- 1Changzhou Maternal and Child Health Care Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, China
- 2Department of Gynecology and Obstetrics, Third Affiliated Hospital of Suzhou University, Changzhou, China
- 3Department of Nursing, the Affiliated Changzhou NO.2 People's Hospital of Nanjing Medical University, Changzhou, China
Background: To determine the risk factors for cesarean section (CS) and adverse fetal outcomes (AFOs) in patients with intrahepatic cholestasis of pregnancy (ICP) based on the severity of maternal hypercholanemia.
Methods: A hospital-based retrospective cohort study was performed between January 1, 2015, and December 31, 2019. A total of 227 nulliparous women with a singleton fetus complicated by ICP were included. The patients were divided into two groups according to the levels of total bile acids, that is, mild (10 μmol/L < total bile acids < 40 μmol/L) and severe (≥40 μmol/L). The patients' clinical characteristics and fetal outcomes were assessed.
Results: Among the 227 eligible women, 177 (78.0%) were allocated to the mild group and 50 (22.0%) were in the severe group. Women with severe ICP also had a significantly higher incidence of planned and unplanned CS compared with mild ICP subjects (52.0% vs. 23.7% and 22.0% vs. 6.8%, respectively; p < 0.001). The indications for CS showed that fetal intolerance (65.4% vs. 14.3%) was higher in severe ICP compared with mild ICP (p < 0.001). Severe ICP was associated with an increased risk of preterm delivery (p < 0.001), low birthweight (p = 0.001), and neonatal intensive care unit (NICU) admission (p < 0.001). Women with severe ICP (OR 6.397, 95%CI 3.041–13.455, p < 0.001) or preeclampsia (OR 12.434, 95%CI 5.166–29.928, p < 0.001) had increased risks of AFOs compared to controls.
Conclusions: Severe ICP and preeclampsia are associated with a higher incidence of AFOs.
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is characterized by unexplained maternal pruritus, raised serum bile acids (>10 μmol/L) and/or elevated liver transaminases (ALT > 35 U/L and/or AST > 35 U/L) during the late second and third trimester of pregnancy (1–3). Currently, the etiology of ICP is not fully understood but may be affected by hormonal, genetic, and environmental factors (4).
Although ICP was thought to be a relatively benign condition, recent studies have indicated that ICP has adverse effects for both the mother and fetus, including a notably higher risk of developing preeclampsia and gestational diabetes mellitus (GDM) (5, 6). Related to the fetus, ICP is associated with increased risks for preterm delivery (7), meconium staining of the amniotic fluid (MSAF) (8), neonatal intensive care unit admission (NICU) (3), and stillbirth (9), especially in cases of severe ICP.
According to previous studies, serum total bile acid levels >10 μmol/L and <40 μmol/L are considered mild ICP, while severe ICP is defined as maternal total bile acid levels ≥40 μmol/L, which is associated with higher maternal and neonatal complications (3, 5). Glantz A, et al. recently demonstrated that adverse pregnancy outcomes did not occur until maternal serum bile acids were >40 μmol/L (10). Previous studies reported that stillbirth in ICP tended to happen after 36 weeks of pregnancy (11). To prevent stillbirth, induction of labor has become common practice, especially in severe cases of patients. A large retrospective cohort study demonstrated that the risks of emergency caesarean section (CS) or fetal asphyxia were not increased after induction of labor in women with ICP during gestational weeks 37–39 (12), suggesting that this practice does not add additional risk. While the risks of adverse outcomes for both mothers and babies have started to be elucidated in women with ICP, the spectrum of ICP severity may differentially impact these risks and warrants further exploration.
The aim of this study was to evaluate maternal and fetal outcomes associated with severe ICP compared to mild ICP. Specifically, we set out to analyze potential differences in the indications for planned and unplanned CS and the rates of adverse fetal outcomes (AFOs) between women with severe ICP compared to women with mild ICP.
