
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 21 March 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1127020
In utero drug exposure is a significant public health threat to the well-being and normal development of the neonate. Recently, testing of umbilical cord tissue (UCT) has been employed to measure illicit drug exposure, as drugs used by the mother during the third trimester may be retained in the UCT. Focus has also been given to potential adverse health effects among drug users, resulting from exposure to pharmacologically active adulterants and cutting agents in the street drug supply. The in utero effects of these substances have not been well studied in humans, nor has their presence been demonstrated as a means for assessing adverse health effects in the neonate. Here, we describe the application of a novel test method to analyze UCT for the presence of more than 20 common adulterating/cutting substances via LC/Q-TOF. In total, 300 de-identified UCT samples were analyzed–all had previously tested positive for cocaine or opiates. Generally, the positivity rates of individual compounds were similar between the Cocaine and Opiates Subgroups, apart from levamisole, xylazine, dipyrone (metabolites), and promethazine. Many of the adulterants used in the street drug supply do have legitimate medicinal/therapeutic uses, including several of the compounds most frequently detected in this study. Caffeine and lidocaine were the most frequently identified compounds both individually (>70% each) and in combination with each other. Alternatively, levamisole, an adulterant with no legitimate therapeutic use, was present in 12% of cases. Importantly, this data demonstrates that the detection of traditional drugs of abuse may serve as indicators of potential in utero exposure to toxic adulterating substances during gestation. While there is cause for concern with respect to any unintentional drug exposure, illicit drug use during pregnancy, including uncontrolled dosing, poly-adulterant consumption, and the interactions of these drug mixtures, produces a significant public health threat to the neonate which warrants further study.
In utero drug exposure has been recognized as a significant public health threat to the well-being and normal development of the neonate (1, 2). Currently, 25 states and the District of Columbia require health care professionals to report suspected prenatal drug use (3). While reporting policies, consequences of reporting, and treatment options vary between states (4), 8 states require testing for prenatal drug exposure when drug use is suspected (3). Consequently, physicians frequently order monitoring and drug testing of pregnant women whose drug-use habits have been identified as putting the health of the mother and fetus at risk (5, 6). This monitoring may be done during pregnancy via urine and/or hair drug testing of the mother to assess recent or long-term drug exposure, respectively. Traditionally, monitoring has been performed following birth, by the collection and testing of meconium, for evidence of maternal drug exposure during the pregnancy. More recently, testing of umbilical cord tissue (UCT) to measure illicit drug exposure has been employed for this purpose. It has been demonstrated that drugs used by the mother during the third trimester may be retained in the cord tissue, and the detection of drugs in the tissue can be used to identify neonates for follow-up monitoring to assess potential health impacts (7), such as agitation, neonatal abstinence syndrome (NAS) (8) and drug withdrawal (9). Additionally, UCT offers several advantages over meconium as a toxicological sample, including immediate availability following birth, larger specimen volume, homogeneous composition, and ease of collection. In contrast, meconium may be released prematurely, before or during delivery, or excreted as a heterogeneous mixture over the first several days of the infant's life, making proper collection for testing purposes difficult (7, 10). Current drug testing practices for UCT have focused on the traditional drugs of abuse, including cocaine, heroin, and methamphetamine; substances with the greatest perceived risk for neonatal health effects.
Recently, additional attention has been given to the potential adverse health effects among drug users resulting from exposure to pharmacologically active adulterants and cutting agents in the street drug supply (11–13). Cutting agents are defined as bulking agents, or pharmacologically inactive diluents added to street drugs for the purpose of adding bulk to dilute the drug and increase the number of doses that can be sold from a given weight of active illicit drug. Adulterants are used for the same purpose but are differentiated in that they have pharmacological activity that may enhance, counter, or alter the illicit drug's effects or exert additional adverse effects on the drug user. The most common cutting agents are sugars and starch (14). Common adulterating substances include levamisole, aminopyrine, diltiazem, and phenacetin, as well as acetaminophen, caffeine, diphenhydramine, quinine, tramadol and xylazine (14, 15). Since the effects of these substances can be significant, and are typically ingested unknowingly by the drug user, they have been designated as Toxic Adulterants (11). Other emerging trends in the adulteration of the illicit drug supply include the adulteration of traditional street drugs with novel psychoactive substances (NPS) in the form of mixed powders and counterfeit and falsified pills, resulting in additional exposure and risk to the user (16). In recent events, adulteration of street drugs has been achieved by the addition of synthetic cannabinoids (17), fentanyl (18), and anticoagulants (19). A recent review considers the many known and potential adverse effects to the mother and child from exposure to drug adulteration from NPS (20).
The health effects of exposure to many of these substances on the fetus in utero have not been well studied in humans, with the exception of caffeine (21, 22) and acetaminophen (23, 24). Anecdotal reports of adverse effects resulting from maternal exposure to some substances have been identified, such as metamizole (dipyrone) (25) and ketamine (26). There are also concerning indications from animal studies of the potential for effects on gestation for toxic adulterating substances such as xylazine (27), tramadol (28), ketamine (29), and quetiapine (30). The persistence of toxic adulterating substances in umbilical cord tissue has likewise not been demonstrated as a potential means for assessing the corresponding adverse health effects on the neonate.
This report describes the application of a previously validated LC/Q-TOF method to analyze a large population of UCT samples for the presence of common toxic adulterating substances. Focus was given to adulterating and cutting agent compounds to better identify the scope and breadth of in utero drug exposure. The method was used to assess 300 UCT samples collected following delivery from drug-exposed mothers. The UCT samples tested positive for opiate or cocaine analytes prior to their use in this study.
Commonly identified adulterating and/or cutting agents from recent studies and analyses of seized drug exhibits (11, 12, 31, 32) were used as the basis for the scope of this analysis. Test analytes of interest include: acetaminophen, aminopyrine and metamizole (dipyrone) breakdown products (4-aminoantipyrine, 4-formylaminoantipyrine, 4-methylaminoantipyrine), benzocaine, caffeine, dextromethorphan, diltiazem, diphenhydramine, ketamine, lidocaine, levamisole, noxiptiline, phenacetin, procaine, promethazine, quetiapine, quinine, tramadol, and xylazine. Internal standards used were acetaminophen-D4, ketamine D-4, and promethazine-D3.
De-identified human UCT samples were provided by United States Drug Testing Laboratories, Inc. (USDTL, Des Plaines, IL). Samples had previously tested positive for cocaine (i.e., benzoylecgonine) or opiate analytes (i.e., 6-monoacetylmorphine (6-MAM), morphine, and/or meconin). The full specimen tissue was rinsed of any external or residual blood with deionized water and patted dry. Approximately one gram was sampled, rinsed in saline solution, and patted dry again to avoid any contamination with residual blood. The sample was cut into small pieces and accurately weighed. A second aliquot was similarly prepared as the duplicate required for standard addition analysis.
Extraction procedures utilizing a standard addition process have been described previously (33). Briefly, duplicate samples were prepared in plastic culture tubes. For each sample, one tube remained unaltered (e.g., blank sample) while the other tube was fortified with a mixture of all 21 analytes of interest (e.g., spiked sample). All tubes received an aliquot of internal standard solution to verify extraction efficacy. Three cleaned, stainless-steel screws were added to each tube, along with acetone/acetonitrile. Tissues were homogenized and then centrifuged. The supernatants were removed, dried, and reconstituted for extraction using solid phase extraction (SPE) techniques. The eluates were collected, evaporated to dryness, and reconstituted for injection. By using the self-controlling method of standard addition, no additional control samples were required for qualitative analysis.
Analysis was performed using liquid chromatography tandem quadrupole time of flight mass spectrometry (LC/Q-TOF) as described previously (33). Samples were analyzed on an Agilent Technologies 1,290 Infinity UHPLC with AJS-ESI 6,545 QTOF with a run time of 48 s, using data dependent acquisition (DDA) for data collection.
