- 1National Center for Clinical Laboratories, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Hospital/National Center of Gerontology, Beijing, China
- 2Department of Gastroenterology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China
- 3Hainan Women and Children's Medical Center, Haikou, China
- 4Department of Maternal and Child Health, School of Public Health, Peking University, Beijing, China
Purpose: To explore the genotypes and allele frequencies of α, β and α+β thalassemias in Li minorities, which resided in Hainan Province of China for a long time.
Methods: In the present study, 1,438 newborns of the Li minority were collected from January 2020 to April 2021. The genotypes of thalassemia were detected by fluorescence PCR and verified by flow-through hybridization PCR analyses. Rare genotypes were detected by restriction fragment length polymorphism electrophoresis and Sanger DNA sequencing.
Results: Among 1,438 participants, 1,024 (71.2%) were diagnosed with any kind of thalassemia. Among all thalassemia carriers, 902 (88.09%) subjects were diagnosed with α-thalassemia, and 18 subtypes of α-thalassemia were detected, with the top three genotypes being −α4.2/αα (25.39%), −α3.7/αα (22.62%) and αWSα/αα (16.96%). Thirty-two (3.13%) patients were β-thalassemia carriers, and 6 types of β-thalassemia genotypes were detected. The top two genotypes were βCD41–42/βN (46.88%) and β−28/βN (18.75%). Additionally, 90 (8.79%) cases were α + β-thalassemia, and the top two genotypes were −α3.7/αα, βCD41–42/βN (30.00%) and −α4.2/αα, βCD41–42/βN (26.67%). Furthermore, two genotypes (−α4.2/HKαα and βCD76 GCT > CCT/βN) were first identified in Hainan Province, and βCD76 GCT > CCT/βN was first identified in China.
Conclusion: Newborns of Li have a higher prevalence of thalassemia for a long period, and further education on the impact of thalassemia, follow-up studies of the clinical manifestation and treatment and proper intervention methods should be designed to reduce the burden of thalassemia and enhance the quality of life in Li newborns.
1. Introduction
Thalassemias are a group of inherited monogenetic blood disorders (α, β, γ, δ, δβ, and εγδβ) that are distributed worldwide. There are two main types of thalassemias, alpha thalassemia (α-thalassemia) and beta thalassemia (β-thalassemia) (1). Thalassemia α and β are particularly spread in regions from the Mediterranean basin to the Middle East, Indian subcontinent, Southeast Asia, Melanesia, and the Pacific Islands (2–4). The severity of thalassemias can vary from mild anemia to stillbirth, which is dependent on the impaired number of corresponding hemoglobin chains (α and/or β). The imbalanced α/β-globin chain ratio was attributed to ineffective erythropoiesis and finally resulted in anemia (5, 6). Previous studies have suggested that thalassemia could be screened and prenatally diagnosed, and it was the most cost-effective method to reduce the occurrence of severe symptoms among newborns (7, 8). Recently, a meta-analysis indicated that the prevalence of thalassemia in China is mainly distributed in southern China, especially in Guangxi (19.10%), Guangdong (11.9%) and Hainan (12.95%) provinces (9, 10). Those three cities had a high prevalence above 10% in China and a more specific prevalence of α-thalassemia (11).
Ethic Li is a Chinese minority and mainly lives in Hainan Province. They were considered the earliest residents on Hainan Island. The population size of the Li minority reached 1.6 million in 2020 (12). The tradition and economic status of the Li people remain similar due to their concentrated residence. Certain studies have shown that the Li people have a higher prevalence of thalassemia than other minorities (13–15). However, most studies have been carried out in Li's adults, in whom the genotypes might be different from those of newborns due to survivor bias, which hampers precise and immediate treatment marking and proper prevention policy design.
Therefore, in the present study, we describe the frequencies of α- and β-thalassemia and specific genotypic mutation frequencies in newborns of the Li minority in Hainan Province.
