
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 11 April 2023
Sec. Pediatric Surgery
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1131629
This article is part of the Research Topic Pediatric Transplantation View all 4 articles
 Min Xu1,2
Min Xu1,2 Chong Dong1,2
Chong Dong1,2 Chao Sun1,2
Chao Sun1,2 Kai Wang1,2
Kai Wang1,2 Wei Zhang1,2
Wei Zhang1,2 Hong Qin1,2
Hong Qin1,2 Chao Han1,2
Chao Han1,2 Yang Yang1,2
Yang Yang1,2 Fubo Zhang1,2
Fubo Zhang1,2 Zhen Wang1,2
Zhen Wang1,2 Weiping Zheng1,2
Weiping Zheng1,2 Xinzhe Wei1,2
Xinzhe Wei1,2 Wei Gao1,2*
Wei Gao1,2* Zhongyang Shen2,3
Zhongyang Shen2,3
Background: Donor shortage is an important limitation of liver transplantation (LT). Split liver transplantation (SLT) may increase the sources of donors and reduce the problem of organ shortage. However, there are no standard criteria of the selection of SLT donor, especially regarding the donor age.
Methods: We retrospectively analyzed the clinical data of children who received initial SLT between January 2015 and December 2021. Based on the age of donors, the patients were divided into groups A (1–10 years old; n = 26), B (10–45 years old; n = 87), and C (45–55 years old; n = 27). The short-term (<1 year after SLT) outcomes of the recipients were analyzed.
Results: A total of 140 patients received SLT from 122 donors. The 1-, 3- and 12-month patient survival rates in group A were 100.0%, and the graft survival rates were 92.3%. The 1-, 3- and 12-month survival rates of patient and graft in group B were 97.7%, 96.6%, and 95.0%, respectively, and in group C were 85.2%, 85.2%, and 81.1%, respectively. The patient survival rate was significantly lower in group C than in groups A and B (p = 0.0082). There was no significant difference in graft survival between the three groups (p = 0.0545).
Conclusions: Similar results were obtained for pediatric SLT with donors <10 years old and 10–45 years old. Pediatric SLT can be performed with older donors (45–55 years) after strict donor selection and selection of appropriate recipients.
Pediatric patients with end-stage liver disease often require pediatric liver transplantation (LT) (1, 2). However, donor shortage is an important problem that restricts the widespread use of pediatric LT (3, 4). The use of living donor liver transplantation (LDLT) and split liver transplantation (SLT) may increase the sources of donors and reduce the problem of organ shortage (5). In particular, SLT allows a greater number of recipients to benefit from a limited number of donors. Under appropriate conditions, a single liver graft can be shared by two recipients (6, 7), which significantly increases the availability of donors and provides more possibilities for patients on the waiting list.
Many studies have reported that SLT can achieve similar survival results as LDLT and whole liver transplantation (WLT) (1, 8–10). Thus, SLT is safe and effective in pediatric recipients when appropriate donors and recipients are selected. However, there are no global standard criteria of the selection of SLT donor, especially regarding the donor age. For example, Italian guidelines suggest that donors should have an age of 10–50 years, stable hemodynamics, intensive care unit (ICU) stay ≤5 days, transaminase level ≤3 times of normal, and no steatosis on ultrasound scan (11). In contrast, British and Korean guidelines require donors to be younger than 40 years (12, 13). In our country, the consensus proposed that the donor age of SLT should be less than 50 years, stable hemodynamics, intensive care unit (ICU) stay ≤5 days, transaminase level ≤3 times of normal, and total bilirubin level ≤2 times of normal (14). Although the criteria vary between centers, older donors are vulnerable to ischemia-reperfusion injury (15) and graft loss (16, 17). Hence, it is necessary to determine the appropriate age of donors for spitting.
The purpose of this study was to compare the short-term (within 1 year after LT) clinical efficacy and prognosis of different age donors in children with SLT.