Agilent MassHunter Qualitative Analysis 10.0 software was used for data analysis. Retention time (RT, minutes), database scores (DBS), and library search scores (LSS) were used to determine positivity for all drugs in the scope based on validated criteria established during method development (33). A sample was considered positive if it met or exceeded the established criteria for RT, DBS, and LSS. Any results that met or exceeded the LSS, but did not meet RT or DBS criteria were manually reviewed for positivity. Results which did not meet these criteria were reported as none detected.
In total, 300 UCT samples were received from USDTL and analyzed using the method described previously (33). From this population, 183 (61%) samples were reported positive for cocaine, and 117 (39%) were positive for opiates (morphine, 6-MAM and/or meconin). All 117 opiate positive samples were positive for the presence of morphine. Of note, no samples provided were positive for both opiates and cocaine.
With the 300 samples tested, 293 UCT samples (>97%) were found positive for one or more of the adulterant analytes within the scope of testing. Table 1 lists the positivity rate for each adulterant analyte in the scope of the assay. Positivity rates were calculated as a percentage of the number of patients testing positive for that adulterating substance within the applicable test population: Total Cohort, Cocaine Subgroup, and/or Opiates Subgroup. Seven UCT samples were found to be none detected for the full scope of analytes—3 samples from the Cocaine Subgroup and 4 samples from the Opiates Subgroup.
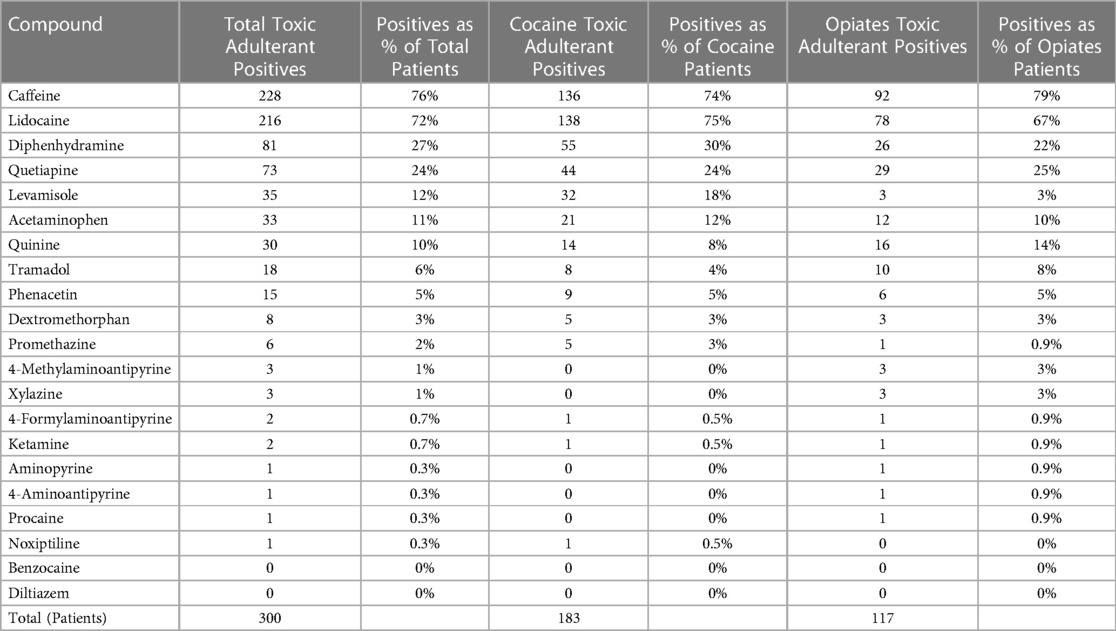
Table 1. Positivity of adulterants as totals and percentages (%) of total numbers of patients in total cohort, cocaine subgroup, and opiates subgroup, respectively.
Results were analyzed to determine the frequency of poly-substance cases (Figure 1), with the overall presence of 2 adulterating substances being the most common poly-drug combination seen (n = 108, 36%) by the Total Cohort. The Cocaine Subgroup saw poly-drug combinations ranging from 0 to 6 different adulterating substances. However, more than 80% (n = 151) of the Cocaine Subgroup contained between 1 and 3 adulterants, with the presence of 2 and/or 3 adulterating substances representing more than two-thirds of the cocaine positive samples. The Opiates Subgroup contained between 0 and 7 different adulterating substances with the presence of 2 drugs being the most common combination (n = 47, 40%). Combinations of 4 or more adulterants were more commonly seen in the Opiates Subgroup (21%) than the Cocaine Subgroup (16%).
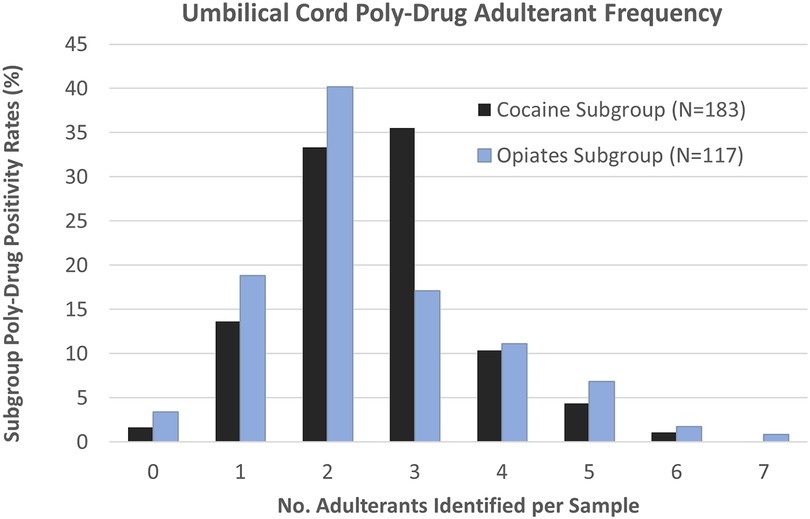
Figure 1. Umbilical cord tissue poly-drug adulterant frequency in Cocaine (black) and Opiates (blue) Subgroups. Individual samples were found to contain anywhere from 0 to 7 adulterating substances.
The positivity rate for each analyte was also compared between the two subgroups to determine whether the presence of an analyte was more commonly associated with one drug subgroup over the other as shown in Figure 2. The graph demonstrates these occurrences with several of the adulterating substances only being found in the Opiates Subgroup population. These include xylazine, 4-methylaminopyrine, procaine, 4-aminoantipyrine, and aminopyrine. Conversely, noxiptiline, levamisole, promethazine, and diphenhydramine were more predominant (>67%) in the Cocaine Subgroup, suggesting that cocaine may be more commonly adulterated with these substances versus opiate formulations. It is also important to point out that the larger population of cocaine positive samples used in this data set may skew some analyte data towards the Cocaine Subgroup. Among these, the positivity rates of caffeine, lidocaine, quetiapine, acetaminophen, phenacetin, and dextromethorphan are higher in the Cocaine Subgroup, though it is unclear if this is a trend from the data or due to the larger cocaine positive population surveyed. Alternately, quinine and tramadol are slightly associated with the Opiates Subgroup.
