
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 15 June 2023
Sec. Pediatric Pulmonology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1137478
This article is part of the Research Topic Current Understanding and Management of Childhood Asthma View all 4 articles
Background: Subcutaneous immunotherapy (SCIT) has been proved to be effective and safe in adult asthma. But it is still controversial in children.
Object: To evaluate the efficacy and safety of SCIT in asthmatic children with allergy to house dust mite.
Method: We searched the databases of Cochrane Library, EMBASE and MEDLINE (from 1 January 1990 to 1 December 2022). Two reviewers independently screened studies, extracted data and critically appraised the risk of bias. We used the Revman 5 to synthesize the effect sizes.
Results: We finally selected 38 eligible studies including 21 randomized controlled trials to evaluate the efficacy and safety of SCIT and 17 observational studies to assess the safety. The results revealed that short-term asthma symptom scores were declined with a standardized mean difference (SMD) of −1.19 (95% CI: −1.87, −0.50) in 12 researches with high heterogeneity. Short-term asthma medication scores were decreased with SMD −1.04 (95% CI: −1.54, −0.54) in 12 heterogeneous researches. One study showed no significant reduction in combined symptom and medication scores without providing details. No studies we reviewed reported long-term efficacy. SCIT resulted in an obviously increased risk of adverse reactions compared with placebo. For secondary outcomes, SCIT improved life quality and reduced the numbers of annual asthma attacks and allergen-specific airway hyperreactivity, but without significant improvement in pulmonary function, asthma control or hospitalization.
Conclusions: SCIT can reduce the short-term symptom scores and medication scores regardless of different treatment duration or mono/polysensitization, but with an increased incidence of local and systemic adverse effects. Further studies on pediatric asthma are needed to evaluate the long-term efficacy, and clarify the effectiveness of SCIT in specific population using mix allergen extracts or with severe asthma. Overall, it is recommended for children with mild-moderate HDM-driven allergic asthma.
Allergic asthma (AA) has been one of the most common chronic diseases among children with an uprising prevalence in recent years. Most patients can benefit from avoidance strategies, drug treatment and allergen immunotherapy (AIT) (1). However, house dust mite (HDM), one of the most relevant triggers of allergic diseases worldwide, is difficult to be avoided (2). Thus, it is necessary to treat allergic disease due to HDM by using the other two approaches. AIT is a highly attractive therapy method to AA by its disease-modifying effect which can exist for a long time after discontinuation (1, 3–5). The main administrations of AIT are subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). Limited trials compared the outcomes of SCIT with SLIT head-to-head, offering low-grade evidence to support greater benefits of SCIT (6–9). In indirect comparison to SLIT, SCIT appeared to be more effective in controlling asthma symptoms and decreasing medication use (10). However, regarding the low adherence, individual variation and safety, the evidence supporting SCIT in the pediatric population is still insufficient and controversial (1, 11, 12), making it remain underused in children (13).
In order to solve these problems, several high-quality trials have provided evidence on the efficacy of AIT in asthmatic children (14–16). However, it is still difficult to draw robust conclusions due to the disparities in outcome definitions and the heterogeneity of interventions when analyzing pooled data (9). Considering the limitations of meta-analyses, the way of focusing on studies of single-product or sub-population may hold promise (1). So, it might be possible to come to more convincing or homogeneous conclusions by focusing exclusively on HDM extracts for pediatrics with mite allergy, while no meta-analyses has reported such relevant results to date. Therefore, we conducted the research to pool data in regard to the asthmatic children allergic to HDM, which may be helpful to promote the rational use of SCIT in clinical practice.
According to the guidelines for systematic reviews, two reviewers screened studies, extracted data and critically appraised the risk of bias all alone. And when necessary, they would consult a third reviewer. A detailed description of the methods has previously been posted online (17).
We searched the databases of Cochrane Library (both Cochrane Systematic Reviews and The Cochrane Central Register of Controlled Trials), MEDLINE and EMBASE (from 1 January 1990 to 1 December 2022) according to the principle of PICOS. All references we searched were uploaded to the reference management software named NoteExpress (v3.5.0.9054, Aegean Software, Beijing, China) for initial deduplication and management. Two reviewers independently screened eligible studies that meet the following inclusion criteria:
Population: children under 18 years of age with allergic asthma (diagnosed by physicians according to accepted diagnostic criteria) and evidence of clinically relevant allergic sensitization to house dust mites as determined by an objective biomarker (e.g., skin prick test or specific-IgE).
Intervention: any way of SCIT (e.g., conventional SCIT, rush SCIT or cluster SCIT) using specific allergen extracts of house dust mite with/without other allergens.
Comparator: placebo or conventional drug therapy when comparing the effectiveness of SCIT. There could be no comparison group when comparing the safety of SCIT in descriptive studies.
Outcomes: short-term (during treatment) and long-term (at least one year after discontinuation of SCIT) efficacy assessed by the improvement of asthma symptoms and medication use as the primary outcomes as well as the safety reported by incidence of adverse effects; quality of life using the Asthma Quality of Life Questionnaire (AQLQ) or other appropriate tools, pulmonary function, asthma control showing the extent to which the various manifestations of asthma were reduced or removed by SCIT (18), and specific or nonspecific bronchial provocation test as secondary outcomes.
Study Design: Randomized controlled trials (RCTs) were included to assess the efficacy of SCIT in asthmatic children and these were supplemented with descriptive studies for the evaluation of adverse effects. Systematic reviews and meta-analysis were also selected for further screening.
If only adults or partially (<60% of totality) eligible participants meet all the inclusion criteria involved, such a study would be excluded. Besides, the language of original articles was limit in English and Chinese.
Two reviewers independently extracted information from the original papers by a previously designed data extraction sheet. During the step, risk of bias assessment was simultaneously processed on each randomized controlled trial (RCT) using the Cochrane Collaboration's tool (19). Those descriptive studies about the safety of SCIT were analyzed separately without quality assessment. The discrepancies were discussed together and settled by a third reviewer if disagreements remained.
The outcomes we focused on were the efficacy and safety of SCIT in pediatric asthma. For the efficacy outcomes which were mainly continuous variables, we used the mean difference (MD), or SMD if appropriately, to represent the effect size with a 95% confidence interval (CI). And for the safety, as a dichotomous variable, relative risk (RR) of local and systemic adverse reactions were applied. We used Revman 5.0 (version 5.4.1) to synthesize these effect sizes. Heterogeneity is quantified using I2 and categorized as no importance (0% ≤ I2 ≤ 30%),mild heterogeneity (30% < I2 < 50%), moderate heterogeneity (50% ≤ I2 ≤ 75%) and substantial heterogeneity (75% < I2 ≤ 100%). If the heterogeneity was significant (I2 ≥ 50%), the random effect model would be selected.
In light of the large heterogeneity of previous RCTs about the relevant topic, subgroups analyses seemed to be sensible and necessary to investigate and reduce the heterogeneity. Lots of previous studies have indicated that some population demographics such as monosensitization or polysensitization, severity of asthma, treatment duration, etc., can influence the effectiveness of SCIT (20). Therefore, subgroup analyses were undertaken to compare: monosensitization vs. polysensitization, mild or moderate vs. severe asthma, the duration of treatment, and single HDM allergen vs. mixed allergens. In accordance with EMA suggestion, we defined a single HDM allergen as one allergen or mixed homologous allergens of mites (e.g., Dermatophagoides farinae, Dermatophagoides pteronyssinus and Blomia tropicalis) and mixed allergens as a mixture of different species (e.g., Dermatophagoides species, grass pollen and Alternaria alternata).