We retrospectively analyzed the clinical data of pediatric patients who received initial SLT at the Department of Pediatric Transplantation, Tianjin First Central Hospital, between January 2015 and December 2021. The patients were followed up until June 30, 2022. A total of 1,343 recipients underwent 1,360 LTs, among which 925, 258, and 24 cases of LDLT, WLT, and reduced-size LT, respectively, were excluded. We also excluded 10 cases of domino LTs. Among the remaining 143 cases of SLT, 3 cases of re-SLT were excluded. Finally, 140 cases were included in the analysis. We divided the patients into three groups based on the donor age: A (1–10 years), B (10–45 years), and C (45–55 years) (Figure 1). This study was approved by the institutional review board of Tianjin First Central Hospital (approval number: 2023DZX09).
Demographic data of the recipients and donors were compared between the three groups. The short-term (<1 year after SLT) outcomes and complications of the recipients were compared between the groups and the risk factors of graft loss were analyzed.
In situ splitting refers to splitting the liver parenchyma, followed by reperfusion and cold preservation (3). In comparison, in ex situ splitting, perfusion is performed, followed by splitting on the back table. Biopsy were obtained routinely after perfusion with University of Wisconsin (UW) solution or histidine-tryptophan-ketoglutarate (HTK) solution. Macrovesicular steatosis refers to steatosis in which large lipid droplets appear within hepatocytes. Macrovesicular steatosis was graded pathologically: mild <30%, moderate 30%–60%, severe >60% (18).
For younger donors (donor age: 1–10 years), the left lateral segment (LLS) will receive the allocation of the celiac axis or left hepatic artery. Whereas for the portal vein (PV), the trunk was allocated to the extended right lobe (ERL). Similarly, the trunk of the bile duct was allocated to the ERL.
Piggyback LT was performed in all recipients. The portal vein was anastomosed end-to-end with continuous or interrupted sutures. The hepatic artery was sutured with 8-0 or 9-0 non-absorbable sutures under the microscope. Roux-en-Y choledochojejunostomy was performed in all cases.
The EAD definition proposed by Olthoff et al. (18) was used, which was based on the occurrence of one or more of the following indicators: (1) Total bilirubin (TBIL) ≥10 mg/dl (171 µmol/L) on postoperative day 7 (POD 7); (2) international normalized ratio ≥1.6 on POD7 without anticoagulant use; and (3) alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels >2,000 U/ml within the first seven postoperative days.
We expressed categorical variables as frequencies or percentages, and performed χ2 test or Fisher's exact probability tests, where appropriate. Shapiro–Wilk test was used to determine whether the measurement variables conformed to a normal distribution, and if so, they were expressed as means ± standard deviation (x ± SD) and analyzed by Student's t-test. If the data were not parametric, they were presented as medians (interquartile range, IQR) and compared using the Kruskal–Wallis test. The Kaplan–Meier method was used for survival analysis, and the log-rank test was used to compare the differences in survival distributions between the groups. The multivariable analysis was used to determine the effect of patient and donor variables on graft survival using the Cox's model and Enter method. p < 0.05 was considered statistically significant. We analyzed the data using SPSS 20.0 software (IBM Corp., Armonk, NY, USA).
A total of 140 patients received LTs from 122 donors and were divided into three groups: 26 (18.6%), 87 (62.1%), and 27 (19.3%) patients in groups A, B, and C, respectively. The median age of the recipients at the time of transplantation was 8.5 (6.5–30.3) months and the median body weight was 7.6 (6.7–12.0) kg. Cholestatic diseases (116/140, 83.9%) were the most common disease that necessitated LT. The main cause of death of donors in group C was stroke (70.4%), while that in groups A and B was brain trauma (46.7% and 55.0%, respectively). Tables 1, 2 compare the basic characteristics of recipients and donors between the three groups.