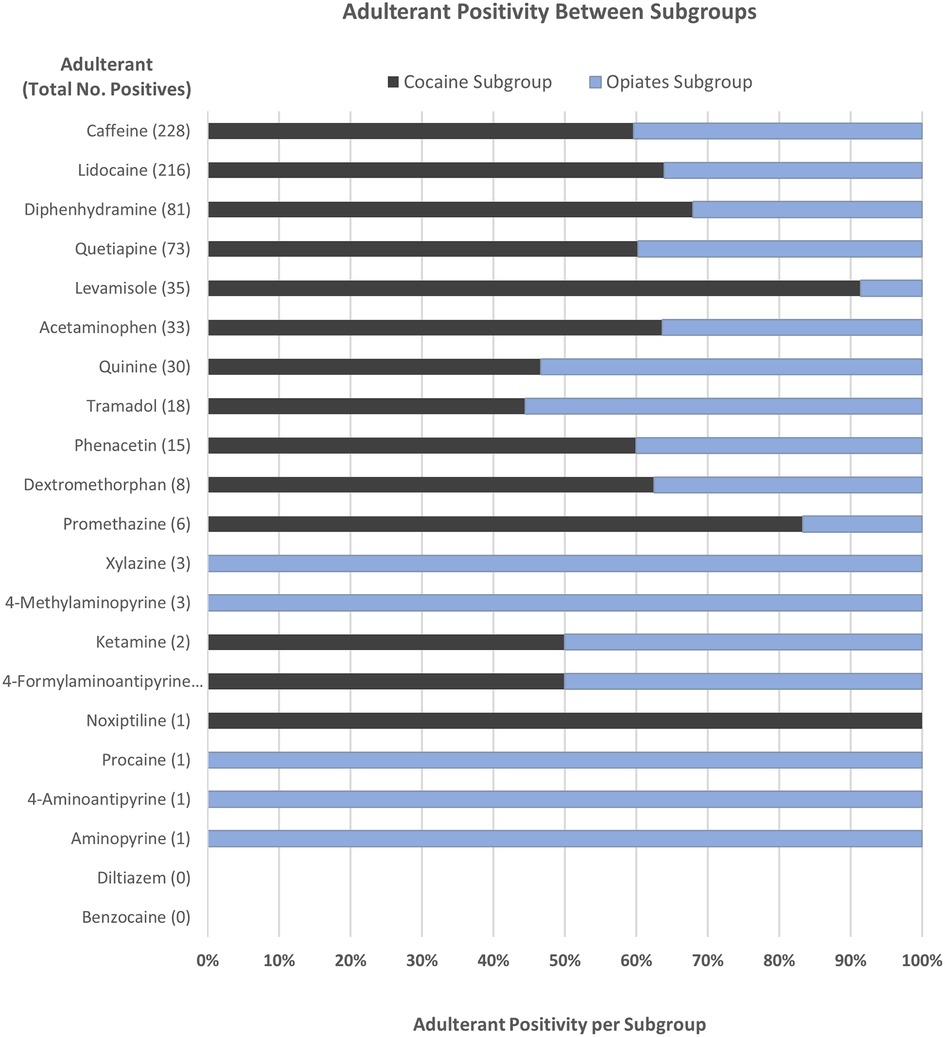
Figure 2. Comparison of toxic adulterant positivity between the Cocaine (black) and Opiates (blue) Subgroups. Positivity rates were determined as a percentage of the total number of positive cases for each adulterant.
The subgroups were analyzed separately to determine the most common adulterant combinations encountered (Figures 3, 4). Overall, the combination of caffeine and lidocaine was the most encountered finding for both the Cocaine Subgroup (n = 38, 20%) and the Opiates Subgroup (n = 28, 23%). Figure 3 illustrates that the next most common Cocaine Subgroup finding was lidocaine-only (n = 12, 6.5%) followed by combinations of caffeine and lidocaine with diphenhydramine (n = 11, 6.0%), quetiapine (n = 10, 5.4%), and levamisole (n = 7, 3.8%), respectively. The presence of caffeine-only was seen in 3.8% (n = 7) of the Cocaine Subgroup. In contrast to this, caffeine-only was the second most common finding (n = 13, 11%) for the Opiates Subgroup (Figure 4). As shown by Figure 4, caffeine-only was followed by the triplet combination of caffeine, lidocaine, and quetiapine (n = 8, 6.8%); lidocaine-only (n = 6, 5.1%); and combinations of quetiapine with either caffeine (n = 4, 3.4%) or lidocaine (n = 3, 2.5%).
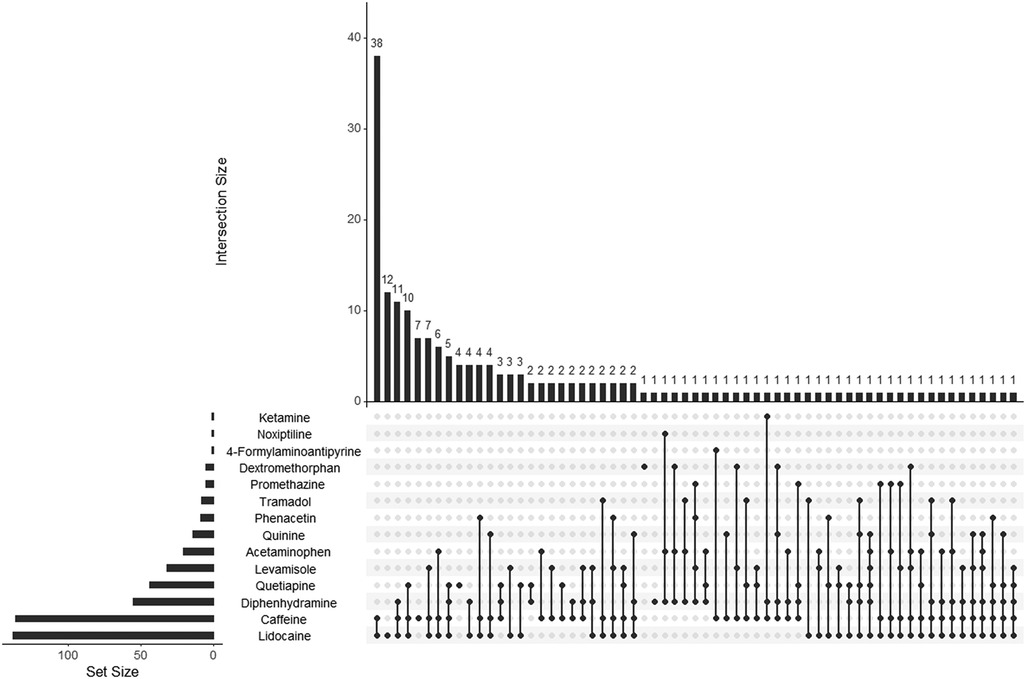
Figure 3. UpSet plot showing adulterant frequency (set size) and poly-drug intersections in umbilical cord tissue from the Cocaine Subgroup. The set size bar graph indicates the total number of positive cases identified for that individual adulterant within the Cocaine Subgroup. Poly-drug combinations, or intersections, are illustrated by the dot diagrams with connecting lines indicating adulterants found in combination with one another. A lone dot indicates a single drug finding. The frequency of each intersection is shown by the histogram at the top of the plot with the frequency number indicated for each combination seen. For example, the Cocaine Subgroup contained 138 lidocaine positive cases, 12 of which were positive for lidocaine-only. The following scope adulterants were not identified in any Cocaine Subgroup samples tested and were not included in the plot: 4-methylaminoantipyrine, xylazine, aminopyrine, 4-aminoantipyrine, procaine, benzocaine, and diltiazem.
