
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 15 March 2023
Sec. Pediatric Surgery
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1131190
An otherwise healthy 4-month-old girl presented to the community health service center because her abdomen was distended. Over the next 2 months, the girl's abdomen gradually became more distended. Her examination was notable for abdominal distention with a large, mobile, non-tender abdominal mass. Abdominal ultrasound images and subsequently obtained CT images showed a large, circumscribed cystic and solid mass. This led to the presumptive diagnosis of teratoma of the mesentery. The mass was completely resected during a laparotomy. The pathology, along with the surgical findings and imaging, led to the final diagnosis.
Teratomas are common tumors in infancy, and they display a mixture of tissues from at least two of the three germ layers. The most common sites are the midline and gonads of the body, such as the sacrocaudal area (57%), gonadal area (29%), mediastinum (7%), and retroperitoneum (4%) (1). Mesenteric teratomas are very rare, occurring in approximately 1/1,000,000 live births (2). Only a few cases have been published and described in contemporary literature (3–13). While a mesenteric cyst is a common mass of mesenteric origin and has a morbidity rate of 1 in every 20,000 pediatric hospital admissions, the diagnosis of mesenteric teratoma can be easily missed at the initial stage. (14). An asymptomatically increasing abdominal girth or a palpable mass is usually accompanied by other symptoms such as vomiting and constipation (7). Here, we describe a case of a patient with mesenteric teratoma.
A 6-month-old girl was admitted to our hospital because of an abdominal mass. Approximately 2 months previously, the patient's parents had noticed that her abdomen had gradually become distended, but there were no other complaints. She was initially taken to the community health service center. On examination at the health service center, the patient appeared to be in normal condition. The patient's weight was 6.0 kg, the abdomen was mildly distended but soft, and there were normal bowel sounds and no apparent tenderness or mass on palpation. The examination concluded without any further diagnosis or prescribed treatment.
The patient had been born by cesarean section because of preeclampsia after 38 weeks of gestation. She weighed 3.2 kg at birth and was fed breast milk. Supplements were added, and mixed feeding had begun when she was 5 months old. The patient's childhood immunizations were up to date. She had no known medical conditions, had not received any additional medications, and had no known allergies. She lived with her parents and grandparents. There was no family history of cancer.
On examination at the hospital, the patient was placid. Her temperature was 36.6°C, pulse was 128 beats per minute, respiratory rate was 30 breaths per minute, and oxygen saturation was 98% while she was breathing ambient air. The patient's weight was 8.5 kg (89th percentile), her height was 69 cm (93rd percentile), and her head circumference was 42.0 cm (44th percentile). The abdomen was soft and distended with a circumference that measured 50 cm. On palpation, there was a large mobile non-tender abdominal mass that measured approximately 19 cm × 15 cm. There were no other abnormal physical findings. Blood levels of glucose, sodium, potassium, chloride, creatinine, total protein, albumin, globulin, and lactate dehydrogenase were normal, as were the results of coagulation and liver function tests. The other test results are given in Table 1.
An ultrasonography of the abdomen confirmed the presence of a mass in the patient's enterocoelia that measured 15.9 cm × 7.9 cm × 17.1 cm. The mass presented as a mixture of echoes and was well circumscribed; it mainly consisted of fat echo (multiple linear hyperechoic areas inside isoechoic areas) with many cysts of various sizes (the largest one measured 6.2 cm × 3.6 cm × 4.4 cm) and several hyperechoic areas with acoustic shadowing. The large retroperitoneal vessels were squeezed and shifted to the back of the patient, but they were not wrapped, and she did not exhibit any other abnormalities relating to any other organs.
The ultrasonography indicated that the mass in the abdomen of the patient was significant in size and would be difficult to remove. Therefore, we needed to perform a computer tomography (CT) scan to clarify the origin of the mass and identify the surrounding vessels.
