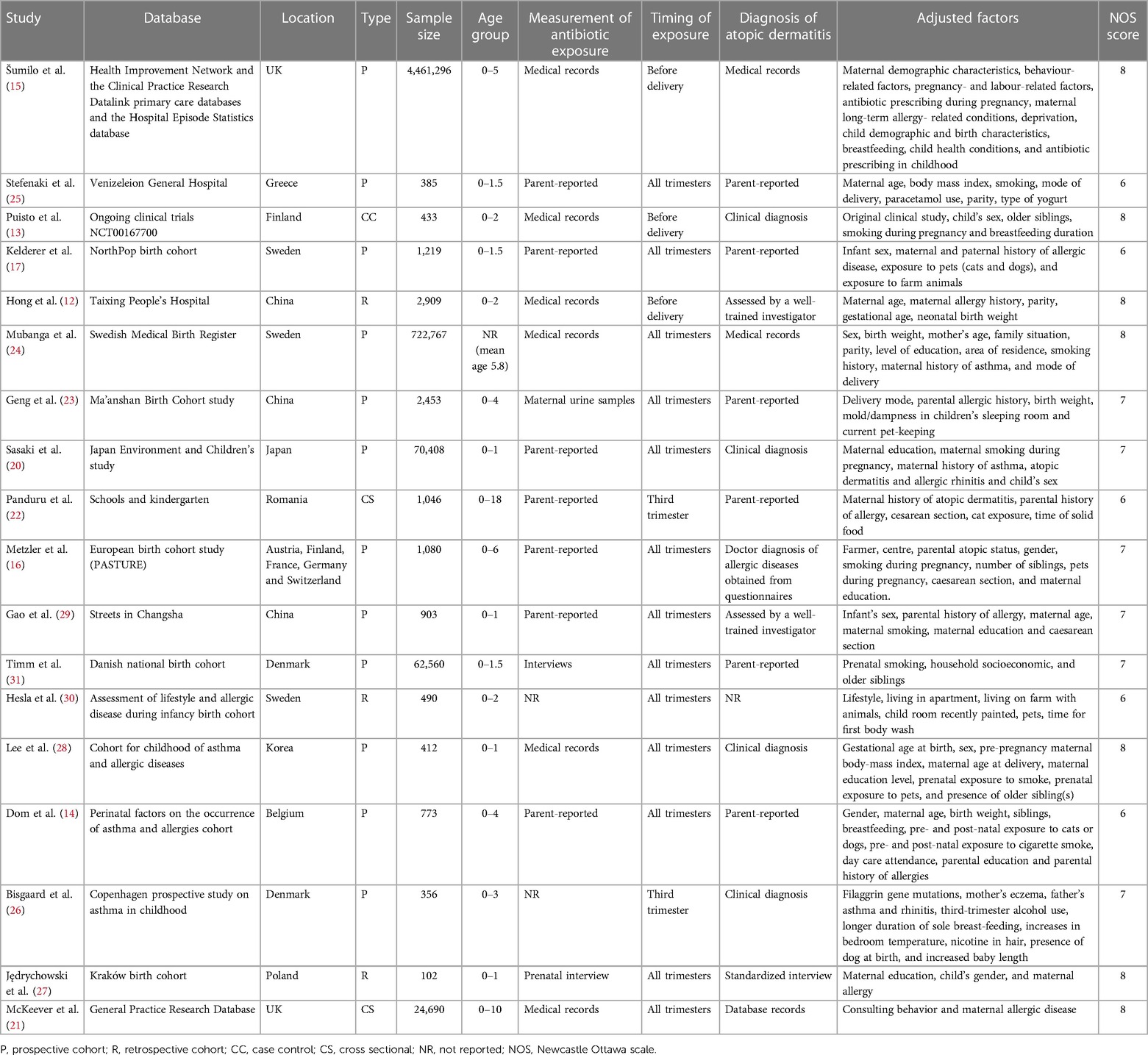
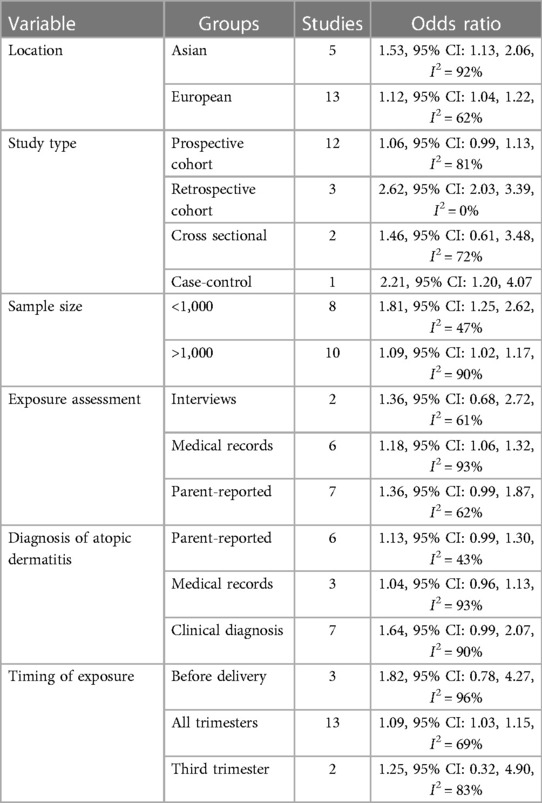
All 18 studies were from Asian or European countries (Table 1). Most of the papers were prospective cohort studies, except for three retrospective cohort studies, two cross-sectional studies, and one case-control study. The studies were published between 2002 and 2022. The total mother-child pair number was 5,354,282. Exposure to antibiotics was determined either by medical records, interviews, and urine samples, or was parent-reported. Most studies did not differentiate the timing of antibiotic exposure. Two studies reported third-trimester exposure, and three studies described pre-delivery exposure. Diagnosis of AD was either clinical or parent-reported or obtained from medical records or interviews. All studies used different confounding