The left lateral segment (LLS) was the main type of graft used in groups B and C. In contrast, the LLS and extended right lobe (ERL) accounted for half of the grafts each in group A. Meanwhile, in terms of surgical techniques, the grafts in group A were mainly split ex situ, while almost all the grafts in groups B and C were split in situ (p = 0.000). In addition, the organ preservation solution used in groups A and C was mainly the University of Wisconsin (UW) solution, while an equal number of grafts in group B used UW solution and histidine-tryptophan-ketoglutarate (HTK) solution (p = 0.003). The median cold ischemia time (CIT) in group A was 520.0 min, which was significantly longer than that in groups B and C (153.0 and 209.0 min, respectively; p = 0.000; Table 3). The proportion of steatosis in grafts in group C was 11.1%, which was higher than that in the other two groups; however, there was no significant difference between the three groups (p = 0.517). There was no significant difference in the graft-to-recipient weight ratio (GRWR) among the three groups.
The ALT and AST of the three groups were significantly increased on the first day after LT. The median AST peak level in groups A and C were 1,280.0 (585.9–2,811.3) and 1,490.8 (680.0–2,987.2) U/L, which was not significantly different from that in group B [981.4 (503.7–1,707.0) U/L; p = 0.121]. There was no significant difference in the peak ALT level among the three groups [p = 0.123; 582.6 (267.7–1093.0), 523.0 (313.3–948.2), and 709.5 (471.8–1582.8) in groups A, B, and C, respectively]. The TBIL was not significantly different between the three groups on days 1, 3, and 14 after LT. However, on day 7 after LT, the TBIL level was higher in group C than that in the other two groups (p = 0.035). As shown in Figure 2, the ALT, AST, and TBIL levels in the three groups decreased to normal 14 days after the LT, with no significant difference among the groups (p = 0.336, 0.333, and 0.083, respectively).
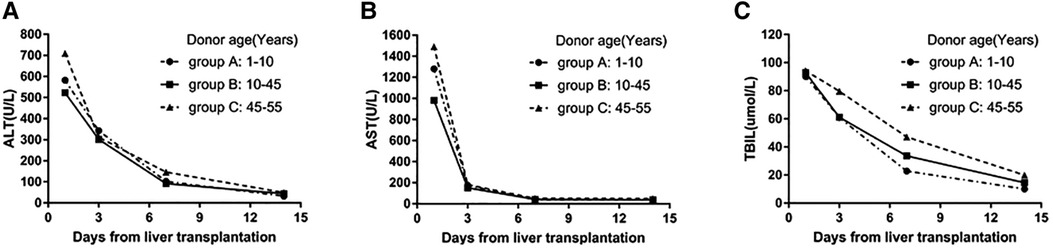
Figure 2. (A) Change in the median ALT values on days 1, 3, 7, and 14 after LT among the three groups. (B) Change in median AST levels on days 1, 3, 7, and 14 after LT in the three groups. (C) The change in median TBIL values on days 1, 3, 7, and 14 after LT in the three groups.
The incidence of vascular complications was 16.4% (23/140), and that of biliary complications was 8.6% (12/140). The incidence of hepatic artery thrombosis (HAT) in group A was 7.7%, which was higher than that in the other two groups, but there were no significant differences among the three groups (p = 0.116). The incidence of hepatic vein stricture and biliary leakage was highest in group B (10.3% and 8.0%, respectively), but was not significantly different compared with the other two groups (p = 0.483 and 0.404, respectively). Overall, the incidence of EAD was 28.9% (39/140), with no significant difference among the three groups (p = 0.111). The complications in each group are shown in Table 4.
A total of 3 recipients underwent retransplantation, including 2 in group A and 1 in group C (Table 5). The main causes of retransplantation were HAT and portal vein thrombosis (PVT). In group A, one patient had graft loss due to HAT and PVT, and another had HAT. Both patients underwent retransplantation and survived. In group C, one recipient underwent retransplantation due to PVT and graft dysfunction, and finally died of graft failure 1 month after the operation.