Methods
The data for the retrospective cohort study were obtained from January 1, 2015, to December 31, 2019 at the Changzhou Maternal and Child Health Care Hospital in Jiangsu (China), where 10,000–12,000 deliveries per year are performed. Information on maternal demographics, medical comorbidities, and AFOs was obtained from medical records for evaluation. Women with fasting serum bile acid levels of more than 10 μmol/L and pruritus of unexplained cause were included in the study. Since multiparas and women with twin pregnancies might influence the mode of delivery directly, only nulliparous women with a singleton fetus were included in the study. Women were excluded if they had viral or autoimmune hepatitis, liver or biliary disease, and fetal chromosomal or structural abnormalities. The study protocol was approved by the Ethical Committee of the Hospital.
The patients were screened for ICP in pregnancy utilizing ICD- 10 code (O26.6). The women received ursodeoxycholic acid (UDCA) therapy, and fetal monitoring was performed more than once per day before delivery, except in cases where delivery occurred before ICP diagnosis. For the primary analysis, ICP severity was defined according to maternal total bile acids levels as mild (10 μmol/L < total bile acids < 40 μmol/L) or severe (≥40 μmol/L).
Similar to previous studies (11, 13), the AFOs assessed included green staining of the placenta, an Apgar score of less than 7 at 5 min, preterm birth, placental abruption, neonatal unit admission, and stillbirth in this study. Preterm birth was defined as a gestational age before 37 completed gestational weeks. Information regarding the occurrence of GDM, gestational hypertension, preeclampsia, premature rupture of membranes (PROM), and other pathologies was also retrieved from the medical records.
Planned CS was defined as the intended mode of delivery, whereas all other CS were classified as unplanned. The indications for planned CS were severe preeclampsia, anatomic abnormality, estimated macrosomia, fetal intolerance of labor, and elective. The indications for unplanned CS were arrest of descent/dilation, non-reassuring fetal heart tracings (FHT), PROM + anatomic abnormality, and PROM + MSAF.
A two-tailed t-test was used to analyze continuous variables, and the χ2 or Fisher's exact test was used to analyze categorical variables. A logistic regression analyse was also performed to assess the risks of AFOs. Our data met the calculated sample size for AFOs which was from 17 to 757 in each group for 80% and 90% power, and a 95% confidence interval. Statistical significance was defined as p-values < 0.05, and the data were analyzed using the statistical software package SPSS 22.0.
Results
Two hundred twenty-seven pregnancies (177 mild and 50 severe ICP) met the inclusion criteria. The demographic and obstetric characteristics of the patients in the mild group and severe group are presented in Table 1. The analysis showed that maternal age, pregestational body mass index, and gestational diabetes mellitus, hypertension, preeclampsia, premature rupture of membranes, and meconium-stained amniotic fluid rate were not significantly different between the two groups (Table 1).
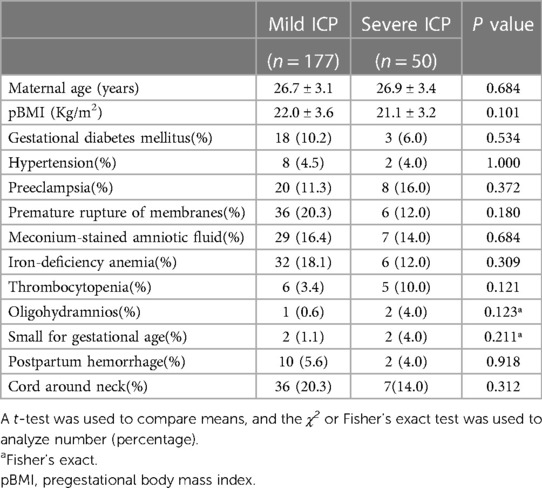
Table 1. Obstetric and demographic characteristics of patients with mild and severe intrahepatic cholestasis of pregnancy.