variables in the adjusted data analysis. The studies of McKeever et al. (21) and Mubanga et al. (24) used hazard ratios to report the association between antibiotic exposure and risk of AD while all other studies used OR. Due to limitations of data conversion the hazard ratios were pooled in the meta-analysis. The NOS score of the studies was between 6 and 8.
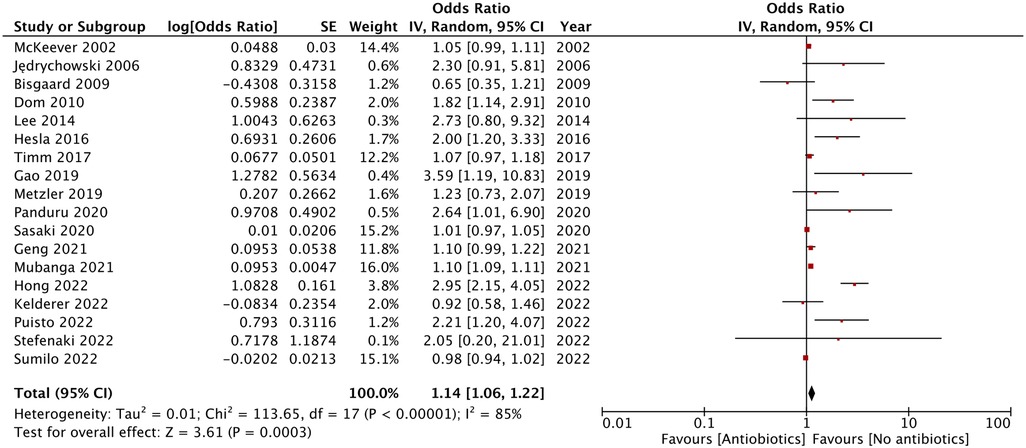
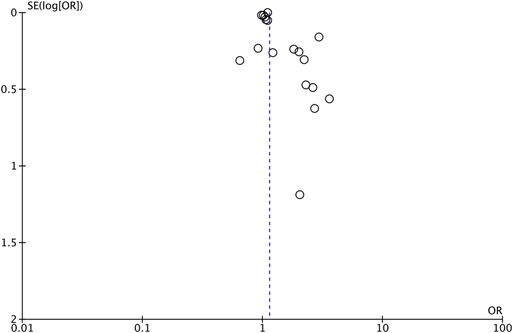
As shown by the results of the meta-analysis of all 18 studies, maternal exposure to antibiotics was associated with increased risk of AD in childhood (OR: 1.14, 95% CI: 1.06, 1.22, I2 = 85%, p = 0.0003) (Figure 2). The funnel plot did not demonstrate any major asymmetry (Figure 3). There was no change in the significance of results on the exclusion of any study during sensitivity analysis.