In this study, there were 9 cases of recipient death: none in group A, 4 (4/87, 4.6%) in group B, and 5 (5/27, 18.5%) in group C (Table 6). The causes of death included graft failure, accident, chronic rejection, interstitial pneumonia, and sepsis. Four patients died within 30 days after LT, including 1 case of graft failure, 1 case of interstitial pneumonia, and 2 cases of sepsis.
As shown in Figure 3A, the 1-, 3- and 12-month overall patient survival rates were 95.7%, 95.0%, and 93.5%, respectively; and the 1-, 3- and 12-month overall graft survival rates were 94.3%, 93.6%, and 92.0%, respectively. The 1-, 3- and 12-month patient survival rates in group A were all 100.0%, and graft survival rates were all 92.3%. The 1-, 3- and 12-month patient survival rates in group B were 97.7%, 96.6% and 95.2%, respectively, and the graft survival rates were similar. And the The 1-, 3- and 12-month survival rates for both patients and grafts in group C were 85.2%, 85.2%, and 81.3%, respectively. The patient survival rate was significantly lower in group C than in groups A and B (p = 0.0094). There was no significant difference in graft survival between the three groups (p = 0.0592).
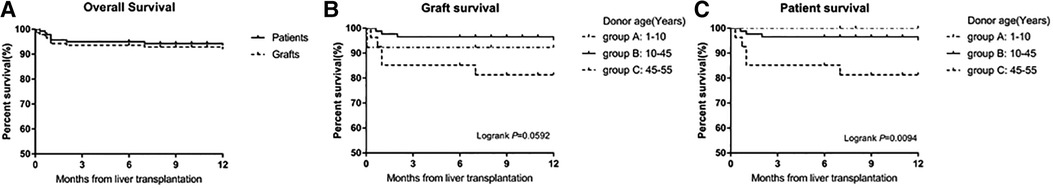
Figure 3. (A) Survival of patients and grafts for 140 children who underwent liver transplantation. (B) Survival of grafts in the three groups. There were no significant differences in the graft survival rates among the three groups (p = 0.0592). (C) Survival rates of the recipients in the three groups. The patient survival rates were significantly lower in group C than in groups A and B (p = 0.0094).
In the univariate analysis, increased blood loss significantly increased the risk of graft loss (p = 0.031; Table 7). However, PVT was a significant factor affecting graft survival (p = 0.015).
Factors with p-value <0.2 in the univariate analysis were included in the multivariate analysis. Table 7 shows the risk factors affecting graft survival in the Cox multivariable analysis. The results showed that donor age >45 years [hazard ratio (HR) = 4.429, p = 0.040] and postoperative PVT (HR = 20.555, p = 0.028) were independent risk factors of graft loss.
SLT significantly alleviates the problem of organ shortage (6). The influence of donor age on the recipient outcomes is unclear, and there are no clear guidelines regarding donor age (19). Some studies have shown that donors aged 60–80 years can safely donate their liver with good results; however, these studies were conducted in WLT (20, 21). There is no unified standard for splitting, especially with regard to the donor age. Donor age significantly affects the survival and prognosis of grafts (12, 17), but there is no clear standard for splitting (12). In this study, we compared the early clinical outcomes and prognosis of SLT with donors of different ages to evaluate the splitability according to the different ages of donors.
In this study, the peak ALT and AST levels were significantly increased in groups A and C after LT, although there was no significant difference compared with the level in group B. This may be related to the significantly prolonged CIT in group A, which results in hepatic ischemic injury. However, although the CIT was shorter in group C than in group A, the vulnerability to ischemic injury caused by the older donors in group C may explain the significantly increased AST level (22). In addition, the transaminase levels in each group decreased to the normal value after 2 weeks, with no significant difference between the groups. Furthermore, there was no difference in the recovery of liver function between the three groups. However, the decrease in TBIL in group C was slower than that in groups A and B. The TBIL level on day 7 after the operation was significantly higher in group C than that in the other groups, which may be due to the delayed recovery of liver function in cases of older donors.