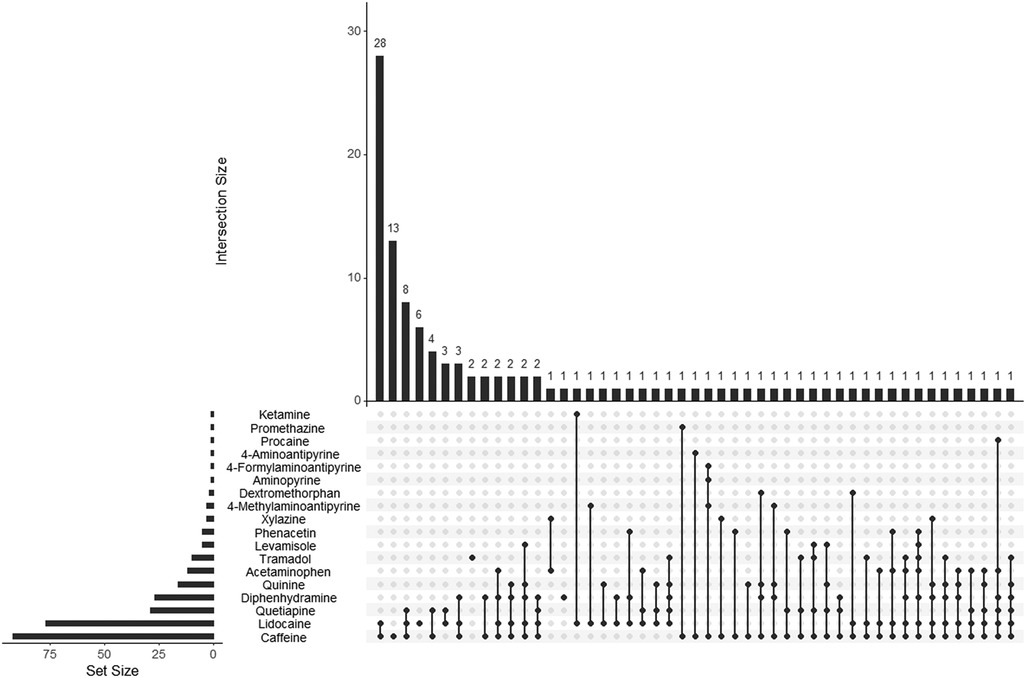
Figure 4. UpSet plot showing adulterant frequency (set size) and poly-drug intersections in umbilical cord tissue from the Opiates Subgroup. The set size bar graph indicates the total number of positive cases identified for that individual adulterant within the Opiates Subgroup. Poly-drug combinations, or intersections, are illustrated by the dot diagrams with connecting lines indicating adulterants found in combination with one another. A lone dot indicates a single drug finding. The frequency of each intersection is shown by the histogram at the top of the plot with the frequency number indicated for each combination seen. For example, the Opiates Subgroup contained 92 caffeine positive cases, 28 of which were positive for the combination of caffeine and lidocaine. The following scope adulterants were not identified in any Opiates Subgroup samples tested and were not included in the plot: noxiptiline, benzocaine, and diltiazem.
Many of the substances known to be used as toxic adulterants in the street drug supply also have legitimate legal or therapeutic uses, including several of the compounds most frequently detected in this study. However, little is known about the impacts and adverse effects for many of these compounds on the fetus/neonate, particularly when consumption exceeds dietary and therapeutic recommendations. Unlike other specimens in the various fields of toxicology, UCT drug testing does not currently have established concentration ranges or cutoff levels. As such, results from this study are reported qualitatively and it is not possible to draw direct conclusions about any toxic effects experienced by the neonates in this study. This would require further investigation with careful monitoring of the neonate's symptoms and development, as well as establishing a non-drug exposed maternal control group for comparison.
Caffeine may be consumed via a wide variety of commercial products, including soft drinks, mixers, coffee, tea, energy drinks and chocolate, or taken therapeutically to treat maladies including headaches and drowsiness. Lidocaine is a common anesthetic administered during medical procedures, including childbirth. Quinine can be found as the primary flavoring ingredient in tonic water and as a common prescription treatment for malaria. Due to their presence in commercial products and routine therapeutic uses, presence of these compounds in a UCT sample cannot be definitively attributed to adulterated street drug consumption. Additionally, common adulterating agents, such as acetaminophen, dextromethorphan, and diphenhydramine are available as conventional over-the-counter (OTC) analgesics, antitussives, and decongestants/sleep aids, respectively. In terms of combinations of adulterants, the most common overall combination involving caffeine and lidocaine (n = 66, 22%) cannot be attributed definitively to illicit drug use.
While these non-prescription drugs are safe in moderation, the doses in illicit street drug preparations are uncontrolled and unknown to the user, putting them at risk of adverse effects. Equally concerning are studies which have shown that excessive consumption of more ubiquitous compounds during pregnancy, such as caffeine and acetaminophen, may increase risks of both fetal growth restrictions and developmental issues (21, 34–36). From this study, the data clearly shows that these compounds are incorporated into the UCT, providing direct exposure to the neonate. What remains unclear is what impact these uncontrolled and unknown doses may have on the neonate both short term and long-term.
Consideration must also be given to drugs that are prescribed as part of routine dental and medical care. For example, lidocaine is administered locally during dental procedures and also during childbirth for pain relief, nerve blocking, and for treating peritoneal stretching and episiotomies (37, 38). Similarly, fentanyl is a common operative analgesic that can also be found routinely in epidurals for pain relief during labor and delivery (39). Ketamine (40), procaine (41), and benzocaine (42) can be administered as anesthetics during medical and dental procedures, and tramadol may be used as a prescription treatment for moderate to severe pain (43). Noxiptiline, a tricyclic antidepressant developed in the 1970s in Europe (44), is not approved or marketed in the United States (45). Complicating things even further, several of the adulterating agents can be prescribed for the treatment of chronic conditions, including the antipsychotic quetiapine, the calcium channel blocker diltiazem, and the multi-purpose drug promethazine. Thus, the presence of an adulterating compound in UCT, alone or in combination, is unable to be fully interpreted without consideration of prenatal and perinatal care. In this study, cases were de-identified as to the donor, such that no clinical histories on the patients were available to differentiate illicit exposure from legitimate routine, therapeutic, or dietary sources of these substances.
Quinine was present in 10% of all cases (n = 30), and in 8% and 14% of the Cocaine and Opiates Subgroups, respectively. While present in the diet as a component of drink mixers, and used routinely in the treatment of malaria, quinine is also commonly used to cut or adulterate cocaine and heroin (11, 12), and this is reflected in the data. When combined with heroin, this substance mimics the “rush” by the hypotensive effect and the bitter taste is similar to that of heroin (46). Quinine can result in cardiovascular toxicity including abnormal heart rhythms and hemolysis (47), or produce other adverse effects if taken in excess. For example, high doses of quinine have been used in some countries in attempts to induce abortions. This resulted in a number of adverse effects for the mothers and neonates in cases where the drug failed to induce an abortion, including blindness, deafness, hemoglobinuria, reversible renal failure, and maternal death (48).
Diphenhydramine is generally considered safe to consume during pregnancy when taken therapeutically to treat symptoms such as allergic rhinitis, headache, insomnia, and postpartum depression (49, 50). However, these studies did not assess outcomes for potentially high doses of diphenhydramine, as might be observed from chronic administration of adulterated cocaine or heroin. Of note, diphenhydramine has been cited in the literature as having potential risks to the health of the mother and/or fetus (51–53). Hyperemesis gravidarum (HG), an extreme persistent nausea during pregnancy, is frequently treated with chronic antihistamine administration. One report indicates that poor neonatal outcomes were significantly greater in women with this condition and were positively associated with gestational hypertension, early start of HG symptoms, and antihistamine use (54). While a few studies have suggested the risk of birth defects resulting from antihistamine use during the first trimester, the results have been inconsistent and there is an overall lack of strong evidence to conclude that any birth defects are associated with therapeutic antihistamine exposure during early pregnancy (55–57). Other authors have called for more studies of OTC medications during pregnancy (52, 58).
Quetiapine (Seroquel®) is a potent second-generation antipsychotic drug used to treat schizophrenia in adults and children. It can be subject to abuse in its own right (59), and has recently been identified as a potential toxic adulterating agent due to having been detected in 10 seized drug exhibits in Kentucky and Vermont (12). Quetiapine was found in UCT in 73 (24%) of the cases in this study, although it cannot be determined if this was from prescription use, from adulteration of street drugs, or a combination of both. In studies regarding women prescribed quetiapine during their first trimester of pregnancy, the authors found no increased risk for major congenital malformations in the infant (60, 61). Case reports identify numerous examples of children (62, 63), adults (64, 65), and a pregnant mother (66) surviving acute quetiapine poisonings—their most common symptoms being tachycardia, QT prolongation, and loss of consciousness. Quetiapine-associated overdose deaths have also been reported (65, 67, 68).