The results of subgroup analyses are shown in Table 2. The significance of results did not change based on study location (Asian or European) or sample size of the studies (>1,000 or <1,000). In terms of study type, the results remained significant for retrospective studies but not for prospective or cross-sectional studies. Subgroup analysis based on exposure assessment or diagnosis of AD demonstrated a tendency of increased risk of AD but this increase became statistically insignificant in multiple subgroups. When segregated based on the timing of exposure, the results remained significant for studies on all trimesters. However, there was no association between antibiotic exposure in the third trimester or just before delivery and the risk of childhood AD.
Discussion
Over the past two decades, numerous studies have attempted to assess the association between maternal exposure to antibiotics and allergic diseases in children (5–8). Nevertheless, the underlying pathophysiological mechanisms of this association are still unclear. One hypothesis states that the type of microbial exposure received by the infant in-utero is responsible for shaping the intestinal flora and the immune system of the child. Research has shown that microbial colonization in the fetus starts as early as the 11th week of gestation. Therefore, any alteration of microbial flora by antibiotics could increase the risk of chronic allergic diseases (9–11). Using the mouse model, Alhasan et al. (32) showed that exposure to antibiotics during the gestational period correlated with marked dose-dependent changes in maternal and pup microbiota and was associated with a proportionately increasing asthma severity. Another study has reported that maternal exposure to antibiotics led to significant alteration of intestinal microbiota with reduced biodiversity of Lactobaccillus and Bifidobacterium (33). Both Lactobaccillus and Bifidobacterium are probiotics. Recent studies showed that early life exposure to these strains can reduce the risk of AD (34). However, the results of the studies in support of this hypothesis are still inconclusive. Corresponding clinical studies have also produced mixed results. While some studies showed that risk of AD increased after maternal exposure to antibiotics (12–14), others found no significant association (15–17).
Considering these lack of consensus, the results of our meta-analysis are of clinical significance, as it was based on a detailed and comprehensive literature search that included higher number of studies compared to previous reviews of Cait et al. (6), Zhong et al. (8), and Huang et al. (7) which included only five, seven, and seven studies, respectively. All three of these previous reviews noted a statistically significant increase in the risk of AD with maternal exposure to antibiotics, with an effect size of 1.28, 1.62, and 1.93, respectively. While our results are in agreement with these studies, we also demonstrate that the risk of AD is very modest at 14% with a narrow CI of 6%–22%. Credibility of our results is further strengthened by their stability, as indicated by the sensitivity analysis.
The primary and most significant limitation of the meta-analysis is its high (85%) heterogeneity. This inter-study heterogeneity may be explained by the varied study populations and methodological differences in the included studies. Multiple subgroup analysis that was performed to assess the source of heterogeneity showed that the results were not affected by the study location and sample size. A slightly increased risk of AD was noted in Asian vs. European studies (53% vs. 12%) but this could be due to a smaller proportion of Asian studies in the review. Also, most of the studies included in the review were predominantly prospective cohort studies. The risk of AD was increased in both study types but just lost statistical significance in the case of only prospective studies.
Assessment of exposure and outcomes can also be a major source of bias. Assessment of antibiotic exposure from medical records and clinical diagnosis of AD is more reliable that self-reported data which can be a subject of recall bias. There is always a possibility of mistaken reporting of drugs used by the women, or reporting other symptoms for AD. However, no marked difference was noted in the subgroup analyses, and all results remained statistically significant or showing a tendency of increased risk of AD.