After the identification and screening, we included 38 eligible researches (21 RCTs for efficacy or safety and 17 non-RCTs about the safety of SCIT) (7, 8, 14–16, 21–53) (Figure 1). Among these, five double-blind and placebo-controlled (DBPC) trials assessed both the efficacy and safety of the SCIT in HDM-sensitized asthmatic children. Each original article exclusively focused on children except two studies including both children and adults. Most included subjects suffered from mild to moderate asthma according to GINA guideline. In addition to asthma, some patients were concomitant with allergic rhinoconjunctivitis or eczema in the majority of included studies. Characteristics of included researches are summarized in Supplementary Tables S1,S2. The risk of bias assessment is shown in Supplementary Table S3.
Based on the literature reviewed and guidelines recommended, we included asthma symptom scores, asthma medication scores, and combined symptom and medication scores (CSMS) as the primary outcomes. A total of 14 studies evaluated the efficacy of SCIT using asthma symptom scores, but only 12 of them reported relevant data. In all reported studies, the four basic asthma symptoms (cough, wheezing, breathlessness and dyspnea) were assessed and recorded on a 4-point scale, except for three studies in which the maximum score was 5 or 20 points (24, 29, 30). In addition, visual analog scale was also recorded to evaluate the severity of asthma and rhinitis symptom in five studies, which revealed a significant improvement. However, we did not pool data from these studies because only two of them reported the variance in detail. For the definition of asthma medication scores, it varied in most included trials, depending on the type and dosage of medication, and the rating scale ranged from 2 to 10 points. As recommended in guidelines, CSMS is an appropriate outcome to evaluate clinical effectiveness and was reported in one study as the sum of asthma medication scores and asthma symptom scores. In order to compare the efficacy of SCIT in various trials, the mean change in these primary outcomes between baseline and the last follow-up visit was calculated as the effect variable.
Twelve trials reported on the short-term asthma symptom scores which were obtained during the SCIT treatment to evaluate the efficacy of SCIT. We pooled data from those studies and the SMD was −1.19 (95% CI: −1.87, −0.50; see Figure 2), indicating that SCIT could improve symptom scores significantly compared to placebo or medication therapy. The efficacy was confirmed after excluding studies at high risk of bias (ROB). However, substantial heterogeneity was obviously among studies (I2 = 94%).
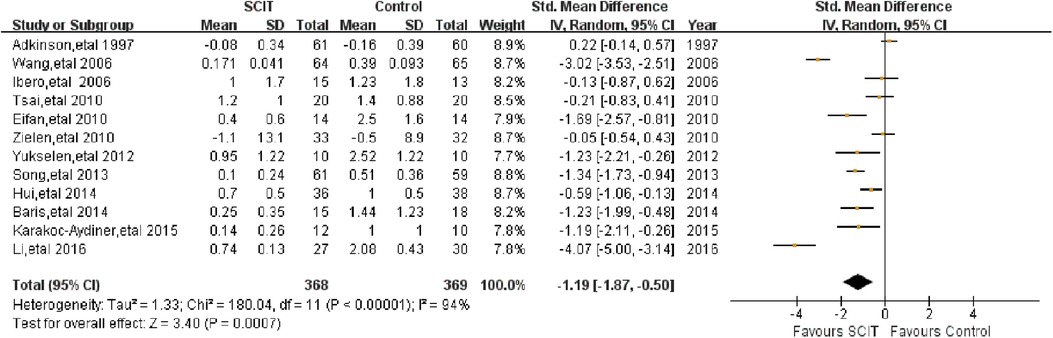
Figure 2. Meta-analysis of RCTs for short-term asthma symptom scores comparing SCIT and control groups (random-effects model).
Mild-moderate asthma vs. moderate-severe asthma: this analysis suggested an apparent efficacy of SCIT on mild-moderate asthma with SMD −1.44 (95% CI: −2.17, −0.70) while SMD 0.08 (95% CI: −0.30, 0.47) on moderate-severe asthma (see Figure 3A). This indirect comparison indicated that mild cases could benefit more compared with severe asthmatic children. However, the result was still doubtful because of the large heterogeneity and the small quantity of studies in subgroups.
Single allergen vs. mixed allergens: the evidence of benefit for SCIT was found obviously in patients using single allergen with SMD −1.32 (95% CI: −2.01, −0.63), but still lacking in those with mixed allergens SMD 0.22 (95% CI: −0.14, 0.57) (see Figure 3B). It seemed that single HDM SCIT could be more effective on the asthma symptom control than mixed allergens SCIT, which still needs further researches to support.
Mono-sensitivity vs. poly-sensitivity& Treatment duration: there is evidence of large benefit of HDM SCIT both in mono-sensitized patients and poly-sensitized population. Similar result was showed in patients under SCIT treatment for more than or less than 3 years, supporting the effectiveness of SCIT during different duration. However, high heterogeneity still existed after subgroup analyses.
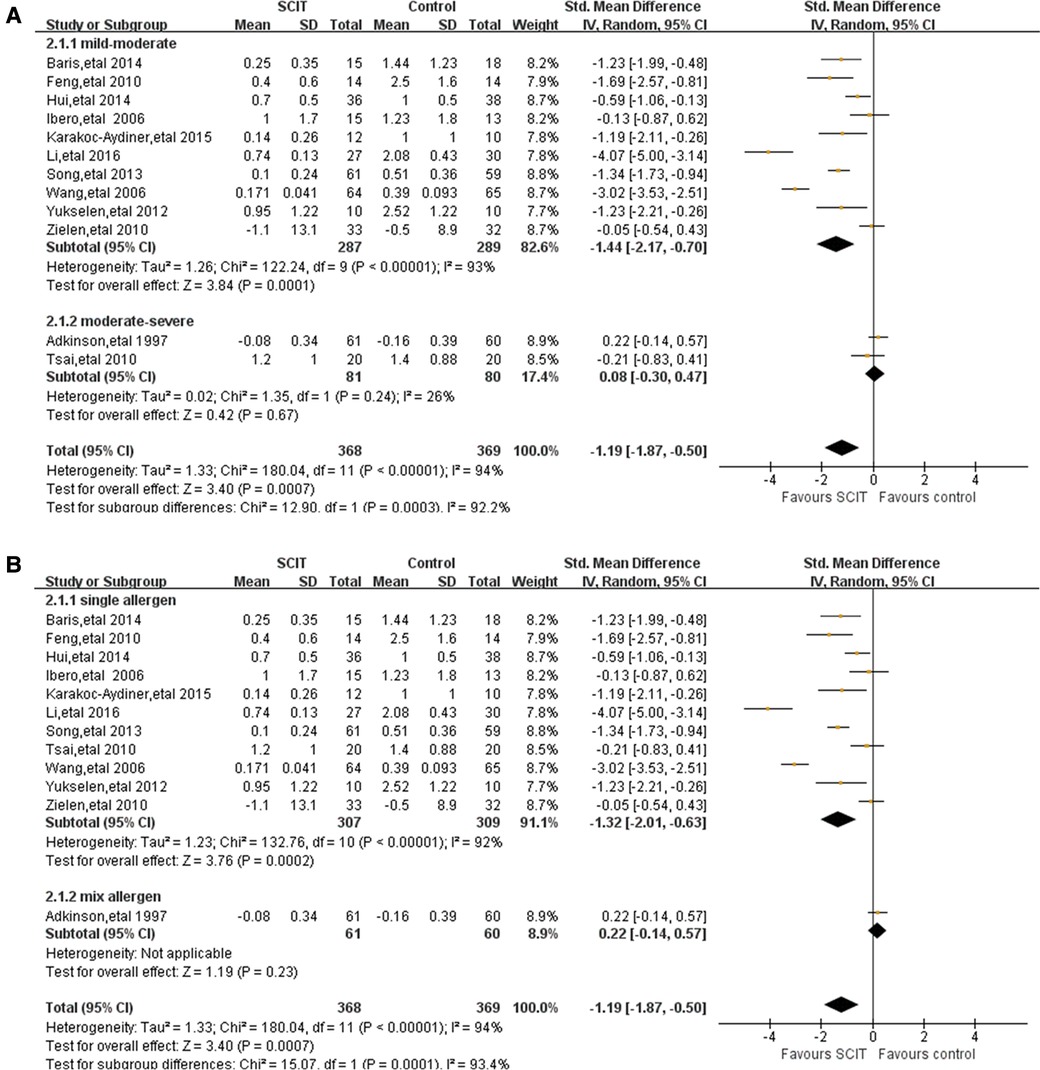
Figure 3. Subgroup analyses of short-term asthma symptom scores comparing SCIT and control groups: (A) mild-moderate asthma vs. moderate-severe asthma; (B) single allergen vs. mix allergens.