In terms of postoperative complications, group A had the highest incidence of HAT within 1 year after LT, although there was no statistical difference when compared with the other two groups. The two recipients who developed HAT in group A were small body weight recipients (5.3 kg and 5 kg). The thrombosis in these two patients with small body weight may be caused by small vessel diameter or vessel mismatch (23–26). Therefore, anastomosis of the hepatic artery must be performed carefully, preferably with use of a microscope. Some studies have shown that SLT can increase the incidence of biliary complications (27, 28). However, in this study, the incidence of biliary complications (including bile leakage and biliary stricture) within 1 year after operation was 8.6% (12/140), which was lower than the 13.6% reported by the Society of Pediatric Liver Transplantation (29). In the present study, the incidence of EAD was highest in group C and lowest in group B, but there was no significant difference between the three groups. In a previous study, Olthoff et al. (18) concluded that donor age older than 45 years significantly increased the risk of developing EAD (RR = 1.94, 95% CI: 1.21, 3.92), and they also confirmed that EAD increased the risk of graft loss (RR = 7.4, 95% CI: 3.4, 16.3). Unlike the results of the aforementioned study, in the present study, the EAD was not a risk factor for graft survival. Furthermore, other research has suggested that donor age, allograft steatosis, donor liver mass, and DCD (donation after cardiac death) status are associated with the development of EAD (30).
The 1-year survival rate of the recipients in group A was 100%. Moreover, even though the median CIT was nearly 9 h in group A, these excellent results were still achieved, and the 1-year graft survival rate was over 90%. Although there is still some controversy surrounding SLT using younger donors (<10 years), our study shows good results with this subset of donors. It follows that even SLT using younger age donors can lead to good outcomes with use of improved surgical techniques as well as refined postoperative care. These findings differ from studies that show that prolonged CIT may significantly increase the risk of graft loss (12, 27, 31, 32). Recent data from Europe show that increasing the CIT from 6 to 10 h corresponds to an HR of 1.33 (12). Similarly, Lozanovski et al. reported that the influence of CIT shows a difference when comparing between ischemia time <10 h and ≥10 h (32). In the Cox univariate analysis in the present study, CIT was not the main factor affecting the survival of recipients. However, our previous studies have shown that prolonged CIT can lead to poor prognosis in recipients of WLT. In univariate and multivariate analysis, graft type, hepatic artery thrombosis, portal vein stenosis, hepatic vein stenosis and biliary complications were not the risk factors affecting the short-term survival of the graft. Actually, however, it is well known that HAT can negatively affect graft survival. That may be explained by the small sample size of this study. In contrast, in the multivariate analysis of the present study, donor age >45 years (HR = 4.429) and postoperative PVT (HR = 25.550) were the main risks of graft loss.
Previously, we have reported that donors younger than 7 years old have achieved good results in SLT, which shows that carefully selected pediatric donors can be safely and effectively used in SLT in children (33). In this study, we included recipients who received donors aged 1–55 years, of which 26 recipients received pediatric donors aged 1–10 years. The 26 recipients received the LLS and ERL of 15 donors, and good results were achieved. Nevertheless, pediatric donors still have many risk factors, such as a high incidence of HAT, which necessitates paying attention to protecting the hepatic artery of the donor and recipient in the process of splitting the liver, as well as closely monitoring the blood flow of the hepatic artery after surgery. In addition, for younger donors, according to the previous experience of our center, the weight of the donor selected for the split should be >15 kg, which can ensure the recipient has a good prognosis.