According to the altered gut microbiota theory, the timing of maternal antibiotic exposure should be an important variable influencing the risk of AD. Theoretically, very early exposure to antibiotics should have minimal impact as there is still enough to restore the maternal microbiota. However, research shows that certain classes of microorganisms, eliminated by antibiotics, are not restored even after nine months (35). Literature is scarce on the association of trimester-wise exposure to antibiotics and the risk of AD. Therefore, our meta-analysis included only few such studies and showed that third-trimester exposure and intrapartum exposure to antibiotics were not associated with an increased risk of AD. These results must be considered in light of changed guidelines that recommend the use of antibiotics before skin incision in caesarean sections (15). However, studies also suggest that the mode of delivery is an important confounder and the correlation between intrapartum antibiotic treatment and AD is increased for vaginally delivered infants but not for infancts, delivered by cesarean sections (12). Similarly, while Mubanga et al. (24) have shown a modest increase in the risk of AD that was associated with maternal antibiotics in all three trimesters, Geng et al. (23) have shown that the timing of antibiotic exposure is confounded by the type of antibiotic. They noted that sulfamethazine exposure in the first trimester and ciprofloxacin exposure in the second trimester increased the risk of AD in offspring. Further research on the timing of maternal antibiotic exposure and the risk of AD is needed.
This review should be interpreted with the following limitations. The pathophysiology of AD is complex with a number of confounding factors such as maternal smoking, socioeconomic status, parental history of allergy, infant gender, birth weight, delivery mode, breastfeeding, pet contact, etc., (7). While our study used adjusted data, there was variability in covariates among studies, and many known and unknown confounders were missed. Secondly, it is possible that the interaction was affected by the multiple variables related to the antibiotic treatment regimen. This review was unable to assess how the timing of exposure, the dose, the number of prescriptions, and the type of antibiotic alter the risk of AD. Thirdly, rigorous recoding of exposure and outcome was not followed in all studies. Medical records could be prone to errors while self-reported data can be erroneous due to recall bias. More objective methods for both exposure and outcome assessment should be utilized by future studies to generate rigorous evidence. Lastly, there was a predominance of European studies in the review and this may affect the general applicability of the results.
In terms of the clinical significance, our review demonstrated that while maternal exposure to antibiotics led to a higher risk of AD in offspring, the increased risk was modest at 14% (6%–22%). Given the high inter-study heterogeneity and bias in the included studies, our results do not recommend avoiding antibiotics in pregnancy. The prescription of antibiotics in pregnancy should be carefully considered after evaluating the risk vs. benefit ratio. Most of the times antibiotics are needed for improving the overall health of the female and prevent any adverse effects of the infectious disease on the fetus. However, mothers should be advised that any intake of antibiotics during pregnancy may lead to a small risk of AD in the child who needs to be closely monitored for early diagnosis and treatment.
Conclusions
The results of this meta-analysis suggest that maternal exposure to antibiotics may lead to a modestly increased risk of AD in childhood. The evidence is limited by high interstudy heterogeneity and bias in exposure and outcome assessment. Future studies are needed to explore if the timing of exposure, the dose, the number of prescriptions, and the type of antibiotic affect this association.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MW and XY: conceived and designed the study. MW: was involved in the literature search and data collection. MW and XY: analyzed the data. XY: wrote the paper. and XY: reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1142069/full#supplementary-material.