Twelve heterogeneous studies reported the asthma medication scores in short term. The pooled data demonstrated a statistically significant reduction in asthma drug usage with SMD −1.04 (95% CI: −1.54, −0.54) (Figure 4), which was confirmed again after the sensitivity analysis.
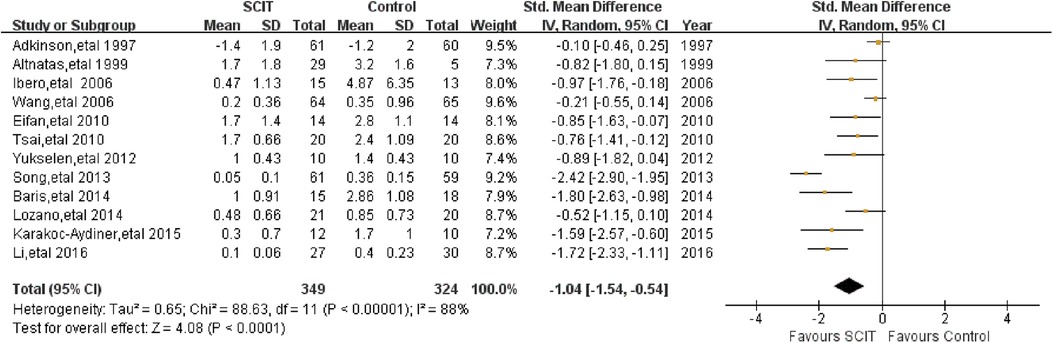
Figure 4. Meta-analysis of RCTs for short-term asthma medication scores comparing SCIT and control groups (random-effects model).
Mild-moderate asthma vs. moderate-severe asthma: the analysis revealed that SCIT is apparently efficacious in mild to moderate cases with SMD −1.18 (95% CI: −1.75, −0.61; I2 = 87%), but not in moderate to severe asthmatic cases with SMD −0.37 (95% CI: −1.01, 0.26; I2 = 68%).
Single allergen vs. mixed allergens: there is evidence of SCIT beneficial to patients with single allergen SMD −1.14 (95% CI: −1.66, −0.62; I2 = 85%) and a possible benefit in those with mixed allergens SMD −0.10 (95% CI: −0.46, 0.25). This result needs to be cautiously interpreted on account of limited studies in subgroups (only one study about mixed allergens).
Mono-sensitivity vs. poly-sensitivity & Treatment duration: both obvious efficacy of SCIT can be found in either sub-group. Those who received SCIT lasting more than 3 years could benefit more than the opposite.
Only one study showed no significant reduction in CSMS without providing data in details (21).
No relevant studies we reviewed reported these two outcomes which were evaluated at least one year after discontinuation of SCIT.
Five DBPC trials assessed adverse events (AEs) of the SCIT in HDM-sensitized asthmatic children. 3 mildly heterogeneous trials of those reported the local AEs and 4 moderately heterogeneous trials reported the systemic AEs. Compared to placebo, SCIT could increase the risk of local and systemic AE, with RR 4.37 (95% CI: 1.81, 10.57) and 2.90 (95% CI: 1.09, 7.71) respectfully (Figure 5). Subgroup analysis was impractical in those limited studies. In the total 33 researches for the safety, varied in participants characteristics or interventions, the incidence of local AE ranged from 1.3% to 64.8% of total injections, while systemic AE mostly accounted for less than 5% which were mainly mild or moderate without dead cases (see Supplementary Table S2). Most participants in those researches were elder than 5 years old and suffered from mild-moderate asthma. Single HDM allergen/allergoid with aluminum as adjuvant was the most common formulation. One study in Japan showed higher incidence of systemic AE up to 10.4% in patients under 5 years old, which might be exaggerated because it only reported the adverse events in induction phase (46). There are also some other studies evaluating the safety in children younger than 5 years old, which did not show the significant difference in contrast to elder children (21, 50).
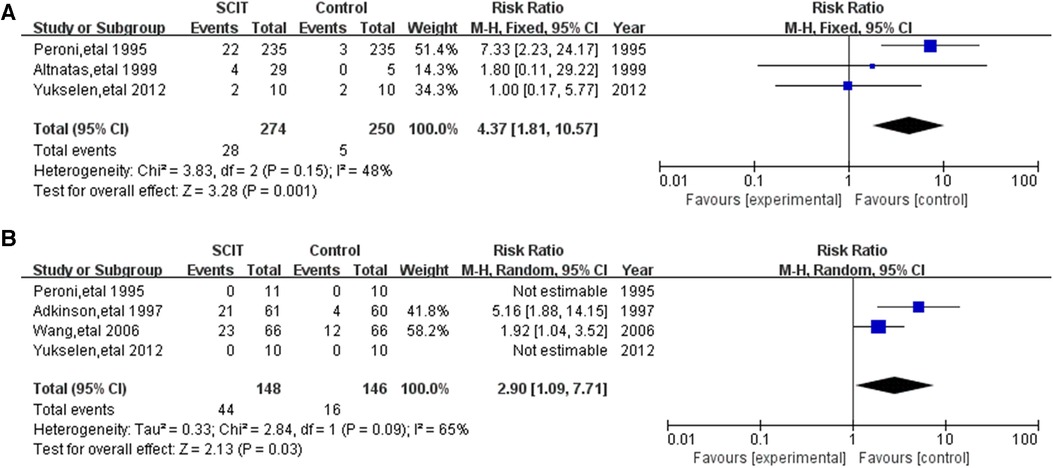
Figure 5. Meta-analysis of double-blind and placebo controlled trials for local and systemic adverse events comparing placebo groups: (A) local adverse events (fix-effects model); (B) systemic adverse events (random-effects model).