The donors we previously selected for SLT were younger than 45 years old. Recently, however, due to the shortage of donors, we began to expand the age selection criteria of donors. In this study, the oldest donor we selected was 55 years old, which is higher than the standard of most centers. In the present study, the survival rate of donors older than 45 years was the lowest; however, this rate does not impact the use of this age group of donors for splitting. First, the number of these donors is small and vulnerable to interference. Second, we performed SLT in 14 cases with donors older than 45 years in 2021, and achieved good short-term survival (no graft loss or recipient death) and few postoperative complications, indicating that older donors may not be the limiting factor in splitting. However, the follow-up time in this study was 1 year; hence, it does not reflect the long-term outcome of the recipients. According to the experience of our center, donor candidates older than 45 years are suitable for splitting, provided there is reasonable selection of donors and recipients; that is, for example, the donor has no hypotension before operation, no or little use of pressor drugs, ALT and AST are less than three times the upper limit of normal, ICU stay time is less than 5 days, and there is no evidence of systemic infection and no moderate-to-severe fatty lesions. Severely ill recipients should not be selected. Furthermore, the choice of in situ splitting can considerably reduce the damage to vessels and bile ducts and bleeding after reperfusion. Careful postoperative care and regular monitoring are essential. These measures may reduce the risk of graft loss and reduce the incidence of complications.
However, this study has several limitations. First, this is a single center retrospective study with a limited sample size. Second, we only reported the short-term outcomes for the recipients and lacked the results of long-term follow-up. Finally, we reported that 140 recipients received 122 donors due to the fact that there was a part of the graft that was allocated to other centers. And adult recipients were not included in the study. Therefore, no information was available for these recipients, which also leads to mismatches in the number of donors and recipients.
In this study, the application of SLT in pediatrics with donor age <10 years resulted in similar satisfactory outcomes compared with those of donor age 10–45 years. For the 45–55-year-old donor age group, although patient survival was reduced, both the patient and graft 1-year survival rate exceeded 80%. Under the conditions that donors are selected according to strict criteria and suitable recipients are selected, this subset of donors can also be applied to pediatric SLT. However, the results of long-term follow-up require further study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Tianjin First Central Hospital Medical Ethics Committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
WG: participated in research design and article revision. MX: was responsible for the data analysis and the writing of the paper. XZW: participated in the data analysis. All authors contributed to the article and approved the submitted version.
This research was funded by National natural science foundation of China (grant no. 82170672) and Tianjin Key Medical Discipline (Specialty) Construction Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LT, liver transplantation; LDLT, living donor liver transplantation; SLT, split liver transplantation; WLT, whole liver transplantation; ERL, extended right lobe; LLS, left lateral segment; RL, right lobe; LL, left lobe; ICU, intensive care unit; BMI, body mass index; PELD, pediatric end-stage liver disease; GRWR, graft-to-recipient weight ratio; RBC, red blood cell; CIT, cold ischemia time; INR, international normalized ratio; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PNF, primary nonfunction; EAD, early allograft dysfunction; UW, university of wisconsin; HTK, histidine-tryptophan-ketoglutarate; HAT, hepatic artery thrombosis; PVT, portal vein thrombosis.