The CT scan depicted a huge space-occupying lesion that appeared to be of mixed density, and the mass reached both the liver's lower edge and the pelvic cavity (Figures 1A,B). The lesion had a maximum diameter of 15.0 cm × 13.5 cm × 9.5 cm and showed an evident mass effect containing various densities, such as fluid (CT value −128 HU), fat (CT value 12 HU), soft tissue (CT value 38 HU), and calcification/ossification shadows (CT value 1,220 HU) (Figure 1C). On contrast-enhanced CT, the soft tissue density was found to be enhanced with a CT value of 95 HU, and there was a vascular shadow that could be seen in the lesion. The superior mesenteric artery branches extended inward, while the other large abdominal vessels and branches were compressed (Figure 1D). As the lesion occupied the void of the abdominal cavity, the intra-abdominal pressure increased. Therefore, the internal organs (the intestines and kidneys) that are not entirely fixed tended to be displaced upward, while the relatively fixed organ (the liver) was flattened. On the contrast-enhanced CT scan, the abdominal parenchyma organs were found to be enhanced uniformly, and no lumps or defects were noted.
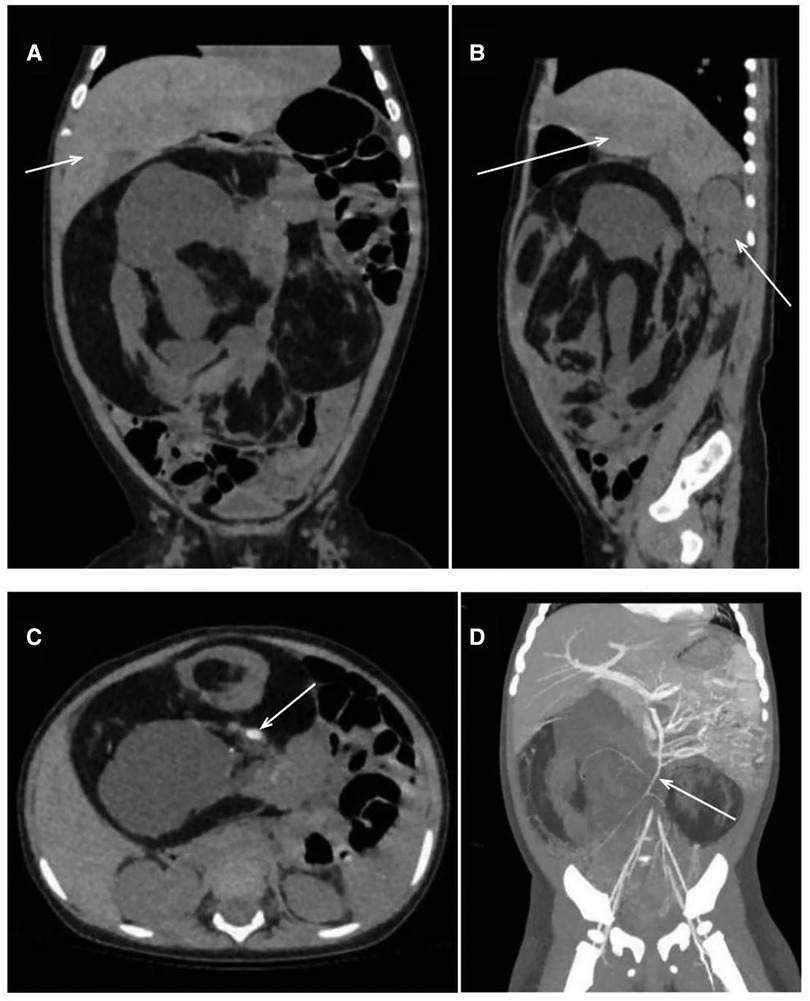
Figure 1. CT obtained at this hospital. CT images (A, B), obtained before the administration of intravenous contrast material, show a multilobulated cystic mass compressing the liver and right kidney (arrows). The CT image (C) shows a mixed density of mass, including a calcified shadow (arrow). The CT image obtained after the injection (D) confirms that the structure was supplied by the superior mesenteric artery (arrow). CT, computed tomography.
Abdominal masses found in children are associated with a broad differential diagnosis because of the various underlying causes; they may originate from one of several possible structures (hepatobiliary system/kidney/pancreas/spleen/adrenal gland/ovarian/mesentery/bowel) (15, 16), and the mass may be found incidentally or with obvious symptoms (abdominal pain/bowel obstruction/fever/early satiety) (17). This 6-month-old patient presented only with a movable abdominal mass.