Nine studies reported FEV1 with moderate heterogeneity. The meta-analysis demonstrated a borderline improvement of SCIT on the FEV1 compared with the placebo or pharmacotherapy by random effects model with MD 3.37 (95% CI: 0.23, 6.51; I2 = 57%). However, the evidence is not robust after sensitivity analysis.
There is mildly heterogeneous evidence of large benefit of HDM SCIT only in mono-sensitized patients MD 5.37 (95% CI: 1.18, 9.57; I2 = 45%, see Figure 6A). Ineffectiveness of SCIT was showed in polysensitized patients, either mild-moderate or moderate-severe asthmatic children and patients treated for different duration. All the participants included in those studies were treated with single allergen of HDM.
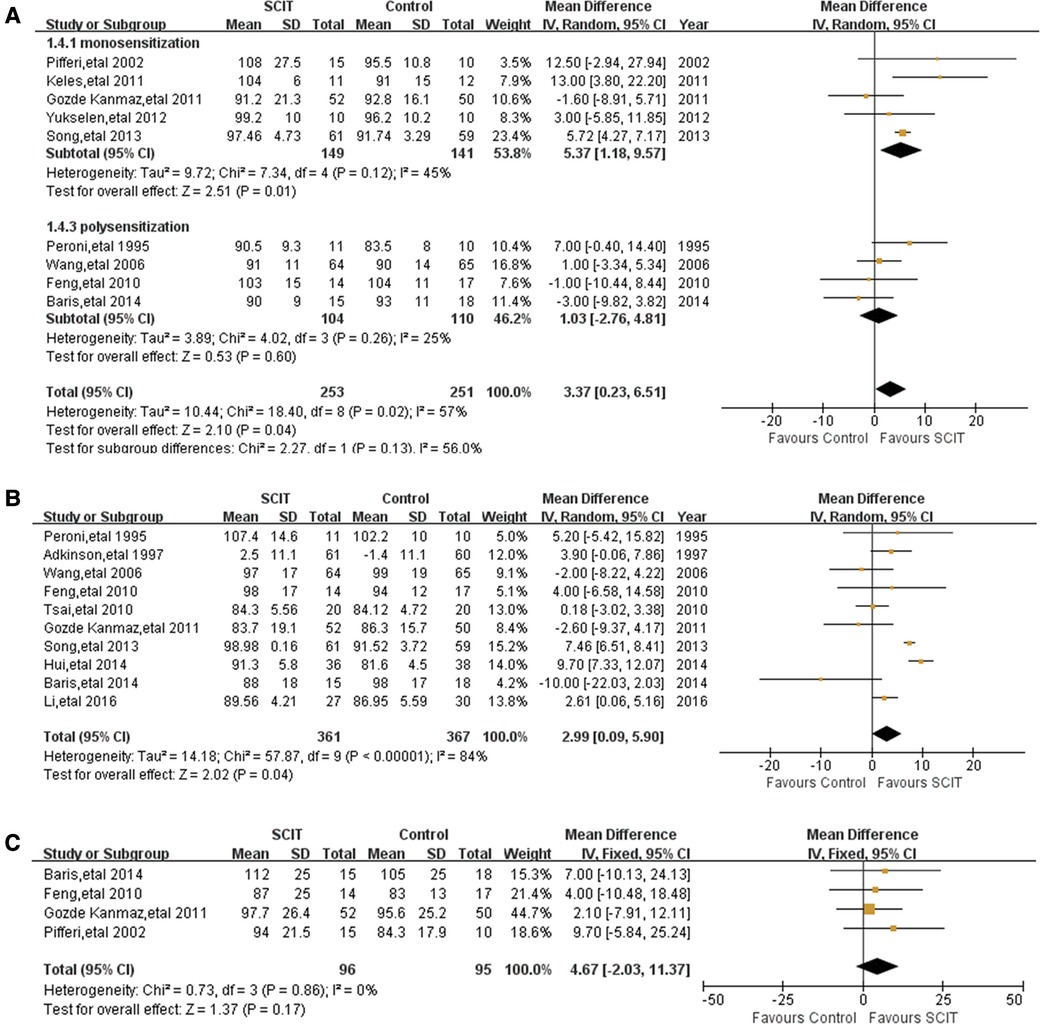
Figure 6. Meta-analysis of RCTs for pulmonary function comparing SCIT and control groups: (A) FEV1 after subgroup analysis of monosensitization vs. polysensitization (random-effects model); (B) PEF (random-effects model).; (C) MMEF (fix-effects model).
Pooled data from 10 trials reporting on PEF demonstrated a marginal improvement of SCIT with a MD of 2.99 (95% CI: 0.09, 5.90; I2 = 84%; see Figure 6B). However, the sensitivity analysis showed the lack of robustness of the finding again. There is no clear evidence supporting that SCIT could apparently improve the value of PEF in a certain population except those treated more than 3 years after subgroup analyses.
Four homogeneous studies reported the value of MMEF, from which the pooled data showed a suggested efficacy (but not confirmed) of SCIT MD 4.67 (95% CI: −2.03, 11.37; see Figure 6C). The evidence was still convincing after sensitivity analysis. Subgroup analyses were not performed because of few studies related.
There were two studies unable to be pooled reporting the Asthma Quality of Life Questionnaire (AQLQ), which revealed an apparent improvement compared with the control group. Another study evaluated the outcome using Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQOL), which also illustrated the efficacy of SCIT in improving asthma related quality of life (21).
Two studies reported the outcome on asthma symptoms control using numerical or categorical tools. Only one article performed the asthma control test (ACT) to assess the asthma control (22). The other research used 11-items of asthma control parameters according to GOAL criteria (54). Neither of them found significant difference between the SCIT and control group regarding asthma control.
Eight studies reported information about asthma exacerbation in different definitions. Three studies, of substantial heterogeneity, reported on exacerbation which was defined by the number of annual asthma attacks. Pooling of data from those studies showed significant difference between the SCIT and control therapy with SMD −1.07 (95% CI: −1.92, −0.22; I2 = 80%), which showed the possible benefit of SCIT in decreasing the number of asthma attacks. Another three studies reported on the number of hospitalizations with moderate heterogeneity, from which the pooled data revealed possible efficacy of SCIT in reducing the rate of hospitalizations: MD −0.07 (95% CI: −0.25, 0.12; I2 = 56%). The sensitivity analyses were not applicable as none of the six studies were found to be at high ROB. Four articles also reported on exacerbation defined in other various ways, which we were unable to pool.
Five mildly heterogeneous studies reported the data of non-specific BPT defined by methacholine PC20 or histamine PC20. Pooling of those data showed an SMD of 0.25 (95% CI: 0.03, 0.46; I2 = 33%), however turning to SMD 0.21 (95% CI: −0.02, 0.44; see Figure 7A) after sensitivity analysis, which indicates possible evidence in favor of SCIT. There were another three studies reporting the change of logPC20 or cold dry air challenge between groups without significant difference as well (16, 27, 34). Three studies performed HDM-specific BPT and the meta-analysis demonstrated an obvious benefit of SCIT with an SMD of 0.90 (95% CI: 0.36, 1.44; see Figure 7B). It appears that SCIT would have a greater effect on allergen specific airway hyperreactivity (AHR) than nonspecific AHR.