1. Mogul DB, Luo X, Bowring MG, Chow EK, Massie AB, Schwarz KB, et al. Fifteen-year trends in pediatric liver transplants: split, whole deceased, and living donor grafts. J Pediatr. (2018) 196:148–53. doi: 10.1016/j.jpeds.2017.11.015
2. Jain AK, Anand R, Lerret S, Yanni G, Chen JY, Mohammad S, et al. Outcomes following liver transplantation in young infants: data from the SPLIT registry. Am J Transplant. (2021) 21:1113–27. doi: 10.1111/ajt.16236
3. Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: current developments. World J Gastroenterol. (2018) 24:5312–21. doi: 10.3748/wjg.v24.i47.5312
4. Perkins JD, Dick AA, Healey PJ, Montenovo MI, Biggins SW, Sibulesky L, et al. New evidence supporting increased use of split liver transplantation. Transplantation. (2020) 104:299–307. doi: 10.1097/TP.0000000000002853
5. Hsu EK, Mazariegos GV. Global lessons in graft type and pediatric liver allocation: a path toward improving outcomes and eliminating wait-list mortality. Liver Transpl. (2017) 23:86–95. doi: 10.1002/lt.24646
6. Lozanovski VJ, Unterrainer C, Döhler B, Süsal C, Mehrabi A. Outcome of extended right lobe liver transplantations. Liver Transplant. (2021) 28(5):807–18. doi: 10.1002/lt.26374
7. Lozanovski VJ, Probst P, Ramouz A, Arefidoust A, Ghamarnejad O, Aminizadeh E, et al. Considering extended right lobe grafts as major extended donor criteria in liver transplantation is justified. Transpl Int. (2021) 34:622–39. doi: 10.1111/tri.13824
8. Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. (2009) 208:682–9. 689–91. doi: 10.1016/j.jamcollsurg.2009.01.023
9. Doyle MB, Maynard E, Lin Y, Vachharajani N, Shenoy S, Anderson C, et al. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. (2013) 217:102–12. 113–4. doi: 10.1016/j.jamcollsurg.2013.03.003
10. Cauley RP, Vakili K, Fullington N, Potanos K, Graham DA, Finkelstein JA, et al. Deceased-donor split-liver transplantation in adult recipients: is the learning curve over? J Am Coll Surg. (2013) 217:672–84. doi: 10.1016/j.jamcollsurg.2013.06.005
11. Angelico R, Trapani S, Spada M, Colledan M, de Ville De Goyet J, Salizzoni M, et al. A national mandatory-split liver policy: a report from the Italian experience. Am J Transplant. (2019) 19:2029–43. doi: 10.1111/ajt.15300
12. Angelico R, Nardi A, Adam R, Nadalin S, Polak WG, Karam V, et al. Outcomes of left split graft transplantation in Europe: report from the European liver transplant registry. Transpl Int. (2018) 31:739–50. doi: 10.1111/tri.13147
13. Park GC, Hwang S, Song GW, Jung DH, Ha TY, Ahn CS, et al. Prognosis of split liver transplantation compared with whole liver transplantation in adult patients: single-center results under the Korean MELD score-based allocation policy. J Korean Med Sci. (2020) 35:e304. doi: 10.3346/jkms.2020.35.e304
14. Group of Transplantation, Chinese Society of Surgery, Chinese Medical Association. Expert consensus on split liver transplantation. Chin J Hepat Surg. (2020) 9(5):429–34. doi: 10.3877/cma.j.issn.2095-3232.2020.05.008
15. Lau NS, Jacques A, McCaughan G, Crawford M, Liu K, Pulitano C. Addressing the challenges of split liver transplantation through technical advances. A systematic review. Transplant Rev. (2021) 35:100627. doi: 10.1016/j.trre.2021.100627
16. Houben P, Döhler B, Weiß KH, Mieth M, Mehrabi A, Süsal C. Differential influence of donor age depending on the indication for liver transplantation—a collaborative transplant study report. Transplantation. (2020) 104:779–87. doi: 10.1097/TP.0000000000002970
17. Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. (2019) 70:745–58. doi: 10.1016/j.jhep.2018.12.009
18. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. (2010) 16:943–9. doi: 10.1002/lt.22091