2. Materials and methods
2.1. Subjects
From January 2020 to April 2021, we collected 1,517 live births of Li in the Hainan Li Autonomous Region. Among all lived births, approximately ninety-five percent of newborns' parents signed the informed consent form and volunteered to participate in thalassemia genotype testing. The age of newborns ranged from 3 to 20 days. The numbers of boys and girls were 743 and 695, respectively. This study was approved by the Ethics Committee for Clinical Investigation of Hainan Women and Children's Medical Center in 2021 (No. 028). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. No potentially identifiable human images or data are presented in this study.
2.2. DNA extraction
According to the People's Republic of China Health Industry Standard, “Basic dataset of children ‘s health—Part 3: Newborn screening” (WS 376.3-2013) (16), the trained health worker of maternal and child health care centers in Hainan Li Autonomous Region collected the heel blood of newborns between 3 and 20 days after delivery. Wiping off the first drop of heel blood, the following drops were dripped vertically on 903-filter paper to generate a spot with a diameter over 8 mm. All dried blood spots were sent to the molecular biology laboratory of Hainan Women and Children's Medical Center for genetic analyses with cold-chain transportation. At least six dried blood spots 3 mm in diameter were punched (Panthera-PuncherTM 9 puncher (Perkin Elmer Co., Ltd, Shanghai, Finland)) and placed in a centrifuge tube for DNA extraction. In the screening stage, DNA extraction was performed with the magnetic bead method (Zeesan Biotech Co., Ltd, Xiamen, China) with a Lab-Aid 824s Nucleic Extraction System (Zeesan Biotech Co., Ltd, Xiamen, China). For the verification stage, genomic DNA was extracted with the spin column method (Hybribio Co., Ltd, Guangzhou, China).
2.3. Screening and verification of thalassemia genotypes
In the screening stage: the fluorescence Polymerase Chain Reaction -melting curve analyses (SLAN-96S Automatic Medical PCR Analysis System, Shanghai Hongshi Medical Technology Co., Ltd, Shanghai, China) was used to detect the genotype of thalassemia including three α-thalassemia gene deletions (−α3.7, −α4.2, –SEA), and three nondeletion α-thalassemia mutations: Hb CS, Hb QS and Hb Westmead and 21 common β-thalassemia mutations [the names given by HGVS are in parentheses following the names of base changes: −90 (C > T) (c.-140C > T), −73 (A > T) (c.-123A > T), −28 (A > G) (c.-78A > G), −29 (A > G) (c.-79A > G), −30 (T > C) (c.-80T > C), −31 (A > C) (c.-81A > C), −32 (C > A) (c.-82C > A), CD14-15 (+G) (c.45_46insG), CD15-16 (+G) (c.48_49insG), CD17 (A > T) (c.52A > T), CD26 (G > A) (c.79G > A), CD30 (A > G) (c.91A > G), IVS-I-1 (G > T) (c.92 + 1G > T), IVS-I-5 (G > C) (c.92 + 5G > C), CD27-28 (+C) (c.84_85insC), CD37 (G > A) (c.113G > A), CD71-72 (+A) (c.216_217insA), CD43 (G > T) (c.130G > T), CD41-42 (-TTCT) (c.124_127delTTCT), IVS-II-5 (G > C) (c.315 + 5G > C) and IVS-II-654 (C > T) (c.316-197C > T)]. The α-thalassemia, β-thalassemia, nondeletion and α+β thalassemia were detected with the thalassemia genotype detection kit (Zeesan Biotech Co., Ltd, Xiamen, China). The polymerase chain reaction (PCR) was carried out with the SLAN-96S Automatic Medical PCR Analysis System (Shanghai Hongshi Medical Technology Co., Ltd, Shanghai, China).
To ensure the accuracy of the genotyping, certain percentage samples were verified with flow-through hybridization analysis (HB-2012A nucleic acid hybridization, Hybribio Co., Ltd, Guangzhou, China). The genotype kit for thalassemia detection (Hybribio Co., Ltd, Guangzhou, China) included three common α-globin gene deletions, 3 nondeletion α-thalassemia mutations and 19 common β-thalassemia mutations. Moreover, the mutation of −α4.2/HKαα was detected by restriction fragment length polymorphism (RFLP) electrophoresis (Hybribio Co., Ltd, Guangzhou, China) and the mutation of β CD76 GCT > CCT(NM_000518.5 (HBB):c.229G > C)/βN was detected by Sanger DNA sequencing (Zeesan Biotech Co., Ltd, Xiamen, China).