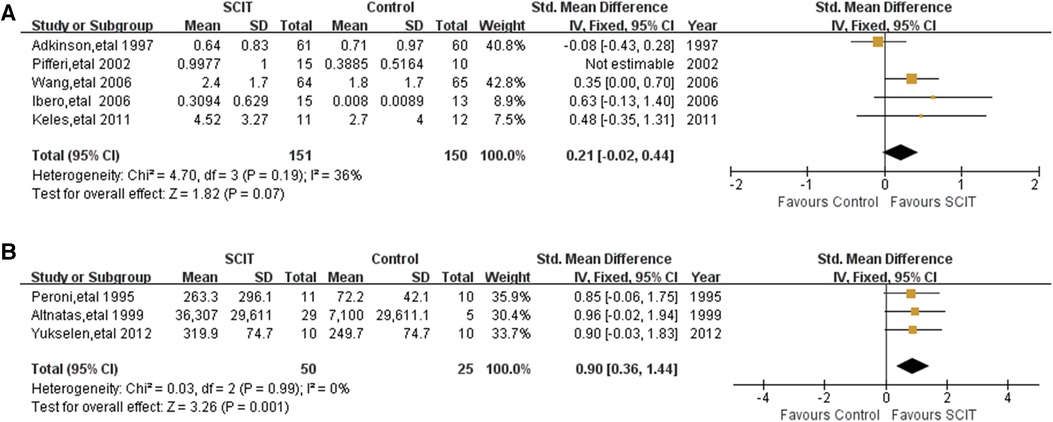
Figure 7. Meta-analysis of RCTs for brochial provocation test comparing SCIT and control groups: (A) nonspecific brochial provocation test (after sensitivity analysis; fix-effects model); (B) allergen-specific brochial provocation test (fix-effects model).
From the pooled data we analyzed, SCIT in HDM-sensitized asthmatic children resulted in significant short-term reductions in asthma symptoms and medication use, regardless of difference in treatment duration and number of allergens sensitized. For secondary outcomes, SCIT could decrease allergen-specific AHR, but without significant improvement in pulmonary function.
Similar efficacy of SCIT in asthma with different allergens in children and adults has also been noted in previous systematic reviews or meta-analysis (6, 10, 55–57). At least three years of SCIT maintenance is recommended in some DBPC trails, which is consistent with our findings that an adequate course of treatment could provide better benefits in reducing medication use and improving PEF compared with less than three years. However, some indirect evidence in our study suggested the low efficacy of SCIT with mixed allergen extracts. The reason might be the ineffective dose concentration of the main clinically relevant allergens (55, 58). In Europe, single or few allergens (homologous only) considered to be most clinically relevant are typically used in polysensitized patients (59–61). Nevertheless, considering the prevalence of polysensitization in patients with allergic rhinoconjunctivitis (AR) or AA, furthermore, the interaction between the multiplicity of allergens and the severity of disease, there is still widespread clinical use of extract mixtures, in Unite States for example (9, 60, 62). More well-designed clinical trials comparing monoallergen or oligoallergen (2 or 3 allergens) with polyallergen immunotherapy strategies are proposed in a head-to-head approach (9). Besides, in the subgroup analysis for the moderate-severe asthma, low-grade evidence supported the ineffectiveness of SCIT in the symptom control and medication usage reduction. It might be due to the confounding factor of mixed allergens or the different phenotypic characteristics of severe asthma (63). Because of the vague population information in the original literature, we could not determine the asthma severity of all subjects and this sub-analysis of asthma severity results in population overlap. Regarding efficacy in patients with severe asthma, which is still controversial, a previous study showed that adults with moderate persistent asthma could benefit more than those with severe asthma after HDM SCIT, supporting better effect of SCIT in patients with mild to moderate asthma than the severe likewise (64). Research on patients with severe asthma but well controlled with drug treatment is still required. However, because severe asthma has been frequently reported as a risk factor for systemic adverse reactions with AIT, especially when uncontrolled, evidence of the effectiveness of AIT on severe asthma is rather limited (65–68). To reduce the risks for these patients, some new emerging therapeutic approaches have been proposed, such as the use of omalizumab (69–71). In addition, the impact of HDM SCIT on quality of life, asthma control and exacerbation in asthmatic children need to be further explored.
Because of the difficulty of performing DBPC studies in asthmatic children, the safety of SCIT was mainly validated in single-arm studies, which showed mild to moderate risk of systemic AEs [mostly Grade I-III according to WAO (72)]. Compared with the results of another systematic review involving asthmatic children and adults without restriction of SCIT allergen types (73), the risks of both local and systemic AEs were higher in our study (RR: 1.4 vs. 4.37 for local AE and RR: 2.45 vs. 2.90 for systemic AE), indicating a potential increased risk of SCIT in asthmatic children allergic to HDM. In contrast to previous studies supporting the significantly greater number of systemic reactions in children under the age of 5 (74), three studies included in this review demonstrated the favourable safety in younger children. Although this may be related to the increased experience of allergists, the result is still not unassailable due to the potentially unrepresentative sample because some studies reporting on the data of children and adults simultaneously which we had to exclude at first. Focusing on those single-arm trials with rather higher incidence of local or systemic AEs, it could be found that the majority was associated with moderate-severe asthma or rush schedule. However, given the considerable variation in methods and the lack of information in some studies, our assumption is casual and needs to be confirmed. It is imperative to offer more high-quality evidence to clarify the risk of SCIT in people of different ages and extracts with different allergen types. In the other hand, prior use of oral antihistamines may prevent the occurrence of adverse reactions. The majority of systemic reactions [86% published in a survey (75)] occurred within 30 min after injections which can be observed in the clinic and treated timely and effectively (76). Consistent with our finding, fatal anaphylaxis is rarely reported under the guidance of a professional medical team (77).
In spite of some new homogeneous conclusions that we have drawn, substantial heterogeneity remained after sensitivity analysis and subgroup analyses, especially on the primary outcomes of short-term symptom scores and medication scores. This heterogeneity can be partly explained by the different scoring schemes used in the original studies, as some objective outcomes did not show heterogeneity (e.g., MMEF or BPT) or turned to homogenous after stratifying (e.g., FEV1 for the mono-/poly-sensitization). EAACI has published the recommendation of standard criteria for the assessment of symptom scores and medication scores in AR (78). However, there is still a lack of relative documents on AA. To facilitate interpretation of future studies, standardization of asthma symptom and medication scores is urgently required. Medication requirements reported as categories may translate into a useful outcome for that (73). In addition, because of the insufficient information, we did not undertake the planned subgroup analyses of the asthma courses and the administration of allergen preparations. Based on the results of our study and previous conclusions, we assumed that a prolonged asthma course would be more likely to trend toward polysensitized state and a complex phenotype, which may preclude the benefit of AIT (62). Many studies have reported the efficacy of novel approaches of SCIT (such as rush SCIT, semi rush SCIT or cluster SCIT), however, in small sample size or open label (79, 80).