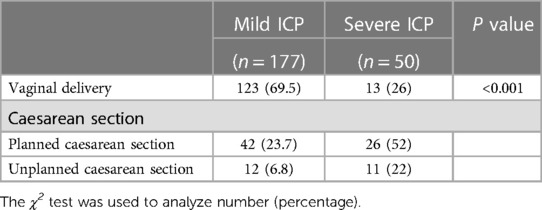
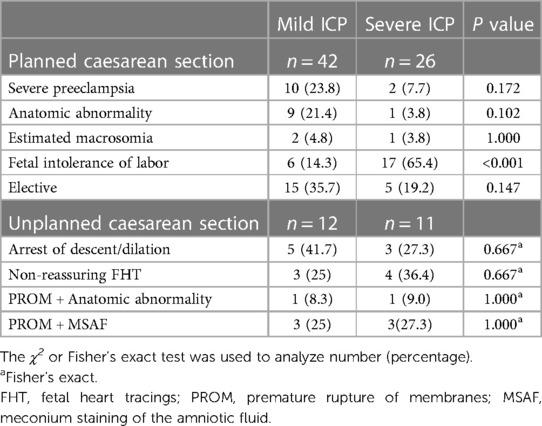
There were significant differences in the mode of delivery found between women with severe ICP compared to those with mild ICP (p < 0.001, Table 2) where a greater proportion of women with severe ICP underwent CS. Furthermore, women with severe ICP had a higher frequency of unplanned CS compared to those with mild ICP (22% vs. 6.8%, p < 0.001). The indications for planned/unplanned CS in women with mild or severe ICP are shown in Table 3. Most planned CS for patients with severe ICP were due to fetal intolerance (65.4%) which had lower incidence (14.3%) in women with mild ICP (p < 0.001). However, the rate of the indications for unplanned CS was not significantly different between the two groups (p > 0.05).
We further analyzed AFOs between the two groups (Table 4). A greater proportion and/or frequency of women with severe ICP had preterm delivery (40.0% vs. 8.5%, p < 0.001) and low birth weight (28.0% vs. 6.8%, p = 0.001) compared to those with mild ICP, Table 4. The incidence of NICU admission (8.5% vs. 32.0%, p < 0.001) and total AFOs (10.2% vs. 42.0%, p < 0.001) between mild ICP and severe ICP groups was significantly lower (Table 4).
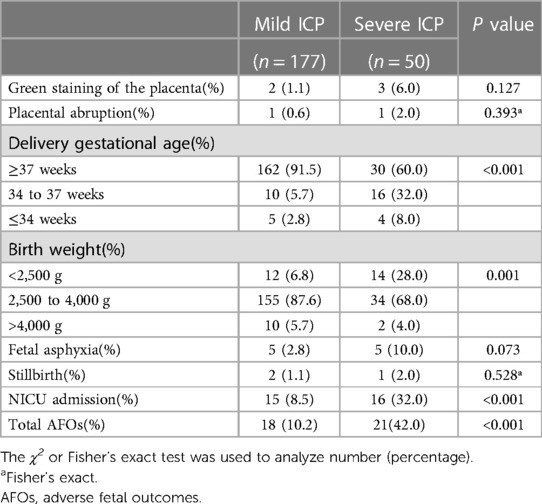
Table 4. Birth weight and adverse fetal outcomes in mild and severe intrahepatic cholestasis of pregnancy.
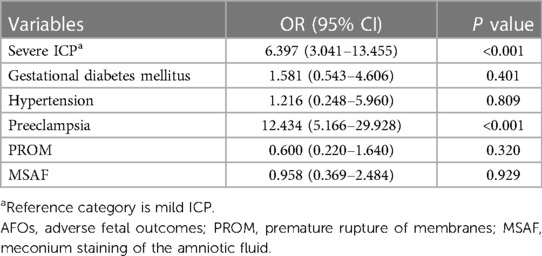
We discovered a strong association between severe ICP and AFOs (OR 6.397, 95%CI 3.041–13.455, p < 0.001) using a logistic regression model (Table 5). Among GDM, hypertension, preeclampsia, MSAF, and PROM, only women with preeclampsia were significantly more likely to have AFOs (OR 12.434, 95%CI 5.166–29.928, p < 0.001, Table 5).