Usually, the common abdominal masses in this age group are neuroblastoma, mesenteric lymphangioma, retroperitoneal teratoma, and ovarian teratoma. Findings on the initial ultrasonography examination suggested that the mass was well circumscribed and had mixed echogenicity (containing fat echo, plentiful cysts, and hyper echoes). The diagnosis of neuroblastoma was excluded because solid masses would have been present anywhere throughout the sympathetic nervous system, with the adrenal gland being the most common primary site. Using CT scans, the diagnosis was clarified because both cystic and solid components could be seen, including fat and calcified/ossific shadows. The diagnosis of mesenteric lymphangiomas was excluded because such tumors are cystic masses without calcification. The diagnosis of teratoma became evident. Therefore, the focus now turned to the origin of the teratoma. Although retroperitoneal teratomas are more common in children, a diagnosis of a retroperitoneal teratoma was excluded because the patient's ultrasonography (US) and CT indicated that the teratoma was located in the abdominal cavity. After the administration of intravenous contrast material, the supplying vessels were recognized as the branches of the superior mesenteric artery, which meant that the mass originated from the mesentery, and the diagnosis of an ovarian teratoma was excluded. Furthermore, the patient had normal ovaries and normal levels of sex hormones.
Our initial impression was that this patient had a large, polycystic mass that seemed to be a mesenteric teratoma. Because mature teratomas are much more common than immature teratomas in infants, mature mesenteric teratomas were first considered in this patient.
After an extensive discussion with her parents, a decision was made to proceed with a laparotomy. The patient had a preoperative diagnosis of a mesenteric teratoma that required complete resection.
The abdomen was entered through a transverse upper abdominal incision, and a pineapple-sized multiple cystic mass was found (Figures 2A,B). The superior mesenteric artery branches not only wrapped around the mass but also gave rise to arteries that had entered the mass. The vessels supplying blood to the mass were ligated, and the vessels supplying blood to the intestine were separated. Then, the mass was resected intact at the radix of the mesentery (Figure 2C). We finally sutured the hiatus of the mesentery (Figure 2D). None of the intestines were removed. The patient was discharged home with an abdominal circumference of 40 cm and a weight of 7.8 kg (70.5th percentile) on postoperative day 7.
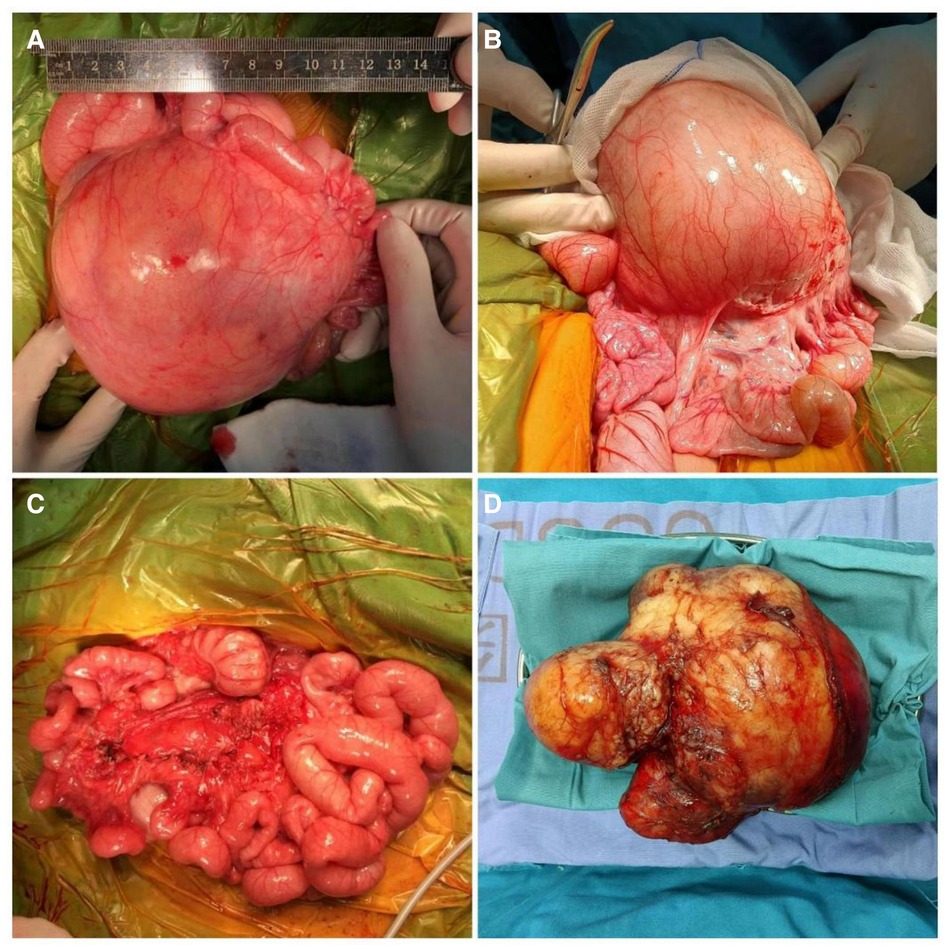
Figure 2. Pictures of a mass obtained during the operation. Pictures (A, B) show that the mass originated from the mesentery. Pictures (C, D) were taken after the mass was resected.