There are still some limitations to be considered in this review. Firstly, the major limitation is that we did not include all data from the potentially eligible population. Several studies involving both children and adults did not report on the relevant data separately, which we had to excluded. In addition, there were remaining 18 references we were unable to retrieve to further screen. We sought the full text or further information from the authors, but received little. Besides, literature written in languages other than English or Chinese, conference reports and potential literature in other databases were not considered in our review, which could result in publication bias. Secondly, we were unable to pool data from all retrieved articles because of the insufficient information and the heterogeneity of approaches. The results of this review, especially for secondary outcomes, may not be representative of all trials, which needs further researches to confirm.
SCIT can reduce asthma symptoms and medication usage and improve the allergen-specific airway hyperreactivity in asthmatic children sensitized to HDM, but with a risk of mainly mild-moderate adverse reactions. The effectiveness of SCIT on lung function, asthma control, exacerbation and long-term efficacy after discontinuation is not conclusive, both in sub-population with mixed allergens and severe asthma, which requires further investigation. Overall, SCIT is still recommended for children with mild-moderate HDM-driven allergic asthma.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CZ: designed the systematic review protocol, assessed articles for inclusion and the risk of bias of included papers, extracted and analyzed data, interpreted the results and drafted the initial manuscript. HX: assessed articles for inclusion and the risk of bias of included papers, extracted and pooled data. SH: analyzed the data and interpreted the results. ZC: provided guidance on study design and interpreted the results. All authors contributed to the article and approved the submitted version.
This review has been funded by the Zhejiang Provincial Education Department. The project is supported by Scientific Research Fund of Zhejiang Provincial Education Department (2021). The grant number is Y202148363.
The authors thank Dr. Yunxian Yu (Department of Epidemiology and Public Health, School of Medicine, Zhejiang University) for his expertise and guidance on systematic review methodology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1137478/full#supplementary-material
SUPPLEMENTARY TABLE S1
General characteristics of the Included Studies about SCIT efficacy.
SUPPLEMENTARY TABLE S2
General characteristics of the Included Studies about SCIT safety.
SUPPLEMENTARY TABLE S3
The risk of bias assessment of included randomized controlled trials.
1. Alvaro-Lozano M, Akdis CA, Akdis M, Alviani C, Angier E, Arasi S, et al. EAACI allergen immunotherapy user’s guide. Pediatr Allergy Immunol. (2020) 31(Suppl 25):1–101. doi: 10.1111/pai.1318932436290
2. Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. (2019) 74:855–73. doi: 10.1111/all.1374931095767
3. Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. (1999) 341:468–75. doi: 10.1056/NEJM19990812341070210441602
4. Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. (2006) 61:198–201. doi: 10.1111/j.1398-9995.2006.01011.x16409196
5. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. (2007) 62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x17620073
6. Kim JM, Lin SY, Suarez-Cuervo C, Chelladurai Y, Ramanathan M, Segal JB, et al. Allergen-specific immunotherapy for pediatric asthma and rhinoconjunctivitis: a systematic review. Pediatrics. (2013) 131:1155–67. doi: 10.1542/peds.2013-034323650298
7. Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Imm. (2012) 157:288–98. doi: 10.1159/000327566
8. Keles S, Karakoc-Aydiner E, Ozen A, Izgi AG, Tevetoglu A, Akkoc T, et al. A novel approach in allergen-specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol. (2011) 128:808–15.e7. doi: 10.1016/j.jaci.2011.04.03321641635
9. Wheatley LM, Wood R, Nadeau K, Liu A, Zoratti E, Bacharier L, et al. Mind the gaps: Clinical trial concepts to address unanswered questions in aeroallergen immunotherapy-An NIAID/AHRQ Workshop. J Allergy Clin Immunol. (2019) 143:1711–26. doi: 10.1016/j.jaci.2019.01.03230731123
10. Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. (2017) 72:1825–48. doi: 10.1111/all.1320828543086
11. Hankin CS, Cox L, Lang D, Levin A, Gross G, Eavy G, et al. Allergy immunotherapy among medicaid-enrolled children with allergic rhinitis: patterns of care, resource use, and costs. J Allergy Clin Immunol. (2008) 121:227–32. doi: 10.1016/j.jaci.2007.10.02618206509
12. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: american academy of allergy, asthma & immunology/European academy of allergy and clinical immunology/PRACTALL consensus report. J Allergy Clin Immunol. (2013) 131:1288–96.e3. doi: 10.1016/j.jaci.2013.01.04923498595
13. Global Initiative for Asthma. GINA guidelines 2022. Global strategy for asthma management and prevention (2022). Available at: http://www.ginasthma.org/.
14. Karakoc-Aydiner E, Eifan AO, Baris S, Gunay E, Akturk E, Akkoc T, et al. Long-term effect of sublingual and subcutaneous immunotherapy in dust mite–allergic children with asthma/rhinitis: a 3-year prospective randomized controlled trial. J Invest Allerg Clin. (2015) 25:334–42.
15. Lozano J, Cruz MJ, Piquer M, Giner MT, Plaza AM. Assessing the efficacy of immunotherapy with a glutaraldehyde-modified house dust mite extract in children by monitoring changes in clinical parameters and inflammatory markers in exhaled breath. Int Arch Allergy Imm. (2014) 165:140–7. doi: 10.1159/000368832
16. Baris S, Kiykim A, Ozen A, Tulunay A, Karakoc-Aydiner E, Barlan IB. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy. (2014) 69:246–53. doi: 10.1111/all.1227824180595
17. Zheng C, Xu H, Chen ZM, Huang SM. Efficacy and safety of subcutaneous immunotherapy in asthmatic children allergic to house dust mite: a meta-analysis and systematic review. PROSPERO 2022 CRD42022320361 Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022320361.
18. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American thoracic society/European respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. (2009) 180:59–99. doi: 10.1164/rccm.200801-060ST19535666
19. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. Available at: https://www.training.cochrane.org/handbook.
20. Canonica GW, Bachert C, Hellings P, Ryan D, Valovirta E, Wickman M, et al. Allergen immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J. (2015) 8:31. doi: 10.1186/s40413-015-0079-726594303
21. de Vos G, Viswanathan S, Pichardo Y, Nazari R, Jorge Y, Ren Z, et al. A randomized trial of subcutaneous allergy immunotherapy in inner-city children with asthma less than 4 years of age. Ann Allergy Asthma Immunol. (2021) 126:367–77.e5. doi: 10.1016/j.anai.2020.12.01633418053
22. Li H, Yang P, Chen X, Sun W, Qu D, Zhao X, et al. A comparative study of sublingual and subcutaneous immunotherapy in mite-sensitive asthmatic children: a single center experience of 90 Chinese patients. Int J Clin Exp Med. (2016) 9:6743–50.
23. Hui Y, Li L, Qian J, Guo Y, Zhang X, Zhang X. Efficacy analysis of three-year subcutaneous SQ-standardized specific immunotherapy in house dust mite-allergic children with asthma. Exp Ther Med. (2014) 7:630–4. doi: 10.3892/etm.2014.146924520258
24. Song W, Lin X, Xie H, Chai R. Evaluation of the efficacy and safety of standardized dust mite allergen specific immunotherapy to children with allergic asthma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2013) 27:1193–6.