Discussion
The present data indicate that women with severe ICP have a greater proportion of CS, including planned and unplanned CS, and a higher incidence of AFOs, compared to women with mild ICP. Percentage of 51.1 more women with severe ICP had fetal intolerance of labor compared to women with mild ICP in planned CS. Preeclampisa might lead to an increase risk of AFOs both in mild and severe ICP.
Tetsuya Kawakita et al. reported that the rate of CS in women with mild or severe ICP was less than 40% (9). In our study, almost 30% of patients with mild ICP underwent CS, while 74% of patients with severe ICP underwent CS. Given that patient preference to choose CS to avoid stillbirth while with severe ICP, the increased incidence of CS in women with a total bile acids level ≥40 μmol/L is not unexpected. Moreover, another study revealed that women with ICP during gestational weeks 37–39 had no increased risk of emergency CS compared to women without ICP; however, this study was limited to gestational weeks 37–39 and lacked data regarding preterm birth (12). As many authors have advocated the implementation of elective early delivery for ICP, especially for severe ICP (11, 14), the rates of planned and unplanned CS in our patients with severe ICP were higher than patients with mild ICP.
Although there are no randomized studies investigating the optimal timing of delivery for women with ICP, a majority of researchers have advocated the implementation of elective early delivery for ICP, especially for those patients with total bile acids levels ≥40 μmol/L (11). Most spontaneous or induced onset of early term labor did not increase the incidence of CS; however, many women with ICP in need of induction of labor underwent CS directly, which may have increased the incidence of CS in our study. Furthermore, to find the potential risk factors associated with CS, we analyzed the indication for these CS cases, which might diverge from current guidelines, containing planned and unplanned CS with different ICP severity. More than half of planned CS with severe ICP occurred due to fetal intolerance of labor, while most unplanned CS with severe ICP occurred for non-reassuring FHT, arrest of descent/dilation, and PROM + MSAF. Although the mechanism for the high rate of these events in severe ICP undergoing CS remains unclear, these indications were the reason the obstetrician chose planned or unplanned CS to prevent poor prognosis. Further research is needed to identify the ideal time of birth with regard to neonatal outcomes and to reduce the rates of CS if possible.
Recent studies have reported that an elevated total bile acids level may cause preeclampsia or GDM (5, 6). Preeclampsia occurs most often 2–4 weeks after the diagnosis of ICP, and still occurred even though total bile acids levels decreased toward the normal range according to a recent study from Raz et al. (5). Missing preeclampsia patients with a high level of total bile acids which returns to normal range later might leading to our study did not find a higher incidence of preeclampsia in women with severe ICP compared to mild ICP. Unexpectedly, our study did not find that GDM was concomitant with ICP severity at initial diagnosis, which is similar to that reported by Dan Shan, et al. (15). Advanced age and increased pregestational BMI may contribute more to the pathogenesis of GDM, in addition to the known effects on bile acid homeostasis.
Previous studies have reported that high bile acid levels could cause MSAF (8, 15). Moreover, previous reports have indicated that MSAF is more likely to occur during later compared to earlier gestation, which may be due to the higher bile acid concentrations in amniotic fluid and a more advanced maturation of the gastrointestinal tract (8). Women with severe ICP might reduce the incidence of MSAF by choosing to deliver before week 37. Although our study did not find MSAF to be associated with an increased risk of AFOs, obstetricians usually make decisions by considering the MSAF and the time to delivery to prevent newborns from developing meconium aspiration syndrome, which can be fatal.
As reported in previous studies, severe ICP as well as preeclampsia are risk factors of AFOs (16, 17), including preterm delivery, low birth weight, NICU admission, and stillbirth. After ORs for predictors of adverse fetal outcomes in ICP, we further found that preeclampsia was the risk factor of AFOs in both mild and severe ICP. Previous studies demonstrated that increased bile acids indeed crossed the placenta with increased oxidative stress contributing to the incidence of AFOs (18). Even if the relationship between preeclampsia and ICP is not considered, preeclampsia can also lead to AFOs through affecting the placental blood supply (19).