Levamisole, phenacetin, metamizole (dipyrone), and xylazine are of interest in this patient study population as they are not approved in the United States for therapeutic use in humans. All four of these drugs were detected in the UCT from this study, representing likely exposure of the mother and the fetus to these toxic adulterants at unknown doses through the illicit drug supply. Importantly, this is the first documentation of the incorporation of these substances into the cord tissue and, consequently, of transfer to the developing fetal tissue.
Levamisole was found predominantly in the Total Cohort in 12% of cases (n = 35); and in cases positive for cocaine at 18% (n = 32), but in only 3% (n = 3) of the opiate positive cases. These findings are consistent with previously described drug seizure reports, such that levamisole is more commonly seen as a cocaine adulterant but can also be added to fentanyl and heroin supplies (11, 12). Levamisole is an antihelminthic agent used in veterinary medicine but has also been used illicitly as a cocaine adulterant since 2002 (69, 70). This timing coincides with levamisole's withdrawal from United States and Canadian pharmaceutical markets due to reports of serious adverse effects (71). The concentration of levamisole in the domestic United States cocaine supply has steadily increased since it was first detected (72). After acute intake, nausea, diarrhea, and dizziness are common effects of this drug. After prolonged intake, muscle pain, headache, fever, insomnia, dizziness, and convulsions can occur. Potential complications associated with use of levamisole-laced cocaine include neutropenia, agranulocytosis, arthralgias, methemoglobinemia, purpura retiform, systemic vasculitis, cutaneous necrosis, intravascular thrombosis, and skin necrosis (73, 74). To the authors' knowledge, the potential adverse effects of this drug to the developing fetus, at doses associated with typical recreational cocaine use, have not been evaluated. Prior studies have shown that doses of levamisole ingested by drug users are within the toxic range, raising the risk of adverse outcomes for both the mother and the fetus (75).
Phenacetin was detected in 5% (n = 15) of the Total Cohort, representing 5% of the Cocaine Subgroup (n = 9) and 5% of the Opiates Subgroup (n = 6), respectively. Phenacetin is a pain-relieving and antipyretic drug, which can metabolize into acetaminophen. Phenacetin was banned in the United States in 1983 by the FDA (76) due to concerns raised about carcinogenesis, nephropathy, and hemolytic anemia in children (77, 78). In more recent years, phenacetin has been observed as a common cutting agent in street drugs (11, 12), especially as a cocaine additive (79–82). Phenacetin's carcinogenicity has been attributed to the metabolic bioactivation of several reactive downstream metabolites and intermediates, all with variable toxicological consequences (83).
Metamizole/dipyrone and aminopyrine have had similar issues to phenacetin as far as pharmaceutical usage, ranging from removal from the United States market in the 1930s (aminopyrine) (84) to being banned by the FDA in 1977 (dipyrone) and 1979 (metamizole) (76). While proven analgesics and antipyretics, both metamizole/dipyrone and aminopyrine were found to cause severe and sometimes fatal agranulocytosis, leading to their removal from markets in many countries (84–87). The compounds also share a common metabolic pathway. Once inside the body, they quickly metabolize to active 4-methylaminoantipyrine, which can be further broken down into a number of different compounds, including active 4-aminoantipyrine and inactive 4-formylaminoantipyrine (88). Due to rapid metabolism of metamizole/dipyrone, attention was focused on detecting the presence of the metabolites and aminopyrine for the purposes of this study. Interestingly, the metabolites were detected in the Total Cohort, but with widely varying results. The primary metabolite, 4-methylaminoantipyrine, was identified in 3 total cases (1%), all of which came from the Opiates Subgroup. The inactive metabolite, 4-formylaminoantipyrine, was detected in 2 total cases (0.7%), one coming from the Cocaine Subgroup and one from Opiates. And finally, active 4-aminoantipyrine was detected in a single case from the Opiates Subgroup—this was the only case found to contain all three metabolites. The single occurrence of aminopyrine also came from a case in the Opiates Subgroup; however, no other metabolites were present concomitantly. In a systematic review of literature regarding the use of metamizole/dipyrone as a pediatric analgesic, the authors could not conclusively determine the risk of agranulocytosis in children due to the limited amount of data available (89). They also could not find evidence to support that metamizole/dipyrone was superior to other non-steroidal anti-inflammatory drugs (NSAIDs) still on the market (87, 89). In general, the drug is not recommended for use with children (87); or if used, treatment duration should be kept as short as possible to minimize any possible adverse reactions (90). No similar reviews or studies could be located for aminopyrine. In one recent case report from Turkey, a 4-year-old boy developed life-threatening agranulocytosis and anemia after receiving dipyrone as treatment for a fever (91).
Xylazine, a veterinary analgesic not approved for human use, was found in 3 cases (1%), all of which were positive for opiates. Xylazine exposure in humans has been associated with hypotension, bradycardia, orthostatic hypotension, and respiratory depression, and with abscess, ulcerations, and other skin lesions in intravenous drug users (92–94). In more recent years, xylazine has emerged as a common drug adulterant (11, 12, 93), often present with heroin, fentanyl, and/or cocaine. At this time, very little is known about xylazine's pharmacokinetics or mechanism of action in humans. However, as an adulterant in heroin, cocaine, and fentanyl, it can potentiate sedation and respiratory depression, increasing risk of overdose (95). Xylazine does not respond to opioid reversal agents such as naloxone, nor is there a known pharmaceutical antidote specific to xylazine (96). What remains clear is that xylazine may be harmful to humans, particularly when taken concomitantly with other drugs which may further potentiate the effects and lead to toxic and/or fatal results (93).
With regards to illicit adulterants, levamisole in combination with caffeine and lidocaine was the most prevalent overall combination (n = 7, 3.8%), all of which were found in the Cocaine Subgroup. This was followed by a combination of phenacetin, caffeine, and lidocaine (n = 4, 2.2%), again only seen by the Cocaine Subgroup. Inasmuch as levamisole was the most common adulterant present in the Cocaine Subgroup (n = 22), there was no consistent pattern in terms of what other drugs it was found concomitant. Similarly, while the Opiates Subgroup predominated with the presence of xylazine and metamizole/dipyrone metabolites, there were no clear patterns of illicit drug combinations seen with this subgroup. Drug metabolism is different in children and adolescents, and illicit drugs and toxic adulterants can cause greater harm even at lower doses in the juvenile and adolescent brains (97, 98). Drugs and adulterants that suppress the immune system (i.e., deplete white blood cells) are an especially significant threat to children as their immune systems are not fully developed (99).
These data clearly demonstrate that substances other than traditional drugs of abuse can be detected in UCT. Additionally, these substances can act as markers for in utero exposure to potentially toxic adulterating substances during gestation where the umbilical cord also tested positive for cocaine or morphine (from heroin). While there is cause for concern with respect to illicit drug exposure and/or unintentional drug exposure at any age, the neonate population remains particularly vulnerable, as impacts and adverse effects are not well understood and may not be readily apparent at birth. Drug use during pregnancy is a recognized significant public health threat to the neonate with illicit drug use being the primary concern. As highlighted by this study, therapeutic, dietary, and illicit compounds can all be identified in combination in UCT through a high-risk neonatal population. Further studies are needed to better understand how uncontrolled dosing of these potentially toxic adulterating substances, the mixing of multiple adulterating substances, and the interaction of adulterants with each other and with illicit drugs of abuse may impact the health and development of the neonate. Thus, the gathering of neonatal outcome data, in terms of adverse events during delivery, neonatal toxicology results, and neonatal development indicators, should be collected to establish the significance of this newly documented exposure.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
TB and BL conceptualized and designed the study. BN performed method development and data collection. BN and FS completed method validation and preliminary data analysis. KM and BN performed additional data analyses and contributed to manuscript writing and review. All authors contributed to the article and approved the submitted version.