The pathological diagnosis was a (Celiac) solid and cystic mature teratoma (WHO 0). The size of the mass was 14.5 cm × 12 cm × 11 cm. Most parts of the surface were covered with capsules, the nature of the surface ranged between dark purple and sallow, and the mass was smooth. In profile, it was solid with cysts of 0.5–5.5 cm in diameter. The capsule contained a bright viscous liquid, and the remaining solid area was sallow and felt greasy to the touch. Cartilaginous, adipose, glandular, and epithelial tissues were observed (Figure 3).
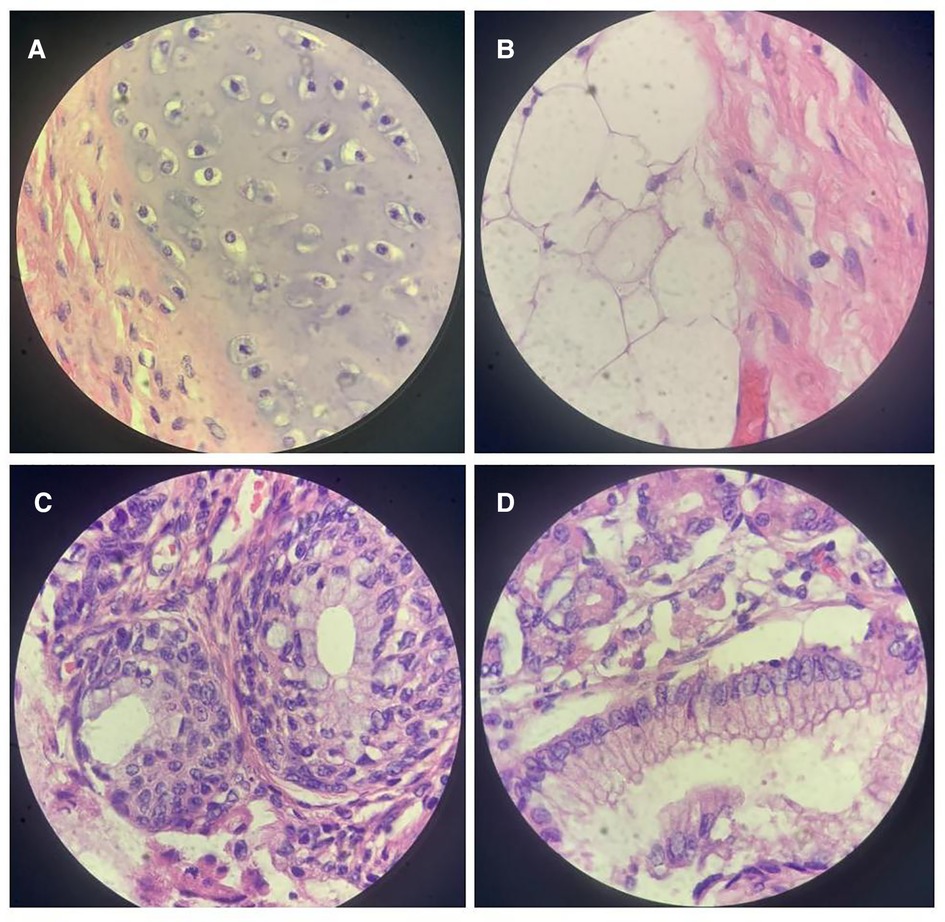
Figure 3. High-power view of the tumor. A microscopic examination revealed cartilaginous tissue (A), adipose tissue (B), glandular tissue (C), and epithelial tissue (D).