2.4. Statistical analysis
Data and results were analyzed using SPSS version 13.0 (IBM Inc., Chicago, United States). To compare the thalassemia gene-carrying rates among sexes and subgroups, the chi-square (χ2) test was used. Statistical significance was set at p < 0.05 for two tails.
3. Results
3.1. The prevalence of thalassemia between girls and boys
The numbers of boys' and girls' carriers were 524 and 500, respectively. Boy and girl thalassemia gene-carrying rates were 70.5% (524/743) and 71.9% (500/695), respectively. There was no statistically significant difference between the gene-carrying rates of thalassemia between boys and girls (χ2 = 0.074, P = 0.786, Table 1).

Table 1. The numbers of newborns and gene-carrying rates of thalassemia of newborns in Li people from January 2020 to April 2021 in the Hainan Li autonomous region.
3.2. The genotypes of α-thalassemia and β-thalassemia
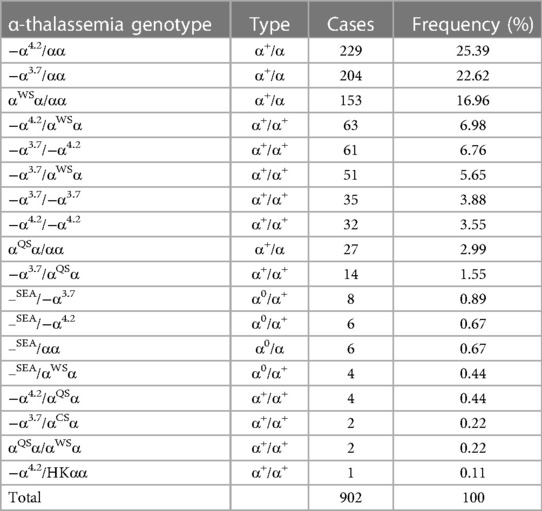
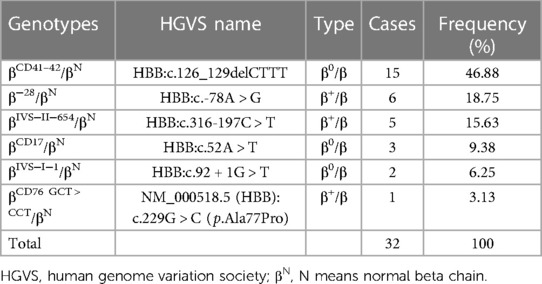
Among 1,438 newborns of Li minorities, 1,024 (71.21%, 1,024/1,438) thalassemia carriers were detected. As shown in Table 2, 902 (88.09%, 902/1,024) cases with α-thalassemia and 18 different types of α-thalassemia were detected. The top three genotypes were −α4.2/αα (25.39%, 229/902), −α3.7/αα (22.62%, 204/902) and αWSα/αα (153/902, 16.96%). For β-thalassemia, 32 (3.14%, 32/1,024) were cases, and six different types of genotypes were detected (Table 3). The top two genotypes were βCD41–42/βN (46.88%, 15/32) and β−28/βN (18.75%, 6/32).
3.3. The genotypes of α+β-thalassemia
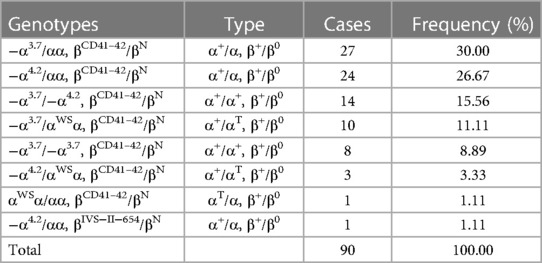
As shown in Table 4, 90 (8.79%, 90/1,024) cases with composite α+β-thalassemia were detected. The two common genotypes were −α3.7/αα+βCD41–42/βN (30.00%, 27/90) and −α4.2/αα+βCD41–42/βN (26.67%, 24/90). Two rare genotypes, −α4.2/HKαα and βCD76 GCT > CCT/βN [NM_000518.5 (HBB):c.229G > C (p.Ala77Pro)]), were detected. The mutation of −α4.2/HKαα was detected for the first time in Hainan Province. The mutation βCD76 GCT > CCT/βN was detected for the first time in China.