References
1. Zhang L, Hou Y, Sun J, Zeng Y. The top 100 most cited articles in the last two decades of atopic dermatitis: a bibliometric analysis. Front Immunol. (2022) 13:7152. doi: 10.3389/FIMMU.2022.949665
2. Bylund S, Von Kobyletzki LB, Svalstedt M, Svensson Å. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. (2020) 100:320–9. doi: 10.2340/00015555-3510
3. Weidinger S, Novak N. Atopic dermatitis. Lancet. (2016) 387:1109–22. doi: 10.1016/S0140-6736(15)00149-X
4. Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Child. (2019) 6:133. doi: 10.3390/CHILDREN6120133
5. Wang S, Zhang R, Li X, Gao Y, Dai N, Wei Y, et al. Relationship between maternal–infant gut microbiota and infant food allergy. Front Microbiol. (2022) 13:4287. doi: 10.3389/FMICB.2022.933152/BIBTEX
6. Cait A, Wedel A, Arntz JL, Duinkerken J, Datye S, Cait J, et al. Prenatal antibiotic exposure, asthma, and the atopic march: a systematic review and meta-analysis. Allergy. (2022) 77:3233–48. doi: 10.1111/ALL.15404
7. Huang FQ, Lu CY, Wu SP, Gong SZ, Zhao Y. Maternal exposure to antibiotics increases the risk of infant eczema before one year of life: a meta-analysis of observational studies. World J Pediatr. (2020) 16:143–51. doi: 10.1007/S12519-019-00301-Y
8. Zhong Y, Zhang Y, Wang Y, Huang R. Maternal antibiotic exposure during pregnancy and the risk of allergic diseases in childhood: a meta-analysis. Pediatr Allergy Immunol. (2021) 32:445–56. doi: 10.1111/PAI.13411
9. Prescott S, Dreisbach C, Baumgartel K, Koerner R, Gyamfi A, Canellas M, et al. Impact of intrapartum antibiotic prophylaxis on offspring microbiota. Front Pediatr. (2021) 9:1386. doi: 10.3389/FPED.2021.754013/BIBTEX
10. Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. (2016) 6:1543. doi: 10.3389/FMICB.2015.01543
11. Al Alam D, Danopoulos S, Grubbs B, Ali NA, MacAogain M, Chotirmall SH, et al. Human fetal lungs harbor a microbiome signature. Am J Respir Crit Care Med. (2020) 201:1002–6. doi: 10.1164/RCCM.201911-2127LE
12. Hong Z, Jing R, Hui L, Kang X, Chunmei Z, Yang W, et al. A cohort study of intrapartum group B streptococcus prophylaxis on atopic dermatitis in 2-year-old children. BMC Pediatr. (2022) 22:693. doi: 10.1186/S12887-022-03758-5
13. Puisto R, Turta O, Rautava S, Isolauri E. Early life exposures and development of allergic disease in infants with familial risk: results from ongoing probiotic intervention trials. Acta Paediatr. (2023) 112:115–21. doi: 10.1111/APA.16518
14. Dom S, Droste JHJ, Sariachvili MA, Hagendorens MM, Oostveen E, Bridts CH, et al. Pre- and post-natal exposure to antibiotics and the development of eczema, recurrent wheezing and atopic sensitization in children up to the age of 4 years. Clin Exp Allergy. (2010) 40:1378–87. doi: 10.1111/J.1365-2222.2010.03538.X
15. Šumilo D, Nirantharakumar K, Willis BH, Rudge GM, Martin J, Gokhale K, et al. Long-term impact of pre-incision antibiotics on children born by caesarean section: a longitudinal study based on UK electronic health records. Health Technol Assess. (2022) 26:vii–63. doi: 10.3310/ZYZC8514
16. Metzler S, Frei R, Schmaußer-Hechfellner E, von Mutius E, Pekkanen J, Karvonen AM, et al. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatr Allergy Immunol. (2019) 30:423–33. doi: 10.1111/PAI.13039
17. Kelderer F, Mogren I, Eriksson C, Silfverdal SA, Domellöf M, West CE. Associations between pre- and postnatal antibiotic exposures and early allergic outcomes: a population-based birth cohort study. Pediatr Allergy Immunol. (2022) 33:e13848. doi: 10.1111/PAI.13848
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
19. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed October 30, 2020)
20. Sasaki M, Sakurai K, Shimojo N, Yamamoto M, Mori C. No association between prenatal antibiotic exposure and atopic dermatitis among Japanese infants. Pediatr Allergy Immunol. (2020) 31:218–21. doi: 10.1111/PAI.13156
21. McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the west midlands general practice database. Am J Respir Crit Care Med. (2002) 166:827–32. doi: 10.1164/RCCM.200202-158OC
22. Panduru M, Epure AM, Cimpoca B, Cozma C, Giuca BA, Pop A, et al. Antibiotics administration during last trimester of pregnancy is associated with atopic dermatitis—a cross-sectional study. Rom J Intern Med. (2020) 58:99–107. doi: 10.2478/RJIM-2020-0006
23. Geng M, Tang Y, Liu K, Huang K, Yan S, Ding P, et al. Prenatal low-dose antibiotic exposure and children allergic diseases at 4 years of age: a prospective birth cohort study. Ecotoxicol Environ Saf. (2021) 225:112736. doi: 10.1016/J.ECOENV.2021.112736
24. Mubanga M, Lundholm C, D’Onofrio BM, Stratmann M, Hedman A, Almqvist C. Association of early life exposure to antibiotics with risk of atopic dermatitis in Sweden. JAMA Netw Open. (2021) 4:e215245. doi: 10.1001/JAMANETWORKOPEN.2021.5245
25. Stefanaki E, Kalaitzidou I, Aristou M, Lakoumentas J, Galanakis E, Xepapadaki X. Prenatal antibiotic exposure increases the risk of infant atopic dermatitis. Data from a Greek cohort. Eur Ann Allergy Clin Immunol. (2022). doi: 10.23822/EURANNACI.1764-1489.266. [Epub ahead of print]36047711
26. Bisgaard H, Halkjær LB, Hinge R, Giwercman C, Palmer C, Silveira L, et al. Risk analysis of early childhood eczema. J Allergy Clin Immunol. (2009) 123:1355-60.e5. doi: 10.1016/J.JACI.2009.03.046
27. Jȩdrychowski W, Gałaś A, Whyatt R, Perera F. The prenatal use of antibiotics and the development of allergic disease in one year old infants. A preliminary study. Int J Occup Med Environ Health. (2006) 19:70–6. doi: 10.2478/V10001-006-0010-0
28. Lee SY, Yu J, Ahn KM, Kim KW, Shin YH, Lee KS, et al. Additive effect between IL-13 polymorphism and cesarean section delivery/prenatal antibiotics use on atopic dermatitis: a birth cohort study (COCOA). PLoS One. (2014) 9:e96603. doi: 10.1371/JOURNAL.PONE.0096603
29. Gao X, Yan Y, Zeng G, Sha T, Liu S, He Q, et al. Influence of prenatal and early-life exposures on food allergy and eczema in infancy: a birth cohort study. BMC Pediatr. (2019) 19:239. doi: 10.1186/S12887-019-1623-3
30. Marell Hesla H, Stenius F, Järnbert-Pettersson H, Alm J. Allergy-related disease in relation to early life exposures-the ALADDIN birth cohort. J Allergy Clin Immunol. (2017) 139:686–8. doi: 10.1016/J.JACI.2016.06.057
31. Timm S, Schlünssen V, Olsen J, Ramlau-Hansen CH. Prenatal antibiotics and atopic dermatitis among 18-month-old children in the Danish national birth cohort. Clin Exp Allergy. (2017) 47:929–36. doi: 10.1111/CEA.12916
32. Alhasan MM, Cait AM, Heimesaat MM, Blaut M, Klopfleisch R, Wedel A, et al. Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy. (2020) 75:1975–86. doi: 10.1111/ALL.14234
33. Milliken S, Allen RM, Lamont RF. The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy, asthma, allergy and obesity in childhood. Expert Opin Drug Saf. (2019) 18:173–85. doi: 10.1080/14740338.2019.1579795
34. Sun M, Luo J, Liu H, Xi Y, Lin Q. Can mixed strains of Lactobacillus and Bifidobacterium reduce eczema in infants under three years of age? A meta-analysis. Nutrients. (2021) 13:1461. doi: 10.3390/NU13051461
Keywords: eczema, allergy, antimicrobials, pregnancy, prenatal
Citation: Wan M and Yang X (2023) Maternal exposure to antibiotics and risk of atopic dermatitis in childhood: a systematic review and meta-analysis. Front. Pediatr. 11:1142069. doi: 10.3389/fped.2023.1142069
Received: 11 January 2023; Accepted: 28 April 2023;
Published: 15 May 2023.
Edited by:
Anne Zajicek, National Institutes of Health (NIH), United StatesReviewed by:
Daniela Podlecka, Medical University of Lodz, PolandRuijie Huang, Sichuan University, China
Boutros Soutou, Université Saint-Joseph, Lebanon
© 2023 Wan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyang Yang eTEwODEwOEAxMjYuY29t
 Mengjie Wan
Mengjie Wan Xiaoyang Yang
Xiaoyang Yang