The present retrospective study has several limitations. Although the patients in our study were treated with UDCA, except for those who delivered before ICP diagnosis, the lack of data regarding the specific time and dose of UDCA use limited our ability to analyze the effect of UDCA on total bile acids levels. A previous study indicated that UDCA treatment appeared to improve the prognosis of both women with ICP and the fetus (20). However, Tetsuya Kawakita, et al. concluded that UDCA was not associated with a reduction in the risk of AFOs (9). Additionally, the number of patients with AFOs was small, which could have been a confounding factor. Since the study presented here was retrospective, we could not evaluate any rare outcomes such as intracranial hemorrhage, and sepsis.
Conclusion
The present retrospective study demonstrates a significantly increased incidence of CS, including planned and unplanned, in women with severe ICP. Severe ICP and preeclampsia were associated with AFOs, suggesting that further prospective studies are warranted to identify the proper treatment to improve fetal outcome.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee of Changzhou Maternal and Child Health Care Hospital. Written informed consent to participate in this study was provided by the patients/participants.
Author contributions
CK, FM, and ZZ contributed equally to this work. CK were responsible for the conception and design of the study. FM and ZZ were responsible for acquisition of data. ZZ performed the formal analysis. FM, ZZ, and CK drafted the manuscript. CK revised and commented the draft, and all authors read and approved the final version of the manuscript.
Funding
The present research was supported by the National Natural Science Foundation of China (NO.81701516), Young Talents Program of Changzhou Commission of Health (CZQM2020098), and Applied Basic Research Program of Science and Technology Commission Foundation of Changzhou (CJ20210135).
Acknowledgments
The authors acknowledge all the women who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Song X, Vasilenko A, Chen Y, Valanejda L, Verma R, Yan B, et al. Transcriptional dynamics of bile salt export pump during pregnancy: mechanisms and implications in intrahepatic cholestasis of pregnancy. Hepatology. (2014) 60:1993–2007. doi: 10.1002/hep.27171
2. Wang F, He Y, Yao N, Ruan L, Tian Z. High levels of serum superoxide dismutase as a biomarker of intrahepatic cholestasis of pregnancy in patients with viral hepatitis B. BMC Pregnancy Childbirth. (2022) 22:444. doi: 10.1186/s12884-022-04776-y
3. Jin J, Pan SL, Huang LP, Yu YH, Zhong M, Zhang GW. Risk factors for adverse fetal outcomes among women with early- versus late-onset intrahepatic cholestasis of pregnancy. Int J Gynaecol Obstet. (2015) 128:236–40. doi: 10.1016/j.ijgo.2014.09.013
4. Jurate K, Rimantas Z, Jolanta S, Vladas G, Limas K. Sensitivity and specificity of biochemical tests for diagnosis of intrahepatic cholestasis of pregnancy. Ann Hepatol. (2017) 16:569–73. doi: 10.5604/01.3001.0010.0294
5. Raz Y, Lavie A, Vered Y, Goldiner I, Skornick-Rapaport A, Landsberg Asher Y, et al. Severe intrahepatic cholestasis of pregnancy is a risk factor for preeclampsia in singleton and twin pregnancies. Am J Obstet Gynecol. (2015) 213:395.e1–8. doi: 10.1016/j.ajog.2015.05.011
6. Liu C, Gao J, Liu J, Wang X, He J, Sun J, et al. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes and preeclampsia. Ann Transl Med. (2020) 8:1574. doi: 10.21037/atm-20-4879
7. Pata O, Vardarelı E, Ozcan A, Serteser M, Unsal I, Saruç M, et al. Intrahepatic cholestasis of pregnancy: correlation of preterm delivery with bile acids. Turk J Gastroenterol. (2011) 22:602–5. doi: 10.4318/tjg.2011.0427