25. Gozde KH, Harmanci K, Razi C, Kose G, Cengizlier MR. Specific immunotherapy improves asthma related quality of life in childhood. Allergol Immunopathol. (2011) 39:68–72. doi: 10.1016/j.aller.2010.04.005
26. Feng H, Xiang L, Shen KL. Dynamical changes of lung function and immunologic markers in asthmatic children receiving specific immunotherapy with standardized house dust mite extract. Zhongguo Dang Dai Er Ke Za Zhi. (2010) 12:715–9.20849721
27. Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. (2010) 40:922–32. doi: 10.1111/j.1365-2222.2009.03448.x20100188
28. Tsai TC, Lu JH, Chen SJ, Tang RB. Clinical efficacy of house dust mite-specific immunotherapy in asthmatic children. Pediatr Neonatol. (2010) 51:14–8. doi: 10.1016/S1875-9572(10)60004-620225533
29. Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immun. (2010) 126:942–9. doi: 10.1016/j.jaci.2010.06.00220624650
30. Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy: Eur J Allergy Clin Immunol. (2006) 61:191–7. doi: 10.1111/j.1398-9995.2005.00913.x
31. Ibero M, Castillo MJ. Significant improvement of specific bronchial hyperreactivity in asthmatic children after 4 months of treatment with a modified extract of dermatophagoides pteronyssinus. J Invest Allerg Clin. (2006) 16:194–202.
32. Pifferi M, Baldini G, Marrazzini G, Baldini M, Ragazzo V, Pietrobelli A, et al. Benefits of immunotherapy with a standardized dermatophagoides pteronyssinus extract in asthmatic children: a three-year prospective study. Allergy: Eur J Allergy Clin Immunol. (2002) 57:785–90. doi: 10.1034/j.1398-9995.2002.23498.x
33. Altintaş DU, Akmanlar N, Güneşer SK, Burgut R, Yilmaz M, Buǧdayci R, et al. Comparison between the use of adsorbed and aqueous immunotherapy material in dermatophagoides pteronyssinus sensitive asthmatic children. Allergol Immunopath. (1999) 27:309–17.
34. Gruber W, Eber E, Mileder P, Modl M, Weinhandl E, Zach MS. Effect of specific immunotherapy with house dust mite extract on the bronchial responsiveness of paediatric asthma patients. Clin Exp Allergy. (1999) 29:176–81. doi: 10.1046/j.1365-2222.1999.00391.x10051720
35. Adkinson NF Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, Bacon JR, et al. A controlled trial of immunotherapy for asthma in allergic children. New Engl J Med. (1997) 336:324–31. doi: 10.1056/NEJM1997013033605029011784
36. Peroni DG, Piacentini GL, Martinati LC, Warner JO, Boner AL. Double-blind trial of house-dust mite immunotherapy in asthmatic children resident at high altitude. Allergy: Eur J Allergy Clin Immunol. (1995) 50:925–30. doi: 10.1111/j.1398-9995.1995.tb02500.x
37. Sala-Cunill A, Almeida-Sánchez ZM, García-Núñez I, Laín S, Martínez-Tadeo JA, Martos MD, et al. Real-world safety and effectiveness evidence of a microcrystalline tyrosine-associated mite allergoid in children and adolescents with allergic rhinitis. Allergol Immunopath. (2021) 49:98–108. doi: 10.15586/aei.v49i4.195
38. Sharkey P, Portnoy J. Rush immunotherapy: experience with a one-day schedule. Ann Allergy Asthma Immunol. (1996) 76:175–80. doi: 10.1016/S1081-1206(10)63419-98595538
39. Liao W, Chen L, Bai J. Systemic reactions to subcutaneous immunotherapy for bronchial asthma and/or allergic rhinitis in children and their risk factors. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22:1204–8. doi: 10.7499/j.issn.1008-8830.200509333172556
40. Liu JL, Ning WX, Li SX, Xu YC, Wu L, Wang YS, et al. The safety profile of subcutaneous allergen immunotherapy in children with asthma in Hangzhou. East China. Allergol Immunopath. (2017) 45:541–8. doi: 10.1016/j.aller.2017.04.002
41. Dai L, Huang Y, Wang Y, Han HL, Li QB, Jiang YH. Serious systemic adverse events associated with allergen-specific immunotherapy in children with asthma. Chin J Contemp Pediat. (2014) 16:58–61. doi: 10.7499/j.issn.1008-8830.2014.01.013
42. Li MR, Wang XN, Jiang HD, Wang QY, Li YC, Lin J, et al. Analysis of adverse reactions induced by subcutaneous immunotherapy against dust mite allergy in 234 cases with allergic rhinitis and asthma. Zhonghua Er Ke Za Zhi. (2012) 50:726–31.23302557
43. Hao C. Safety of specific immunotherapy with standardized house - Mite vaccine in asthmatic children. Allergy: Eur J Allergy Clin Immunol. (2010) 65:707–8. doi: 10.1111/j.1398-9995.2010.02395.x
44. Morais-Almeida M, Arêde C, Sampaio G, Borrego LM. Ultrarush schedule of subcutaneous immunotherapy with modified allergen extracts is safe in paediatric age. Asia Pac Allergy. (2016) 6:35–42. doi: 10.5415/apallergy.2016.6.1.3526844218
45. Schubert R, Eickmeier O, Garn H, Baer PC, Mueller T, Schulze J, et al. Safety and immunogenicity of a cluster specific immunotherapy in children with bronchial asthma and mite allergy. Int Arch Allergy Immunol. (2009) 148:251–60. doi: 10.1159/00016158518849616
46. Hamada M, Saeki K, Tanaka I. Effectiveness and safety of subcutaneous immunotherapy with standardized house dust mite extract for patients under the age of 5 years: a prospective cohort study. Allergol Int. (2021) 70:492–4. doi: 10.1016/j.alit.2021.05.00434108104
47. Akcakaya N, Hassanzadeh A, Camcioglu Y, Cokugras H. Local and systemic reactions during immunotherapy with adsorbed extracts of house dust mite in children. Ann Allergy Asthma Immunol. (2000) 85:317–21. doi: 10.1016/S1081-1206(10)62536-711061476
48. Peñas A, García-González M, Cruz MJ, Valdesoiro L, Boot JD, Larramona H, et al. Observational study of the safety of a cluster schedule for subcutaneous immunotherapy in a pediatric population. J Invest Allerg Clin. (2013) 23:63–5.
49. Xiang L, Liu F, Zhi L, Jiang W, Liu C, Xie H, et al. Safety of semi-depot house dust mite allergen extract in children and adolescents with allergic rhinitis and asthma. Immunotherapy. (2021) 13:227–39. doi: 10.2217/imt-2020-023233317341
50. Tortajada M, Juliá J, Moreno M, García C, Tallón M, Gracia M, et al. Efficacy and safety of specific subcutaneous immunotherapy with dust mites in children under 5 years of age. Allergy: Eur J Allergy Clin Immunol. (2010) 65:707. doi: 10.1111/j.1398-9995.2010.02395.x
51. Wu Y, Long Z, Huang Y, Huang X. Study on safty of standardized specific mite-allergen immunotherapy to children with allergic rhinitis and/or asthma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2011) 25:641–4.