3.4. The allele frequency of α- and β-thalassemia mutations
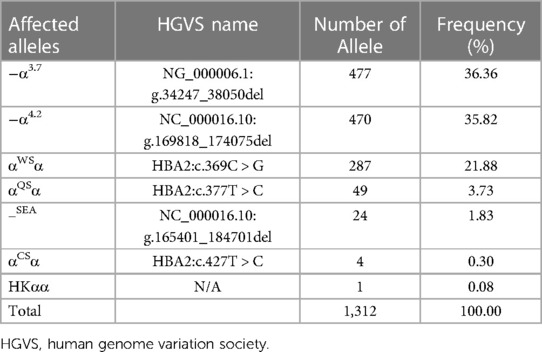
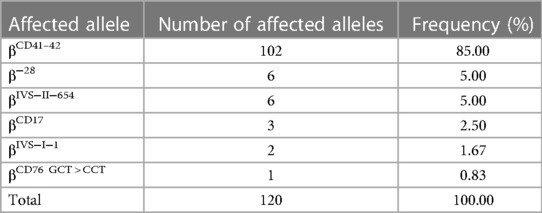
As shown in Table 5, 1,312 chromosomes carrying α-thalassemia gene mutations were identified, including seven α genetic mutations. The top three most frequent mutations were −α3.7 (36.36%, 477/1,312), −α4.2 (35.82%, 470/1,312) and αWSα (21.88%, 287/1,312). As shown in Table 6, 120 chromosomes carrying β-thalassemia gene mutations were identified, including six β gene mutations. The top three most frequent mutations were CD41-42 (85.00%, 102/120), CD41-28 (5.00%, 6/120) and IVS-II-654 (5.00%, 6/120).
4. Discussion
In the present study, we focused on the newborns of the Li minority who have abundant genotypes of thalassemia and are living in the high prevalence malaria region (Li Autonomous Region of Hainan) in China. The rank of thalassemia types was α-thalassemia (88.09%, 902/1,024), β-thalassemia (3.14% (32/1,024) and α + β (8.79%, 90/1,024). Additionally, we first found the HKαα allele and βCD76 GCT > CCT/βN allele in the Li minority.
The data of the present study are in line with those of previous studies, and α-thalassemia is the major type of thalassemia in the Li minority (14, 15). The proportions of different subtypes of α thalassemia and β-thalassemia are mainly consistent with one recent study carried out in childbearing age adults of the Li minority (15), particularly for the top two genotypes in α thalassemia (−α4.2/αα and −α3.7/αα) and top three in β-thalassemia (βCD41–42/βN, β−28/βN and βIVS−II−654/βN). However, the percentages in different subtypes had significant disparities; for example, βCD41–42/βN only accounted for 46.88% in our present study, but it accounted for 90.96% in childbearing age adults of the Li minority (15). A similar scenario was also observed in α thalassemia; −α4.2/αα accounted for 25.39% and −α3.7/αα accounted for 22.62%, but those two subtypes accounted for 17.24% and 17.16%, respectively, in childbearing age adults in the Li minority (15). The plausible reason might be that our sample was only collected from Li minority autonomous regions, and Wang's research was conducted with Li minorities from 19 cities and counties of Hainan. With the development of economic and transportation technology, increasingly more Li travelled around Hainan Province. Interestingly, another recent study carried out with 1to10-year-old children in Guangxi by He revealed that–-SEA and–-SEA/αα were the most frequent genotypes in α thalassemia. Nonetheless, the major type of β-thalassemia is βCD41–42/βN (17). Meanwhile, those major types of thalassemia were consistent with the residents living in the Dongguan region of Guangdong Province (18). However, it seems that the southern part of China has different major genotypes of thalassemia in comparison with Chongqing/Yunnan province/Zhejiang province/northern part of China, where β-thalassemia is the dominant genotype (19–22). These results proved that there were regional differences in thalassemia (23). The possible reason might be the inbreeding and migration of human beings. From the route out of Africa, we do observe that human beings first settled near the coast (24). Li minority was the aborigines of Hainan Island, who had been living on Hainan Island for more than 3,000 years. More genetic immigration tracing could be conducted to gain insight into the history of thalassemia in the Li minority.