The patient recovered well with no subsequent complications and was 9.5 kg (88th percentile) in weight and 73 cm (88th percentile) in height with an abdomen circumference of 49 cm. An ultrasonography revealed that there was no stenosis in the initial segment of the superior mesenteric artery and that blood flow was not restricted. The alpha-fetoprotein (AFP) level dropped to 4.65 ng/mL.
The final diagnosis was a mature teratoma of the mesentery (WHO 0).
Only 12 cases of mesenteric teratoma have been reported worldwide (Table 2). Except for one case that was a fetus, the rest of the cases were patients who ranged in age from 2 months to 9 years, and 8 of the 12 patients were female (58.3%). Half of the patients presented with an asymptomatic abdominal distention/mass, while the other half presented with accompanying symptoms such as vomiting (36.4%), constipation (27.3%), abdominal pain (18.2%), and diarrhea (9.1%). Most of the cases were mature mesenteric teratomas (66.7%), and only one case was multiple mesenteric teratomas.
Reviewing the history of this patient, we noted that she had asymptomatic abdominal distention when she was 4 months old. The patient's weight was 6 kg (29th percentile) at the first visit, and when compared with her birth weight of 3.2 kg (47th percentile), her weight gain was slower than that of infants of the same age. However, 2 months later, the patient's weight increased dramatically from 6 kg (29th percentile) to 8.5 kg (89th percentile), which meant that the weight gain rate significantly accelerated because of unknown factors. The patient's abdominal distention gradually became obvious at the same time, indicating that the mass was growing rapidly.
In previous cases, all patients underwent ultrasonography, and the other imaging examinations were CT, x—ray, and magnetic resonance (MR). However, only three of these patients had been provisionally diagnosed with mesenteric teratoma before surgery. Two patients had been provisionally diagnosed with an ovarian mass and one other as having a tubercular mass. In the remaining patients, there was not sufficient information to determine the origin of the teratoma. While the diagnosis of teratoma can be relatively easy to establish through imaging findings such as ultrasound and CT (the mass contains multiple components such as bone, fat, and fluid), the origin of an abdominal teratoma is more difficult to determine. A mass located on the alimentary tract or related organs strongly suggests the diagnosis of gastrointestinal teratoma (2). The larger the mass is, the more difficult it becomes to determine the source by imaging findings. Combined with the fact that the mass was movable during the physical examination and that the superior mesenteric artery had vascular branches extending into the tumor, the results from further use of the enhanced CT scan indicated that the provisional diagnosis before surgery was a mesenteric teratoma. This provisional diagnosis was confirmed through surgical findings.
The treatment for mesenteric teratoma is surgery, and complete resection of the tumor is warranted. As teratomas always adhere to the root of the mesentery rather than to the distal mesentery, the supplying vessels are relatively independent. Thus, the tumor can be resected without interrupting the continuity of the intestine.
Mesenteric teratomas are very rare teratomas that usually present initially with asymptomatic abdominal distension or digestive tract symptoms, and abdominal ultrasound is the first choice of examination.
In infants, a circumscribed mass located on the alimentary tract or related organs with cystic and solid components, including fat, fluid, and calcified/ossific shadows, suggests mesenteric teratoma.
All mesenteric teratomas should be surgically resected, and enhanced CT should be performed before surgery to clarify the vascular course and ensure that blood flow to the intestines is maintained.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
YJ contributed to the design of the case presentation, drafted the initial manuscript, critically reviewed and revised the manuscript, and was involved in the care of the patient. YZ and YC contributed to the design of the case presentation, critically reviewed and revised the manuscript, and were involved in the care of the patient. JH contributed to the design of the case presentation and critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CT, computed tomography; US, ultrasonography; MR, magnetic resonance.