This study was supported by a collaboration between NMS Labs, the Center for Forensic Science Research and Education (CFSRE) at the Fredric Rieders Family Foundation, and the Colombo Plan Secretariat. Open access funding provided by NMS Labs.
The authors wish to thank Tiffany Chan and Joshua DeBord for their assistance with data analysis and generation of UpSet plots.
At the time of this study, authors KM, BN, FS and BL are/were employed by NMS Labs. At the time of publication, author FS is employed by MOBILion Systems, Inc. Remaining author TB declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huestis MA, Choo RE. Drug abuse's Smallest victims: in utero drug exposure. Forensic Sci Int. (2002) 128:20–30. doi: 10.1016/s0379-0738(02)00160-3
2. Narkowicz S, Płotka J, Polkowska Ż, Biziuk M, Namieśnik J. Prenatal exposure to substance of abuse: a worldwide problem. Environ Int. (2013) 54:141–63. doi: 10.1016/j.envint.2013.01.011
3. Substance Use During Pregnancy. Guttmacher Inst (2023) https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy [Accessed February 8, 2023]
4. Jarlenski M, Hogan C, Bogen DL, Chang JC, Bodnar LM, Van Nostrand E. Characterization of U.S. State laws requiring health care provider reporting of perinatal substance use. Womens Health Issues. (2017) 27:264–70. doi: 10.1016/j.whi.2016.12.008
5. Price HR, Collier AC, Wright TE. Screening pregnant women and their neonates for illicit drug use: consideration of the integrated technical, medical, ethical, legal, and social issues. Front Pharmacol. (2018) 9:961. doi: 10.3389/fphar.2018.00961
6. Concheiro M, Lendoiro E, de Castro A, Gónzalez-Colmenero E, Concheiro-Guisan A, Peñas-Silva P, et al. Bioanalysis for cocaine, opiates, methadone, and amphetamines exposure detection during pregnancy. Drug Test Anal. (2017) 9:898–904. doi: 10.1002/dta.2087
7. Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. (2005) 26:11–4. doi: 10.1038/sj.jp.7211416
8. Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome: advances in diagnosis and treatment. JAMA. (2018) 319:1362–74. doi: 10.1001/jama.2018.2640
9. Wabuyele SL, Colby JM, McMillin GA. Detection of drug-exposed newborns. Ther Drug Monit. (2018) 40:166–85. doi: 10.1097/FTD.0000000000000485
10. Montgomery DP, Plate CA, Jones M, Jones J, Rios R, Lambert DK, et al. Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey. J Perinatol Off J Calif Perinat Assoc. (2008) 28:750–3. doi: 10.1038/jp.2008.97
11. Singh VM, Browne T, Montgomery J. The emerging role of toxic adulterants in street drugs in the US illicit opioid crisis. Public Health Rep Wash DC 1974. (2020) 135:6–10. doi: 10.1177/0033354919887741
12. Fiorentin TR, Krotulski AJ, Martin DM, Browne T, Triplett J, Conti T, et al. Detection of cutting agents in drug-positive seized exhibits within the United States. J Forensic Sci. (2019) 64:888–96. doi: 10.1111/1556-4029.13968
13. Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. (2011) 3:89–96. doi: 10.1002/dta.220
14. Broséus J, Gentile N, Esseiva P. The cutting of cocaine and heroin: a critical review. Forensic Sci Int. (2016) 262:73–83. doi: 10.1016/j.forsciint.2016.02.033
15. Solimini R, Rotolo MC, Pellegrini M, Minutillo A, Pacifici R, Busardò FP, et al. Adulteration practices of psychoactive illicit drugs: an updated review. Curr Pharm Biotechnol. (2017) 18:524–30. doi: 10.2174/1389201018666170710184531
16. Minutillo A, Palmi I, Mastrobattista L. The health threat of drugs of abuse adulteration by new psychoactive substances. Clin Ter. (2019) 170:e425–6. doi: 10.7417/CT.2019.2170
17. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. (2017) 376:235–42. doi: 10.1056/NEJMoa1610300
18. Tobias S, Shapiro AM, Grant CJ, Patel P, Lysyshyn M, Ti L. Drug checking identifies counterfeit alprazolam tablets. Drug Alcohol Depend. (2021) 218:108300. doi: 10.1016/j.drugalcdep.2020.108300
19. Moritz E, Austin C, Wahl M, DesLauriers C, Navon L, Walblay K, et al. Notes from the field: outbreak of severe illness linked to the vitamin K antagonist brodifacoum and use of synthetic cannabinoids—illinois, march-April 2018. MMWR Morb Mortal Wkly Rep. (2018) 67:607–8. doi: 10.15585/mmwr.mm6721a4
20. Chomchai S, Phuditshinnapatra J, Mekavuthikul P, Chomchai C. Effects of unconventional recreational drug use in pregnancy. Semin Fetal Neonatal Med. (2019) 24:142–8. doi: 10.1016/j.siny.2019.01.010
21. Fenster L, Eskenazi B, Windham GC, Swan SH. Caffeine consumption during pregnancy and fetal growth. Am J Public Health. (1991) 81:458–61. doi: 10.2105/AJPH.81.4.458
22. Christensen ZP, Freedman EG, Foxe JJ. Caffeine exposure in utero is associated with structural brain alterations and deleterious neurocognitive outcomes in 9-10 year old children. Neuropharmacology. (2021) 186:108479. doi: 10.1016/j.neuropharm.2021.108479
23. Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, et al. Association of cord plasma biomarkers of in utero Acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism Spectrum disorder in childhood. JAMA Psychiatry. (2020) 77:180–9. doi: 10.1001/jamapsychiatry.2019.3259
24. Bauer AZ, Kriebel D, Herbert MR, Bornehag C-G, Swan SH. Prenatal paracetamol exposure and child neurodevelopment: a review. Horm Behav. (2018) 101:125–47. doi: 10.1016/j.yhbeh.2018.01.003
25. Arruza Gómez L, Corredera Sánchez A, Montalvo Montes J, de Marco Guilarte E, Moro Serrano M. [Intrauterine closure of the ductus arteriosus probably associated with the taking of metamizole during the third trimester]. An Pediatr Barc Spain 2003. (2008) 68:626–7. doi: 10.1157/13123300
26. Su P-H, Chang Y-Z, Chen J-Y. Infant with in utero ketamine exposure: quantitative measurement of residual dosage in hair. Pediatr Neonatol. (2010) 51:279–84. doi: 10.1016/S1875-9572(10)60054-X
27. Sakamoto H, Misumi K, Nakama M, Aoki Y. The effects of xylazine on intrauterine pressure, uterine blood flow, maternal and fetal cardiovascular and pulmonary function in pregnant goats. J Vet Med Sci. (1996) 58:211–7. doi: 10.1292/jvms.58.211
28. Aboulhoda BE, Hassan SS. Effect of prenatal tramadol on postnatal cerebellar development: role of oxidative stress. J Chem Neuroanat. (2018) 94:102–18. doi: 10.1016/j.jchemneu.2018.10.002
29. Zhao T, Li C, Wei W, Zhang H, Ma D, Song X, et al. Prenatal ketamine exposure causes abnormal development of prefrontal cortex in rat. Sci Rep. (2016) 6:26865. doi: 10.1038/srep26865
30. Singh KP, Tripathi N. Prenatal exposure to a novel antipsychotic quetiapine: impact on neuro-architecture, apoptotic neurodegeneration in fetal hippocampus and cognitive impairment in young rats. Int J Dev Neurosci Off J Int Soc Dev Neurosci. (2015) 42:59–67. doi: 10.1016/j.ijdevneu.2015.02.011
31. Drug Enforcement Administration. DEA Intelligence Report: The Heroin Signature Program and Heroin Domestic Monitor Program 2014 Reports. (2016).