Notably, the detection rates of nondeletion α-thalassemia mutations were much higher than those in other regions of China (19–22). The gene mutation frequencies of αWSα, αQSα and αCSα were 21.88%, 3.73% and 0.30%, respectively, which are relatively higher than those in a previous report (13, 25). The reason why the prevalence of nondeletion α-thalassemia was very high might be attributed to two aspects. First, the cases and subjects of many previous reports were collected from health examinations, genetic counselling and prenatal diagnosis, which might omit some carriers with no counselling needs or mild symptoms. Second, we speculated that the traditional culture of the Li minority, consanguineous marriage, was very common in the Li minority, which might be susceptible to inheriting mutations and insufficient attention to the harm of thalassemia due to peer effects.
Two rare genotypes, −α4.2/HKαα and βCD76 GCT > CCT/βN (c.229G > C), were detected in the present study. −α4.2/HKαα was identified for the first time in Hainan Province, and currently, no documents have recorded its impact on erythropoiesis. βCD76 GCT > CCT/βN was identified for the first time in China. βCD76 GCT > CCT/βN (c.229G > C) is a missense mutation. H Wajcman et al. reported only one case of this mutation worldwide before 1990 (26). They believed that it was a new variant of hemoglobin with a greater reduction in oxygen affinity than others. There was no functional evidence of this mutation in the ClinVar database. According to the guidelines of the American College of Medical Genetics (ACMG), this variant is preliminarily considered a deleterious mutation. Follow-up studies need to be performed to observe and record how it impacts the erythropoiesis of carriers, and appropriate invention methods should be conducted when the carrier needs.
For the newborn population, the traditional screening method for thalassemia, which is a combination of complete blood count (CBC) and hemoglobin electrophoresis, has many disadvantages, such as repeated blood sampling, higher cost and longer waiting time. In contrast, thalassemia genetic testing is performed by testing dried blood spots on filter paper, which does not require fresh blood sampling and can be easily retested. Genetic testing is conducive to population screening and has been clinically verified in some studies (27, 28).
In view of the high carrying rates of thalassemia genes in newborns of the Li minority, applicable and feasible interventions should be designed to reduce the prevalence. In the response to the call of “Thalassemia in children: from quality of care to quality of life”, prenatal and neonatal screening of thalassemia disease is critical to alert public awareness of this disease (29). Previous research indicated that children with thalassemia had significantly lower quality of life in the physical, emotional, social and school functioning domains (30, 31). This situation would be even worse in low-resource settings, for instance, Li minority. When children grow up, their general health is impacted as well, with a smaller impact on mental health (32). Additionally, caregivers simultaneously suffer from anxiety and depression (33). Overall, the disease burden is not only limited to the patients themselves. The urbanization index and health care system of Hainan Province are relatively low in comparison with those of Beijing or Shanghai. Compared with other congenital disorders of newborns, the prevalence of thalassemia was high in the Li minority (34). The Chinese National Center for Clinical Laboratories has been ensuring the quality of newborn screening work over the years (35). However, quality of life care should also catch up and be implemented step by step. For instance, public education of the impact of thalassemia disease could be embedded in the pregnancy school to further enhance the voluntary screening rate. During the pregnancy school, let the pregnant women and their husband understand this disease and know the typical syndrome of this disease and get help from the hospital in the proper time are essential as well. Since the health worker force is limited in Hainan as well, online courses would be another way for pregnant women and unmarried young people as well. Additionally, free counseling and proper portion reimbursement of the costing in transfusion-dependent thalassemia could also be designed to bridge the generation gap by policy.