8. Estiú MC, Frailuna MA, Otero C, Dericco M, Williamson C, Marin J, et al. Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid. PLoS One. (2017) 12:e0176504. doi: 10.1371/journal.pone.0176504
9. Kawakita T, Parikh LI, Ramsey PS, Huang CC, Zeymo A, Fernandez M, et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. (2015) 213:570.e1–8. doi: 10.1016/j.ajog.2015.06.021
10. Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. (2004) 40:467–74. doi: 10.1002/hep.20336
11. Puljic A, Kim E, Page J, Esakoff T, Shaffer B, LaCoursiere DY, et al. The risk of infant and fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol. (2015) 212:667.e1–5. doi: 10.1016/j.ajog.2015.02.012
12. Wikström Shemer EA, Thorsell M, Marschall HU, Kaijser M. Risks of emergency cesarean section and fetal asphyxia after induction of labor in intrahepatic cholestasis of pregnancy: a hospital-based retrospective cohort study. Sex Reprod Healthc. (2013) 4:17–22. doi: 10.1016/j.srhc.2012.11.005
13. Rook M, Vargas J, Caughey A, Bacchetti P, Rosenthal P, Bull L. Fetal outcomes in pregnancies complicated by intrahepatic cholestasis of pregnancy in a northern California cohort. PLoS One. (2012) 7:e28343. doi: 10.1371/journal.pone.0028343
14. Lo JO, Shaffer BL, Allen AJ, Little SE, Cheng YW, Caughey AB. Intrahepatic cholestasis of pregnancy and timing of delivery. J Matern Fetal Neonatal Med. (2015) 28:2254–8. doi: 10.3109/14767058.2014.984605
15. Shan D, Hu Y, Qiu P, Mathew BS, Chen Y, Li S, et al. Intrahepatic cholestasis of pregnancy in women with twin pregnancy. Twin Res Hum Genet. (2016) 19:697–707. doi: 10.1017/thg.2016.74
16. Usta A, Turan G, Sancakli Usta C, Avci E, Adali E. Placental fractalkine immunoreactivity in preeclampsia and its correlation with histopathological changes in the placenta and adverse pregnancy outcomes. J Matern Fetal Neonatal Med. (2020) 33:806–15. doi: 10.1080/14767058.2018.1505854
17. Greiner KS, Speranza RJ, Rincón M, Beeraka SS, Burwick RM. Association between insurance type and pregnancy outcomes in women diagnosed with hypertensive disorders of pregnancy. J Matern Fetal Neonatal Med. (2020) 33:1427–33. doi: 10.1080/14767058.2018.1519544
18. Wang J, Lun W, Shi W. Effects of elevated bile acid levels on fetal myocardium in intrahepatic cholestasis of pregnancy, a retrospective study from a neonatal perspective. Clin Res Hepatol Gastroenterol. (2022) 46:102013. doi: 10.1016/j.clinre.2022.102013
19. Licini C, Avellini C, Picchiassi E, Mensà E, Fantone S, Ramini D, et al. Pre-eclampsia predictive ability of maternal miR-125b: a clinical and experimental study. Transl Res. (2021) 228:13–27. doi: 10.1016/j.trsl.2020.07.011
Keywords: intrahepatic cholestasis of pregnancy, adverse fetal outcomes, cesarean section, preeclampsia, serum bile acids
Citation: Kong C, Zhu Z and Mei F (2023) Risk factors associated with cesarean section and adverse fetal outcomes in intrahepatic cholestasis of pregnancy. Front. Pediatr. 11:1136244. doi: 10.3389/fped.2023.1136244
Received: 2 January 2023; Accepted: 19 June 2023;
Published: 30 June 2023.
Edited by:
Suksham Jain, Government Medical College and Hospital, IndiaReviewed by:
Rachana Singh, Tufts University, United StatesJessica Saben, University of Colorado Anschutz Medical Campus, United States
© 2023 Kong, Zhu and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengcai Kong a29uZ2NoZW5nY2FpMTJAMTYzLmNvbQ==
Abbreviations ICP, intrahepatic cholestasis of pregnancy; AFOs, adverse fetal outcomes; CS, cesarean section; NICU, neonatal intensive care unit; pBMI, pregestational body mass index; GDM, gestational diabetes mellitus; MSAF, meconium staining of the amniotic fluid; UDCA, ursodeoxycholic acid; PROM, premature rupture of membranes; FHT, fetal heart tracings.
 Chengcai Kong
Chengcai Kong Zonghao Zhu2
Zonghao Zhu2