32. Inter-American Drug Abuse Control Commission (CICAD). Drug adulterants and their effects on the health of users: A critical review. (2019)
33. Nelson BN, Strathmann FG, Browne T, Cervantes A, Logan BK. Qualitative LC-Q-TOF analysis of umbilical cord tissue via data-dependent acquisition as an indicator of in utero exposure to toxic adulterating substances. J Anal Toxicol. (2022) 46:619–24. doi: 10.1093/jat/bkab094
34. CARE Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. Br Med J. (2008) 337:a2332–a2332. doi: 10.1136/bmj.a2332
35. Thiele K, Kessler T, Arck P, Erhardt A, Tiegs G. Acetaminophen And pregnancy: short- and long-term consequences for mother and child. J Reprod Immunol. (2013) 97:128–39. doi: 10.1016/j.jri.2012.10.014
36. Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J. Acetaminophen Use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. (2014) 168:313–20. doi: 10.1001/jamapediatrics.2013.4914
37. Novikova N, Cluver C. Local anaesthetic nerve block for pain management in labour. Cochrane Database Syst Rev. (2012) 4:CD009200. doi: 10.1002/14651858.CD009200.pub2
38. Kargar R, Aghazadeh-Nainie A, Khoddami-Vishteh HR. Comparison of the effects of lidocaine prilocaine cream (EMLA) and lidocaine injection on reduction of perineal pain during perineum repair in normal vaginal delivery. J Fam Reprod Health. (2016) 10:21–6. PMID: 27385970.
39. Nanji JA, Carvalho B. Pain management during labor and vaginal birth. Best Pract Res Clin Obstet Gynaecol. (2020) 67:100–12. doi: 10.1016/j.bpobgyn.2020.03.002
40. Marland S, Ellerton J, Andolfatto G, Strapazzon G, Thomassen O, Brandner B, et al. Ketamine: use in anesthesia. CNS Neurosci Ther. (2013) 19:381–9. doi: 10.1111/cns.12072
41. Weinschenk S, Mergenthaler C, Armstrong C, Göllner R, Hollmann MW, Strowitzki T. Local anesthetics, procaine, lidocaine, and mepivacaine show vasodilatation but No type 1 allergy: a double-blind, placebo-controlled study. BioMed Res Int. (2017) 2017:9804693. doi: 10.1155/2017/9804693
42. Khair-Ul-Bariyah S, Arshad M, Ali M, Din MI, Sharif A, Ahmed E. Benzocaine: review on a drug with unfold potential. Mini Rev Med Chem. (2020) 20:3–11. doi: 10.2174/1389557519666190913145423
43. Subedi M, Bajaj S, Kumar MS, Yc M. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. (2019) 111:443–51. doi: 10.1016/j.biopha.2018.12.085
44. Lingjaerde O, Asker T, Bugge A, Engstrand E, Eide A, Grinaker H, et al. Noxiptilin (agedal)–a new tricyclic antidepressant with a faster onset of action? A double-blind, multicentre comparison with amitriptyline. Pharmakopsychiatr Neuropsychopharmakol. (1975) 8:26–35. doi: 10.1055/s-0028-1094440
45. National Center for Advancing Translational Sciences (NCATS) Inxight Drugs — NOXIPTILINE. https://drugs.ncats.io/drug/DF7D3NY7EL [Accessed November 5, 2021].
47. Phillips KA, Hirsch GA, Epstein DH, Preston KL. Cardiac complications of unwitting co-injection of quinine/quinidine with heroin in an intravenous drug user. J Gen Intern Med. (2012) 27:1722–5. doi: 10.1007/s11606-012-2089-2
48. Dannenberg AL, Dorfman SF, Johnson J. Use of quinine for self-induced abortion. South Med J. (1983) 76:846–9. doi: 10.1097/00007611-198307000-00007
49. Khazaie H, Ghadami MR, Knight DC, Emamian F, Tahmasian M. Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomized clinical trial. Psychiatry Res. (2013) 210:901–5. doi: 10.1016/j.psychres.2013.08.017
50. Childress KMS, Dothager C, Gavard JA, Lebovitz S, Laska C, Mostello DJ. Metoclopramide and diphenhydramine: a randomized controlled trial of a treatment for headache in pregnancy when Acetaminophen alone is ineffective (MAD headache study). Am J Perinatol. (2018) 35:1281–6. doi: 10.1055/s-0038-1646952
51. Saxén I. Letter: cleft palate and maternal diphenhydramine intake. Lancet Lond Engl. (1974) 1:407–8. doi: 10.1016/s0140-6736(74)93172-9
52. Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A. Use of antihistamine medications during early pregnancy and isolated Major malformations. Birt Defects Res A Clin Mol Teratol. (2009) 85:137–50. doi: 10.1002/bdra.20513
53. Brost BC, Scardo JA, Newman RB. Diphenhydramine overdose during pregnancy: lessons from the past. Am J Obstet Gynecol. (1996) 175:1376–7. doi: 10.1016/s0002-9378(96)70059-5
54. Fejzo MS, Magtira A, Schoenberg FP, MacGibbon K, Mullin P, Romero R, et al. Antihistamines and other prognostic factors for adverse outcome in hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. (2013) 170:71–6. doi: 10.1016/j.ejogrb.2013.04.017
55. Li Q, Mitchell AA, Werler MM, Yau W-P, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract. (2013) 1:666–674.e1. doi: 10.1016/j.jaip.2013.07.008
56. Hansen C, Desrosiers TA, Wisniewski K, Strickland MJ, Werler MM, Gilboa SM. Use of antihistamine medications during early pregnancy and selected birth defects: the national birth defects prevention study, 1997-2011. Birth Defects Res. (2020) 112:1234–52. doi: 10.1002/bdr2.1749
57. Gilboa SM, Ailes EC, Rai RP, Anderson JA, Honein MA. Antihistamines and birth defects: a systematic review of the literature. Expert Opin Drug Saf. (2014) 13:1667–98. doi: 10.1517/14740338.2014.970164
58. Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. (2005) 193:771–7. doi: 10.1016/j.ajog.2005.02.100
59. Bogart GT, Ott CA. Abuse of second—generation antipsychotics: what prescribers need to know. Curr Psychiatry. (2011) 10:77–80.
60. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone. Aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. (2015) 116:315–20. doi: 10.1111/bcpt.12372
61. Cohen LS, Góez-Mogollón L, Sosinsky AZ, Savella GM, Viguera AC, Chitayat D, et al. Risk of Major malformations in infants following first-trimester exposure to quetiapine. Am J Psychiatry. (2018) 175:1225–31. doi: 10.1176/appi.ajp.2018.18010098
62. Catalano G, Catalano MC, Agustines RE, Dolan EM, Paperwalla KN. Pediatric quetiapine overdose: a case report and literature review. J Child Adolesc Psychopharmacol. (2002) 12:355–61. doi: 10.1089/104454602762599916
63. Juhl GA, Benitez JG, McFarland S. Acute quetiapine overdose in an eleven-year-old girl. Vet Hum Toxicol. (2002) 44:163–4. PMID: 12046970.12046970
64. Hustey FM. Acute quetiapine poisoning. J Emerg Med. (1999) 17:995–7. doi: 10.1016/s0736-4679(99)00128-6
65. Ngo A, Ciranni M, Olson KR. Acute quetiapine overdose in adults: a 5-year retrospective case series. Ann Emerg Med. (2008) 52:541–7. doi: 10.1016/j.annemergmed.2008.03.016
66. Paulzen M, Gründer G, Orlikowsky T, Gräf CM, Hoeltzenbein M, Veselinovic T. Suicide attempt during late pregnancy with quetiapine: nonfatal outcome despite severe intoxication. J Clin Psychopharmacol. (2015) 35:343–4. doi: 10.1097/JCP.0000000000000308
67. Langman LJ, Kaliciak HA, Carlyle S. Fatal overdoses associated with quetiapine. J Anal Toxicol. (2004) 28:520–5. doi: 10.1093/jat/28.6.520
68. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. (2002) 159:2114–2114. doi: 10.1176/appi.ajp.159.12.2114
70. Midthun KM, Nelson LS, Logan BK. Levamisole-a toxic adulterant in illicit drug preparations: a review. Ther Drug Monit. (2021) 43:221–8. doi: 10.1097/FTD.0000000000000851
71. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. (2012) 87:581–6. doi: 10.1016/j.mayocp.2012.03.010
72. Substance Abuse and Mental Health Services Administration (SAMHSA). Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug. (2009)
73. Midthun K.M., Mohr A.L.A., Browne T., Martin D.M., Logan B.K. Levamisole: A toxic adulterant found in illicit street drugs. (2020) https://www.npsdiscovery.org/wp-content/uploads/2020/08/Public-Alert_Levamisole_Final2.pdf [Accessed November 3, 2021]
74. Brunt TM, van den Berg J, Pennings E, Venhuis B. Adverse effects of levamisole in cocaine users: a review and risk assessment. Arch Toxicol. (2017) 91:2303–13. doi: 10.1007/s00204-017-1947-4
75. Fiorentin TR, Fogarty M, Limberger RP, Logan BK. Determination of cutting agents in seized cocaine samples using GC-MS, GC-TMS and LC-MS/MS. Forensic Sci Int. (2019) 295:199–206. doi: 10.1016/j.forsciint.2018.12.016
76. List of drug products that have been withdrawn or removed from the market for reasons of safety or effectiveness. Food and drug administration, HHS. Final rule. Fed Regist. (1999) 64:10944–7. PMID: 10557618.10557618
77. Carro-Ciampi G. Phenacetin abuse: a review. Toxicology. (1978) 10:311–39. doi: 10.1016/0300-483x(78)90081-1
78. Peterson RG. Consequences associated with nonnarcotic analgesics in the fetus and newborn. Fed Proc. (1985) 44:2309–13. PMID 3884385.3884385
79. Ladroue V, Dujourdy L, Besacier F, Jame P. IRMS To study a common cocaine cutting agent: phenacetin. Drug Test Anal. (2017) 9:479–84. doi: 10.1002/dta.2137
80. Gameiro R, Costa S, Barroso M, Franco J, Fonseca S. Toxicological analysis of cocaine adulterants in blood samples. Forensic Sci Int. (2019) 299:95–102. doi: 10.1016/j.forsciint.2019.03.005
81. Ribeiro M, Trevizol AP, Frajzinger R, Ribeiro A, Speierl H, Pires L, et al. Adulterants in crack cocaine in Brazil. Trends Psychiatry Psychother. (2019) 41:186–90. doi: 10.1590/2237-6089-2017-0143
82. Fucci N. Phenacetin and cocaine in a body packer. Forensic Sci Int. (2004) 141:59–61. doi: 10.1016/j.forsciint.2003.12.012
83. Taxak N, Chaitanya Prasad K, Bharatam PV. Mechanistic insights into the bioactivation of phenacetin to reactive metabolites: a DFT study. Comput Theor Chem. (2013) 1007:48–56. doi: 10.1016/j.comptc.2012.11.018
84. Aminopyrine, dipyrone and agranulocytosis. Can Med Assoc J. (1964) 91:1229–30. PMID: 14226100.14226100
85. Bailey DN. The unusual occurrence of 4-aminoantipyrine (4-aminophenazone) in human biological fluids. J Anal Toxicol. (1983) 7:76–8. doi: 10.1093/jat/7.2.76
86. Lutz M. Metamizole (dipyrone) and the liver: a review of the literature. J Clin Pharmacol. (2019) 59:1433–42. doi: 10.1002/jcph.1512
87. Rollason V, Desmeules JA. Use of metamizole in children and the risk of agranulocytosis: is the benefit worth the risk? Eur J Anaesthesiol EJA. (2015) 32:837–8. doi: 10.1097/EJA.0000000000000275
88. Volz M, Kellner HM. Kinetics and metabolism of pyrazolones (propyphenazone, aminopyrine and dipyrone). Br J Clin Pharmacol. (1980) 10(Suppl 2):299S–308S. doi: 10.1111/j.1365-2125.1980.tb01813.x
89. de Leeuw TG, Dirckx M, Gonzalez Candel A, Scoones GP, Huygen FJPM, de Wildt SN. The use of dipyrone (metamizol) as an analgesic in children: what is the evidence? A review. Paediatr Anaesth. (2017) 27:1193–201. doi: 10.1111/pan.13257
90. Ziesenitz VC, Erb TO, Trachsel D, van den Anker JN. Safety of dipyrone (metamizole) in children-What's The risk of agranulocytosis? Paediatr Anaesth. (2018) 28:186–7. doi: 10.1111/pan.13312
91. Isik M, Kaya Z, Belen FB, Aktas AT, Tezer H, Gürsel T. Life-threatening agranulocytosis, Anemia, and plasmacytosis after dipyrone use for fever in a child. J Pediatr Hematol Oncol (2014) 36:e46. doi: 10.1097/MPH.0b013e31826e7d9d
92. Mohr A.L.A., Browne T., Martin D.M., Logan B.K. Xylazine: A toxic adulterant found in illicit street drugs. (2020) https://www.npsdiscovery.org/wp-content/uploads/2020/11/Public-Alert_Xylazine-Final.pdf [Accessed November 3, 2021]
93. Ruiz-Colón K, Chavez-Arias C, Díaz-Alcalá JE, Martínez MA. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: a comprehensive review of the literature. Forensic Sci Int. (2014) 240:1–8. doi: 10.1016/j.forsciint.2014.03.015
94. Mittleman RE, Hearn WL, Hime GW. Xylazine toxicity–literature review and report of two cases. J Forensic Sci. (1998) 43:400–2. doi: 10.1520/JFS16155J
95. Wong SC, Curtis JA, Wingert WE. Concurrent detection of heroin, fentanyl, and xylazine in seven drug-related deaths reported from the Philadelphia medical Examiner's Office. J Forensic Sci. (2008) 53:495–8. doi: 10.1111/j.1556-4029.2007.00648.x
96. Kariisa M, Patel P, Smith H, Bitting J. Notes from the field: xylazine detection and involvement in drug overdose deaths — united States, 2019. Morb Mortal Wkly Rep. (2021) 70:1300–2. doi: 10.15585/mmwr.mm7037a4
97. Bell GL, Lau K. Perinatal and neonatal issues of substance abuse. Pediatr Clin North Am. (1995) 42:261–81. doi: 10.1016/s0031-3955(16)38946-5
98. Wagner CL, Katikaneni LD, Cox TH, Ryan RM. The impact of prenatal drug exposure on the neonate. Obstet Gynecol Clin North Am. (1998) 25:169–94. doi: 10.1016/s0889-8545(05)70364-8
Keywords: umbilical cord, adulterant, levamisole, dipyrone, phenacetin, xylazine, neonate
Citation: Midthun KM, Nelson BN, Strathmann FG, Browne T and Logan BK (2023) Analysis of umbilical cord tissue as an indicator of in utero exposure to toxic adulterating substances. Front. Pediatr. 11:1127020. doi: 10.3389/fped.2023.1127020
Received: 19 December 2022; Accepted: 27 February 2023;
Published: 21 March 2023.
Edited by:
Sule Cataltepe, Harvard Medical School, United StatesReviewed by:
Sara Berkelhamer, University of Washington, United States© 2023 Midthun, Nelson, Strathmann, Browne and Logan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kari M. Midthun a2FyaS5taWR0aHVuQG5tc2xhYnMuY29t
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.