Our study is the first to comprehensively profile the genotypes of thalassemia in newborns among the Li minority. Second, neonatal genetics were analysed for thalassemia. This would provide additional information about the spectrum of genotypes of thalassemia for the Li minority. Finally, the accuracy of the present study is relatively high due to the application of screening and verification methods.
However, several limitations need to be addressed in the present study as well. Due to data availability, we could not include all Li minorities in Hainan, which might have different frequencies of certain subtypes of thalassemia. Second, we only decipher the genetic variants of thalassemia, and no clinical manifestation was followed-up, which hampers the design of proper and precise interventions in the public.
5. Conclusion
The newborns of the Li minority have a high prevalenceof thalassemia genotypes. Screening work should accompany education to enhance the awareness and recognition of symptoms of this disease for health care needs. To further enhance the quality of life among thalassemia carriers, follow-up studies need to be designed.
Impact statement
This study was the first comprehensive study using neonatal samples and molecular biology methods to investigate the frequency of genotypes among α-, β- and α+β thalassemia in newborns of the Li minority in southern China. Using the newborn to describe the genotypes and mutation frequency could provide additional information about the genotypes of thalassemia for the Li minority. The newborns of Li people had a relatively higher incidence and rare genotypes of thalassemia than Chinese Han people. Appropriate public health interventions should be designed for this population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee for Clinical Investigation of Hainan Women and Children's Medical Center in 2021 (No. 028). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
ZZ and HW conceived and designed the study; KZ and HS conducted the data analyses; KZ and WW wrote the draft; WW and HX collected samples and performed the lab work; HW comprehensively revised the draft; All authors contributed to the article and approved the submitted version.
Funding
The authors disclosed receipt of the following financial support for the research, authorship and publication of this article: Natural Science Foundation of Hainan province (820MS150), Hainan Health Industry Scientific Research Project (21A200147), Hainan Province Clinical Medical Center (QWYH202175), and the Major Scientific and Technological Projects of Hainan Province (No. ZDKJ2017007).
Acknowledgments
Thanks to all the parents of the newborns who participated in the present study. Thanks for the funding from Hainan Natural Science Foundation (820MS150), Hainan Health Industry Scientific Research Project (21A200147) and Hainan Province Clinical Medical Center (QWYH202175) to support this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HGVS, human genome variation society; alpha thalassemia, α-thalassemia; beta thalassemia, β-thalassemia.
References
1. Viprakasit V, Ekwattanakit S. Clinical classification, screening and diagnosis for thalassemia. Hematol Oncol Clin North Am. (2018) 32:193–211. doi: 10.1016/j.hoc.2017.11.006
2. De Simone G, Quattrocchi A, Mancini B, di Masi A, Nervi C, Ascenzi P. Thalassemias: from gene to therapy. Mol Aspects Med. (2022) 84:101028. doi: 10.1016/j.mam.2021.101028
3. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull W H O. (2008) 86:480–7. doi: 10.2471/blt.06.036673
4. Weatherall DJ. Hemoglobinopathies worldwide: present and future. Curr Mol Med. (2008) 8:592–9. doi: 10.2174/156652408786241375
5. Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. (2018) 391:155–67. doi: 10.1016/S0140-6736(17)31822-6
6. Bansal D, Lal A. Iron metabolism, hemolytic Anemia, and thalassemia. Indian J Pediatr. (2020) 87:56–7. doi: 10.1007/s12098-019-03151-8
7. Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. (2012) 12:457–68. doi: 10.1016/s1473-3099(12)70055-5
8. Harteveld CL, Higgs DR. Alpha-thalassaemia. Orphanet J Rare Dis. (2010) 5:13. doi: 10.1186/1750-1172-5-13
9. Xu X, Zhou Y, Luo G, Liao C, Zhou M, Chen P, et al. The prevalence and spectrum of alpha and beta thalassaemia in guangdong province: implications for the future health burden and population screening. J Clin Pathol. (2004) 57:517–22. doi: 10.1136/jcp.2003.014456
10. Xiong F, Sun M, Zhang X, Cai R, Zhou Y, Lou J, et al. Molecular epidemiological survey of haemoglobinopathies in the guangxi zhuang autonomous region of southern China. Clin Genet. (2010) 78:139–48. doi: 10.1111/j.1399-0004.2010.01430.x
11. Long P, Wang Z, Yang H, Liu Z, Wu B, Zhong G, et al. Generation of nine iPSC lines (HNMUi002-A, HNMUi003-A, HNMUi004-A, HNMUi005-A, HNMUi006-A, HNMUi007-A, HNMUi008-A, HNMUi009-A, HNMUi010-A) from three Chinese families with thalassemia. Stem Cell Res. (2020) 49:102014. doi: 10.1016/j.scr.2020.102014
12. National Bureau of statistics of China. National bureau of statistics of China (2021). Beijing: China Statistic Press (2022).