52. Lange J, Zagórska W, Wójtowicz A, Krauze A. Cluster immunotherapy in children with allergic rhinoconjuncitivitis and/or bronchial asthma and mite allergy - safety of initial immunotherapy - preliminary results from Poland. Allergy: Eur J Allergy Clin Immunol. (2010) 65:264. doi: 10.1111/j.1398-9995.2010.02393.x
53. Businco L, Zannino L, Cantani A, Corrias A, Fiocchi A, La Rosa M. Systemic reactions to specific immunotherapy in children with respiratory allergy: a prospective study. Pediatr Allergy Immunol. (1995) 6:44–7. doi: 10.1111/j.1399-3038.1995.tb00257.x7550765
54. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma ControL study. Am J Respir Crit Care Med. (2004) 170:836–44. doi: 10.1164/rccm.200401-033OC15256389
55. Lu Y, Xu L, Xia M, Li Y, Cao L. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. (2015) 60:269–78. doi: 10.4187/respcare.0339925389355
56. Rice JL, Diette GB, Suarez-Cuervo C, Brigham EP, Lin SY, Ramanathan MJ, et al. Allergen-specific immunotherapy in the treatment of pediatric asthma: a systematic review. Pediatrics. (2018) 141:e20173833. doi: 10.1542/peds.2017-383329572287
57. Erekosima N, Suarez-Cuervo C, Ramanathan M, Kim JM, Chelladurai Y, Segal JB, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. (2014) 124:616–27. doi: 10.1002/llary.2429523832632
58. Haugaard L, Dahl R, Jacobsen L. A controlled dose-response study of immunotherapy with standardized, partially purified extract of house dust mite: clinical efficacy and side effects. J Allergy Clin Immunol. (1993) 91:709–22. doi: 10.1016/0091-6749(93)90190-q8454793
59. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth VWR, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. (2018) 73:765–98. doi: 10.1111/all.1331728940458
60. Migueres M, Davila I, Frati F, Azpeitia A, Jeanpetit Y, Lheritier-Barrand M, et al. Types of sensitization to aeroallergens: definitions, prevalences and impact on the diagnosis and treatment of allergic respiratory disease. Clin Transl Allergy. (2014) 4:16. doi: 10.1186/2045-7022-4-1624817997
61. European Medicines Agency. Allergen products: production and quality issues (2008). Available at: https://www.ema.europa.eu/en/allergen-products-production-quality-issues.
62. Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. (2016) 138:1016–29. doi: 10.1016/j.jaci.2016.06.06127720016
63. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. (2018) 6:545–54.e4. doi: 10.1016/j.jaip.2017.05.03228866107
64. Blumberga G, Groes L, Haugaard L, Dahl R. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy. (2006) 61:843–8. doi: 10.1111/j.1398-9995.2006.01088.x16792582
65. Epstein TG, Murphy-Berendts K, Liss GM, Bernstein DI. Risk factors for fatal and nonfatal reactions to immunotherapy (2008−2018): postinjection monitoring and severe asthma. Ann Allergy Asthma Immunol. (2021) 127:64–9.e1. doi: 10.1016/j.anai.2021.03.01133753219
66. Bernstein DI, Wanner M, Borish L, Liss GM. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990−2001. J Allergy Clin Immunol. (2004) 113:1129–36. doi: 10.1016/j.jaci.2004.02.00615208595
67. Bernstein DI, Epstein T. Safety of allergen immunotherapy in North America from 2008−2017: lessons learned from the ACAAI/AAAAI national surveillance study of adverse reactions to allergen immunotherapy. Allergy Asthma Proc. (2020) 41:108–11. doi: 10.2500/aap.2020.41.20000132122446
68. Aue A, Ho J, Zhu R, Kim H, Jeimy S. Systemic reactions to subcutaneous allergen immunotherapy: real-world cause and effect modelling. Allergy Asthma Clin Immunol. (2021) 17:65. doi: 10.1186/s13223-021-00566-x34229743
69. Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. (2010) 125:383–9. doi: 10.1016/j.jaci.2009.11.02220159249
70. Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. (2014) 25:829–32. doi: 10.1111/pai.1230625387446
71. Stelmach I, Majak P, Jerzynska J, Bojo M, Cichalewski L, Smejda K. Children with severe asthma can start allergen immunotherapy after controlling asthma with omalizumab: a case series from Poland. Arch Med Sci. (2015) 11:901–4. doi: 10.5114/aoms.2015.4854626322106
72. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the world allergy organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. (2010) 125:569–74. 574.e1−574.e7. doi: 10.1016/j.jaci.2009.10.06020144472
73. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. (2010) 8:CD001186. doi: 10.1002/14651858.CD001186.pub2
74. Hejjaoui A, Dhivert H, Michel FB, Bousquet J. Immunotherapy with a standardized dermatophagoides pteronyssinus extract. IV. systemic reactions according to the immunotherapy schedule. J Allergy Clin Immunol. (1990) 85:473–9. doi: 10.1016/0091-6749(90)90157-y2406324
75. Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. Immediate and delayed-onset systemic reactions after subcutaneous immunotherapy injections: ACAAI/AAAAI surveillance study of subcutaneous immunotherapy: year 2. Ann Allergy Asthma Immunol. (2011) 107:426–31.e1. doi: 10.1016/j.anai.2011.05.02022018614
76. Bousquet J, Pfaar O, Togias A, Schunemann HJ, Ansotegui I, Papadopoulos NG, et al. 2019 ARIA care pathways for allergen immunotherapy. Allergy. (2019) 74:2087–102. doi: 10.1111/all.1380530955224
77. Bernstein DI, Epstein T, Murphy-Berendts K, Liss GM. Surveillance of systemic reactions to subcutaneous immunotherapy injections: year 1 outcomes of the ACAAI and AAAAI collaborative study. Ann Allergy Asthma Immunol. (2010) 104:530–5. doi: 10.1016/j.anai.2010.04.00820568387
78. Pfaar O, Demoly P, Gerth VWR, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy. (2014) 69:854–67. doi: 10.1111/all.1238324761804
79. Bousquet J, Calvayrac P, Guerin B, Hejjaoui A, Dhivert H, Hewitt B, et al. Immunotherapy with a standardized dermatophagoides pteronyssinus extract. I. in vivo and in vitro parameters after a short course of treatment. J Allergy Clin Immunol. (1985) 76:734–44. doi: 10.1016/0091-6749(85)90680-34056259
Keywords: subcutaneous immunotherapy, asthma, children, house dust mite, efficacy, safety, systematic review, meta-analysis
Citation: Zheng C, Xu H, Huang S and Chen Z (2023) Efficacy and safety of subcutaneous immunotherapy in asthmatic children allergic to house dust mite: a meta-analysis and systematic review. Front. Pediatr. 11:1137478. doi: 10.3389/fped.2023.1137478
Received: 4 January 2023; Accepted: 1 June 2023;
Published: 15 June 2023.
Edited by:
Bülent Taner Karadağ, Marmara University, TürkiyeReviewed by:
Corrado Pelaia, Magna Græcia University, Italy© 2023 Zheng, Xu, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhimin Chen em1jaGVuQHpqdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.