19. Pratschke S, Bender A, Boesch F, Andrassy J, van Rosmalen M, Samuel U, et al. Association between donor age and risk of graft failure after liver transplantation: an analysis of the eurotransplant database. Transpl Int. (2019) 32:270–9. doi: 10.1111/tri.13357
20. Serrano MT, Garcia-Gil A, Arenas J, Ber Y, Cortes L, Valiente C, et al. Outcome of liver transplantation using donors older than 60°year of age. Clin Transplant. (2010) 24:543–9. doi: 10.1111/j.1399-0012.2009.01135.x
21. Chedid MF, Rosen CB, Nyberg SL, Heimbach JK. Excellent long-term patient and graft survival are possible with appropriate use of livers from deceased septuagenarian and octogenarian donors. HPB. (2014) 16:852–8. doi: 10.1111/hpb.12221
22. Feng S, Lai JC. Expanded criteria donors. Clin Liver Dis. (2014) 18:633–49. doi: 10.1016/j.cld.2014.05.005
23. Ma N, Song Z, Dong C, Sun C, Meng XC, Zhang W, et al. Risk factors of hepatic artery thrombosis in pediatric deceased donor liver transplantation. Pediatr Surg Int. (2019) 35:853–9. doi: 10.1007/s00383-019-04500-6
24. Channaoui A, Tambucci R, Pire A, de Magnée C, Sokal E, Smets F, et al. Management and outcome of hepatic artery thrombosis after pediatric liver transplantation. Pediatr Transplant. (2020) n/a:e13938. doi: 10.1111/petr.13938
25. Lisman T, Porte RJ. Hepatic artery thrombosis after liver transplantation: more than just a surgical complication? Transpl Int. (2009) 22:162–4. doi: 10.1111/j.1432-2277.2008.00762.x
26. Oh CK, Pelletier SJ, Sawyer RG, Dacus AR, McCullough CS, Pruett TL, et al. Uni- and multi-variate analysis of risk factors for early and late hepatic artery thrombosis after liver transplantation. Transplantation. (2001) 71:767–72. doi: 10.1097/00007890-200103270-00014
27. Sasaki K, Firl DJ, McVey JC, Schold JD, Iuppa G, Diago U, et al. Elevated risk of split-liver grafts in adult liver transplantation: statistical artifact or nature of the beast? Liver Transplant. (2019) 25:741–51. doi: 10.1002/lt.25409
28. Battula NR, Platto M, Anbarasan R, Perera MT, Ong E, Roll GR, et al. Intention to split policy: a successful strategy in a combined pediatric and adult liver transplant center. Ann Surg. (2017) 265:1009–15. doi: 10.1097/SLA.0000000000001816
29. Elisofon SA, Magee JC, Ng VL, Horslen SP, Fioravanti V, Economides J, et al. Society of pediatric liver transplantation: current registry status 2011-2018. Pediatr Transplant. (2020) 24:e13605. doi: 10.1111/petr.13605
30. Lee DD, Croome KP, Shalev JA, Musto KR, Sharma M, Keaveny AP, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol. (2016) 15:53–60. doi: 10.5604/16652681.1184212
31. Lee KW, Cameron AM, Maley WR, Segev DL, Montgomery RA. Factors affecting graft survival after adult/child split-liver transplantation: analysis of the UNOS/OPTN data base. Am J Transplant. (2008) 8:1186–96. doi: 10.1111/j.1600-6143.2008.02211.x
32. Lozanovski VJ, Döhler B, Weiss KH, Mehrabi A, Süsal C. The differential influence of cold ischemia time on outcome after liver transplantation for different indications-who is at risk? A collaborative transplant study report. Front Immunol. (2020) 11:892. doi: 10.3389/fimmu.2020.00892
Keywords: pediatric split liver transplantation, donor age, short-term outcomes, complications, graft survival rates
Citation: Xu M, Dong C, Sun C, Wang K, Zhang W, Qin H, Han C, Yang Y, Zhang F, Wang Z, Zheng W, Wei X, Gao W and Shen Z (2023) Impact of donor age on short-term outcomes after pediatric split liver transplantation. Front. Pediatr. 11:1131629. doi: 10.3389/fped.2023.1131629
Received: 25 December 2022; Accepted: 28 March 2023;
Published: 11 April 2023.
Edited by:
Juan A. Tovar, University Hospital La Paz, SpainReviewed by:
Isamu Saeki, Hiroshima University, Japan© 2023 XU, Dong, Sun, Wang, Zhang, Qin, Han, Yang, Zhang, Wang, Zheng, Wei, Gao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Gao Z2Fvd2VpX3RqZmNoQDE2My5jb20=
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.