13. Yao H, Chen X, Lin L, Wu C, Fu X, Wang H, et al. The spectrum of α- and β-thalassemia mutations of the Li people in Hainan province of China. Blood Cells Mol Dis. (2014) 53:16–20. doi: 10.1016/j.bcmd.2014.01.003
14. Yu Y, Lu C, Gao Y, Li C, Li D, Wang J, et al. Molecular Spectrum, ethnic and geographical distribution of thalassemia in the southern area of Hainan, China. Front Pediatr. (2022) 10:894444. doi: 10.3389/fped.2022.894444
15. Wang M, Zhang X, Zhang Y, Xiao M. Prevalence and genetic analysis of thalassemia and hemoglobinopathy in different ethnic groups and regions in Hainan island, Southeast China. Front Genet. (2022) 13:874624. doi: 10.3389/fgene.2022.874624
16. China, N. H. C. o. t. P. s. R. o. Basic dataset of children's Health—part 3: Newborn screening. Beijing: National Health Commission (2013).
17. He S, Li D, Yi S, Huang X, Zhou C, Chen B, et al. Molecular characterization of α- and β-thalassaemia among children from 1 to 10 years of age in guangxi, A multi-ethnic region in southern China. Front Pediatr. (2021) 9:724196. doi: 10.3389/fped.2021.724196
18. Peng Q, Zhang Z, Li S, Cheng C, Li W, Rao C, et al. Molecular epidemiological and hematological profile of thalassemia in the dongguan region of guangdong province, southern China. J Clin Lab Anal. (2021) 35:e23596. doi: 10.1002/jcla.23596
19. Yao X, Yu J, Chen S, Xiao J, Zheng Q, Liu H, et al. Prevalence and genetic analysis of α-thalassemia and β-thalassemia in Chongqing area of China. Gene. (2013) 532:120–4. doi: 10.1016/j.gene.2013.09.031
20. Huang T, Zhang T, Song C, Lin Y, Sang B, Lei Q, et al. Gene mutation Spectrum of thalassemia among children in yunnan province. Front Pediatr. (2020) 8:159. doi: 10.3389/fped.2020.00159
21. Ge Q, Wang Y, Zhang Y, Mu Q, Guo F, Ouyang G [Clinical features of pregnant women with thalassemia in non endemic area]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2020) 28:2022–7. doi: 10.19746/j.cnki.issn.1009-2137.2020.06.037
22. Yang Z, Cui Q, Zhou W, Qiu L, Han B. Comparison of gene mutation spectrum of thalassemia in different regions of China and Southeast Asia. Mol Genet Genomic Med. (2019) 7:e680. doi: 10.1002/mgg3.680
23. Boardman FK, Clark C, Jungkurth E, Young PJ. Social and cultural influences on genetic screening programme acceptability: a mixed-methods study of the views of adults, carriers, and family members living with thalassemia in the UK. J Genet Couns. (2020) 29:1026–40. doi: 10.1002/jgc4.1231
24. Forster P, Matsumura S. Evolution. Did early humans go north or south? Science. (2005) 308:965–6. doi: 10.1126/science.1113261
25. Zhuang J, Jiang Y, Wang Y, Zheng Y, Zhuang Q, Wang J, et al. Molecular analysis of α-thalassemia and β-thalassemia in quanzhou region southeast China. J Clin Pathol. (2020) 73:278–82. doi: 10.1136/jclinpath-2019-206179
26. Wajcman H, Kister J, Marden M, Bohn B, Blouquit Y, Descamps J, et al. Hemoglobin Calais [beta 76 (E20) ala—-pro]: a hemoglobin variant with decreased intrinsic oxygen affinity. Biochim Biophys Acta. (1990) 1096:60–6. doi: 10.1016/0925-4439(90)90013-f