1. Grosfeld JL, Billmire DF. Teratomas in infancy and childhood. Curr Prob Cancer. (1985) 9(9):1–53. doi: 10.1016/S0147-0272(85)80031-3
2. Bowen B, Ros PR, McCarthy MJ, Olmsted WW, Hjermstad BM. Gastrointestinal teratomas: CT and US appearance with pathologic correlation. Radiology. (1987) 162(2):431–3. doi: 10.1148/radiology.162.2.3541031
3. Prieto ML, Casanova A, Delgado J, Zabalza R. Cystic teratoma of the mesentery. Pediatr Radiol. (1989) 19(6–7):439. doi: 10.1007/BF02387647
4. Chiba T, Iwami D, Kikuchi Y. Mesenteric teratoma in an 8-month-old girl. J Pediatr Surg. (1995) 30(1):120. doi: 10.1016/0022-3468(95)90627-4
5. Marcolongo A, Divirgilio G, Bettili G, Camoglio FS, Fasoli L, Marradi P. Immature mesenteric teratoma in a male newborn infant: prenatal ultrasonographic diagnosis and surgical treatment. Prenatal Diag. (1997) 17(7):686–8. doi: 10.1002/(SICI)1097-0223(199707)17:7%3C686::AID-PD686%3E3.0.CO;2-5
6. Ratan SK, Ratan J, Kalra R. Large benign cystic teratoma of the mesosigmoid causing intestinal obstruction: report of a case. Surg Today. (2002) 32(10):922–4. doi: 10.1007/s005950200183
7. Al-Arfaj AA, El-Shawarby MA, Al-Mulhim FA, Lardhi AA. Mesenteric cystic teratoma in children. Saudi Med J. (2003) 24(12):1388. doi: 10.202207/article.14710290
8. Okada T, Sasaki F, Onodera Y, Oonishi S, Ichikawa N, Itoh T. Multiple mesenteric teratomas: usefulness of spiral computed tomography with 3-dimensional reconstruction. J Pediatr Surg. (2006) 41(4):868–71. doi: 10.1016/j.jpedsurg.2005.12.040
9. Rattan KN, Ratan SK, Jhanwar A, Kaushik V, Magu S. Immature mesenteric teratoma causing intestinal obstruction. Indian J Pediatr. (2007) 74(2):207–8. doi: 10.1007/s12098-007-0020-3
10. Singh M, Ratan K, Rani B, Kadian Y, Hasija S. Infected mature teratoma of mesentery in a child. APSP J Case Rep. (2013) 4(2):18 PMID: 24040596.24040596
11. Zhang X, Zhao Y, Chen X, Hua K. Mature mesenteric teratoma in a child: a case report. Clin Exp Obstet Gyn. (2015) 42(5):714. doi: 10.12891/ceog1952.2015
12. Li J, Li S, Xiao D, Song J, Mao J, Yin J. Extratesticular gliomatosis peritonei after mesenteric teratoma: a case report and literature review. J Int Med Res. (2021) 49(9):030006052110470. doi: 10.1177/03000605211047076
13. Ray R, Dey S, Khatun F, Barman S, Das M, Chatterjee U. Adrenal and mesenteric teratomas in infants: common tumors in uncommon sites. J Indian Assoc Pediatr Surg. (2022) 27(3):354–6. doi: 10.4103/jiaps.JIAPS_26_21
14. Kurtz RJ, Heimann TM, Beck AR, Holt JA. Mesenteric and retroperitoneal cysts. Ann Surg. (1986) 203(1):109–12. doi: 10.1097/00000658-198601000-00017
15. Ranganath SH, Lee EY, Eisenberg RL. Focal cystic abdominal masses in pediatric patients. Am J Roentgenol. (2012) 199(1):W1–16. doi: 10.2214/AJR.11.6642
16. Shah U, Goldstein AM, Gee MS, Deshpande V. Case 24-2017. New Engl J Med. (2017) 377(6):574–82. doi: 10.1056/NEJMcpc1616393
Keywords: teratoma, mesentery, infant, surgery, case report
Citation: Jin Y, Zhang Y, Chen Y and Huang J (2023) A 6-month-old girl with mesenteric teratoma: A Case report and literature review. Front. Pediatr. 11:1131190. doi: 10.3389/fped.2023.1131190
Received: 24 December 2022; Accepted: 16 February 2023;
Published: 15 March 2023.
Edited by:
Nikolaos Zavras, University General Hospital Attikon, GreeceReviewed by:
Lovenish Bains, University of Delhi, India© 2023 Jin, Zhang, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinshi Huang anNkcjIwMDJAMTI2LmNvbQ==
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.