27. Wen W, Guo M, Peng HB, Ma L. Optimization and application of a dried blood spot-based genetic screening method for thalassemia in Shenzhen newborns. World Journal of Pediatrics: WJP. (2019) 15:610–4. doi: 10.1007/s12519-018-00222-2
28. Jindatanmanusan P, Riolueang S, Glomglao W, Sukontharangsri Y, Chamnanvanakij S, Torcharus K, et al. Diagnostic applications of newborn screening for α-thalassaemias, haemoglobins E and H disorders using isoelectric focusing on dry blood spots. Ann Clin Biochem. (2014) 51:237–47. doi: 10.1177/0004563213491078
29. Amid A, Saliba AN, Taher AT, Klaassen RJ. Thalassaemia in children: from quality of care to quality of life. Arch Dis Child. (2015) 100:1051–7. doi: 10.1136/archdischild-2014-308112
30. Klaassen R, Barrowman N, Pulcini M, Vichinsky E, Sweeters N, Allen M, et al. Validation and reliability of a disease-specific quality of life measure (the TranQol) in adults and children with thalassaemia major. Br J Haematol. (2014) 164:431–7. doi: 10.1111/bjh.12631
31. Thavorncharoensap M, Torcharus K, Nuchprayoon I, Riewpaiboon A, Indaratna K, Ubol B, et al. Factors affecting health-related quality of life in Thai children with thalassemia. BMC Blood Disord. (2010) 10:1. doi: 10.1186/1471-2326-10-1
32. Porter J, Bowden D, Economou M, Troncy J, Ganser A, Habr D, et al. Health-Related quality of life, treatment satisfaction, adherence and persistence in beta-thalassemia and myelodysplastic syndrome patients with iron overload receiving deferasirox: results from the EPIC clinical trial. Anemia. (2012) 2012:297641. doi: 10.1155/2012/297641
33. Yengil E, Acipayam C, Kokacya M, Kurhan F, Oktay G, Ozer C Anxiety, depression and quality of life in patients with beta thalassemia major and their caregivers. Int J Clin Exp Med. (2014) 7:2165–72.25232402
34. Zhong K, Wang W, He F, Wang Z. The status of neonatal screening in China, 2013. J Med Screen. (2016) 23:59–61. doi: 10.1177/0969141315597715
Keywords: newborns, thalassemia, genotypes, Li minority, Hainan provice
Citation: Zhong K, Shi H, Wu W, Xu H, Wang H and Zhao Z (2023) Genotypic spectrum of α-thalassemia and β-thalassemia in newborns of the Li minority in Hainan province, China. Front. Pediatr. 11:1139387. doi: 10.3389/fped.2023.1139387
Received: 6 January 2023; Accepted: 28 February 2023;
Published: 20 March 2023.
Edited by:
Carsten Heilmann, Juliane Marie Centre, Rigshospitalet, DenmarkReviewed by:
Ammar Husami, Cincinnati Children's Hospital Medical Center, United StatesAnil Pathare, Sultan Qaboos University, Oman
© 2023 Zhong, Shi, Wu, Xu, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wang aHVpd2FuZ0Biam11LmVkdS5jbg== Zhendong Zhao am9lNzkwNDMwQDE2My5jb20=
†These authors have contributed equally to this work
‡ORCID Hui Wang orcid.org/0000-0002-7639-5702
Specialty Section: This article was submitted to Pediatric Hematology and Hematological Malignancies, a section of the journal Frontiers in Pediatrics
 Kun Zhong1,†
Kun Zhong1,† Hui Wang
Hui Wang